Abstract
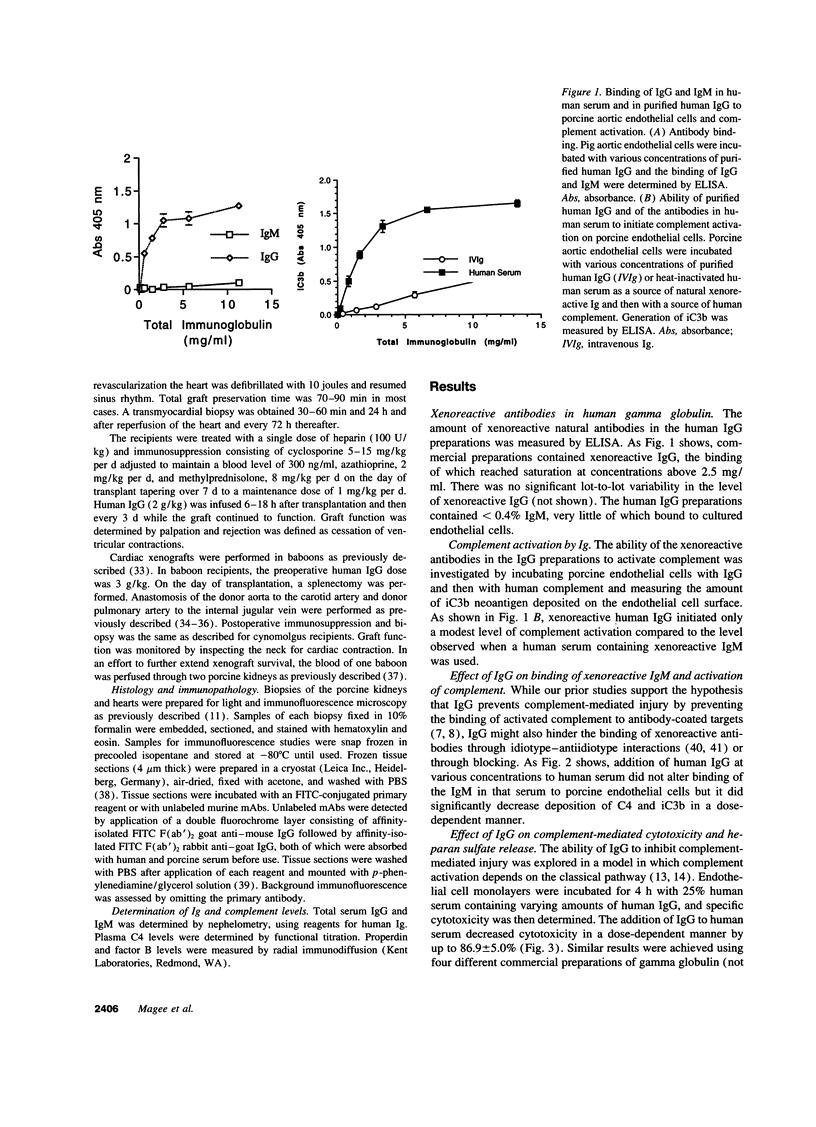
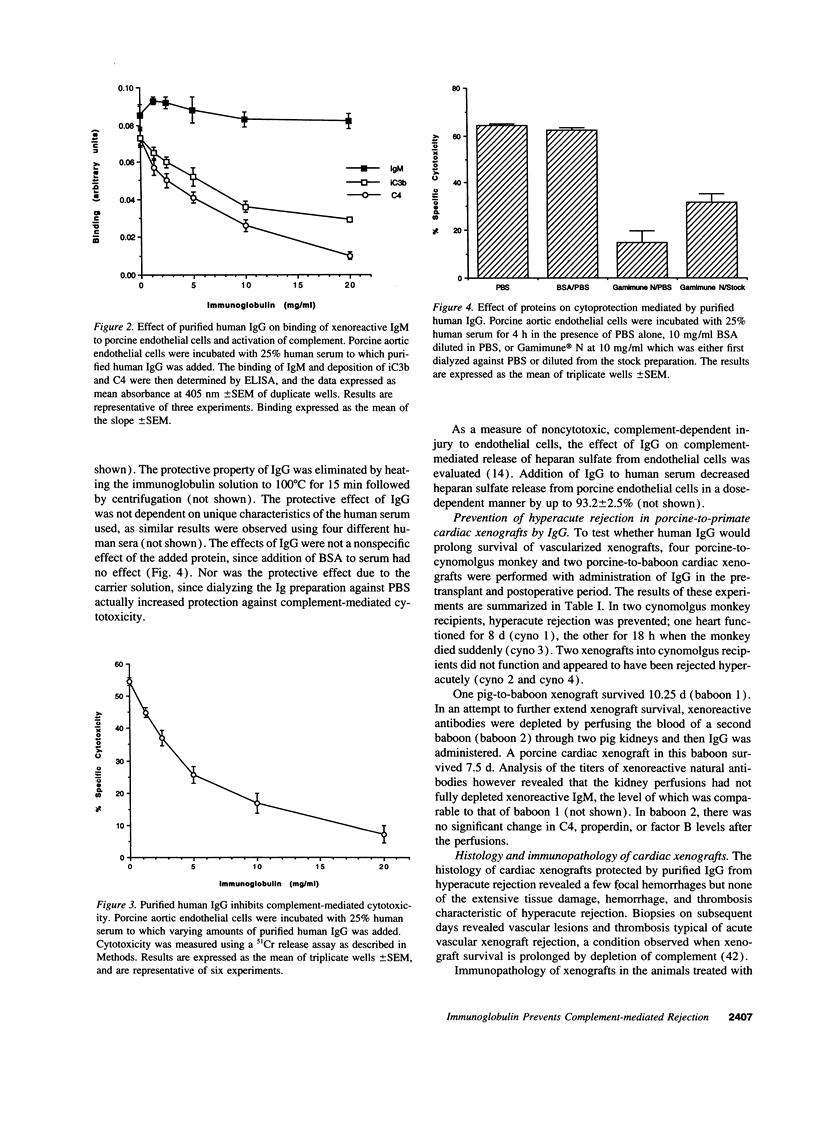
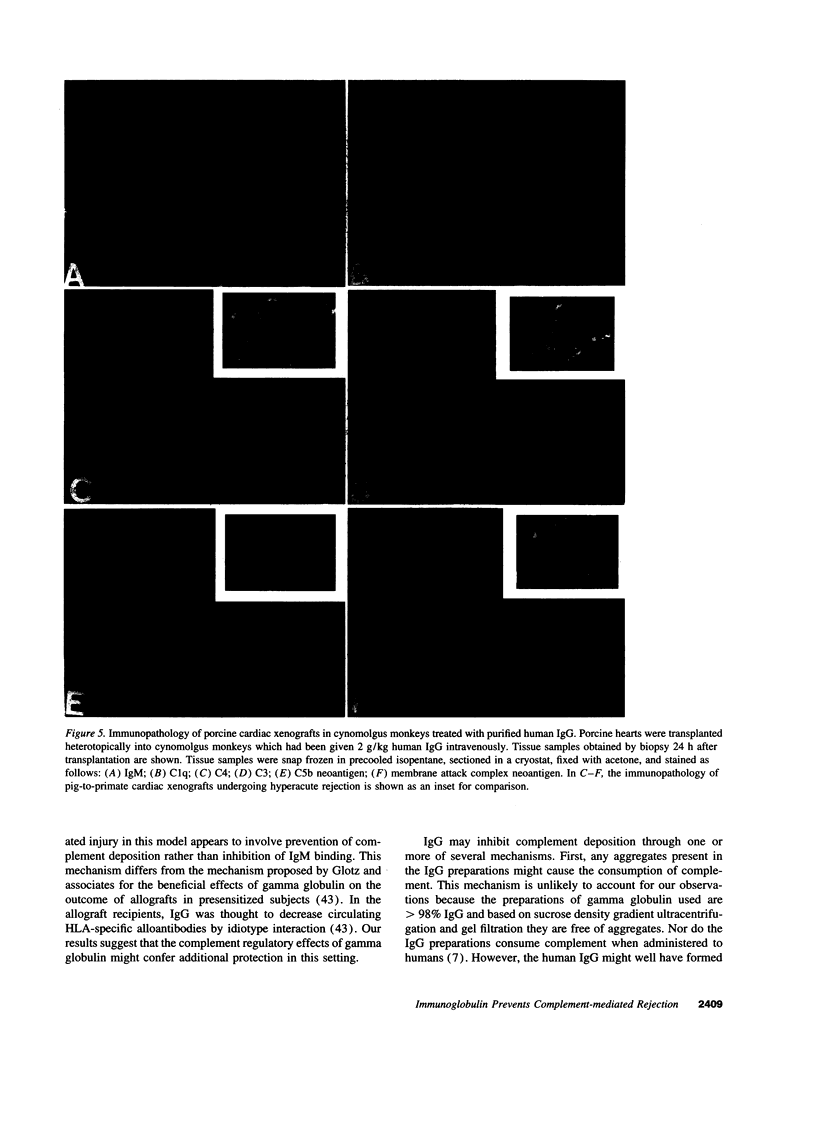
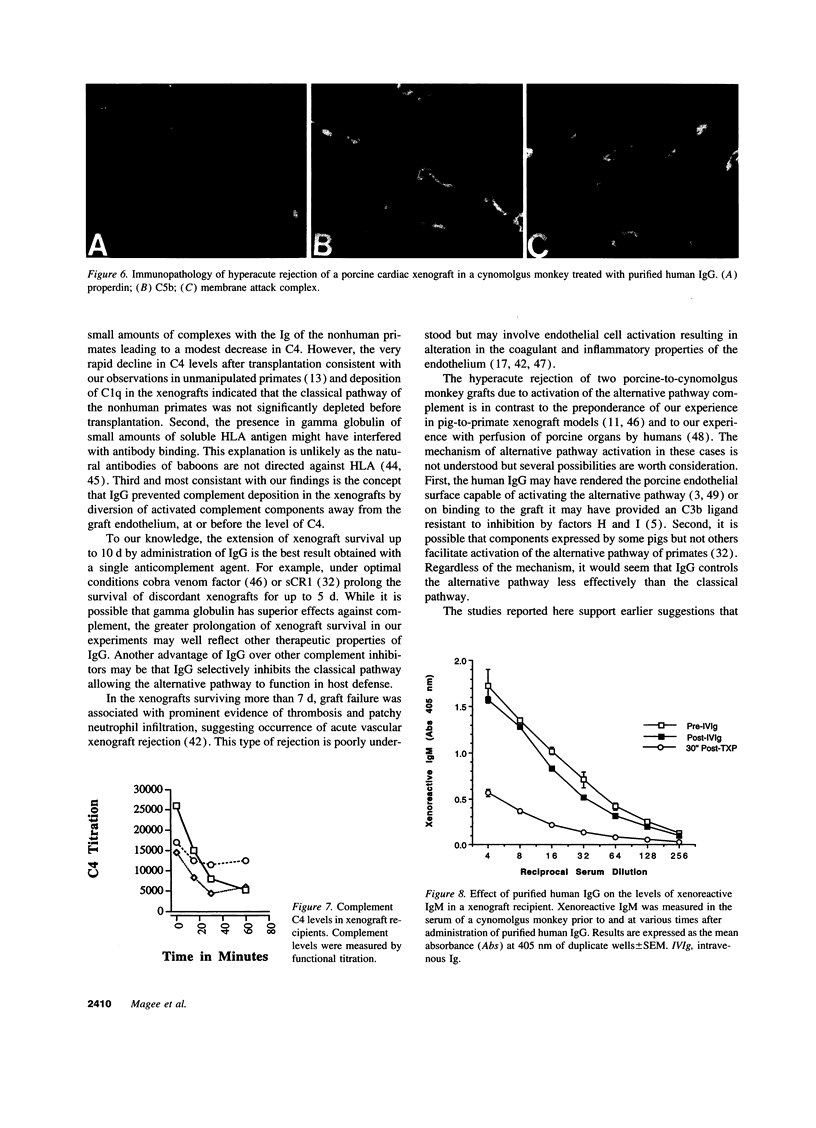
Immunoglobulins regulate the complement system by activating complement on foreign surfaces and diverting reactive complement proteins away from autologous cell surfaces. Based on this model, we explored the ability of Ig to balance complement activation versus control in a pig-to-primate cardiac xenotransplantation model in which the binding of xenoreactive antibodies of the recipient to graft blood vessels and the activation of complement cause hyperacute rejection. Human IgG added to human serum caused a dose-dependent decrease in deposition of iC3b, cytotoxicity, and heparan sulfate release when the serum was incubated with porcine endothelial cells. This decrease was not caused by alteration in antibody binding or consumption of complement but presumably reflected decreased formation of C3 convertase on the endothelial cells. Infusion of purified human IgG into nonhuman primates prevented hyperacute rejection of porcine hearts transplanted into the primates. As expected, the transplants contained deposits of recipient Ig and C1q but not other complement components. The inhibition of complement on endothelial cell surfaces and in the xenotransplantation model supports the idea that IgG regulates the classical complement pathway and supports therapeutic use of that agent in humoral-mediated disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado C. G., Cotterell A. H., McCurry K. R., Collins B. H., Magee J. C., Berthold J., Logan J. S., Platt J. L. Variation in the level of xenoantigen expression in porcine organs. Transplantation. 1995 Jun 15;59(11):1589–1596. [PubMed] [Google Scholar]
- Basta M., Kirshbom P., Frank M. M., Fries L. F. Mechanism of therapeutic effect of high-dose intravenous immunoglobulin. Attenuation of acute, complement-dependent immune damage in a guinea pig model. J Clin Invest. 1989 Dec;84(6):1974–1981. doi: 10.1172/JCI114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. H., Chari R. S., Magee J. C., Harland R. C., Lindman B. J., Logan J. S., Bollinger R. R., Meyers W. C., Platt J. L. Mechanisms of injury in porcine livers perfused with blood of patients with fulminant hepatic failure. Transplantation. 1994 Dec 15;58(11):1162–1171. [PubMed] [Google Scholar]
- Collins B. H., Cotterell A. H., McCurry K. R., Alvarado C. G., Magee J. C., Parker W., Platt J. L. Cardiac xenografts between primate species provide evidence for the importance of the alpha-galactosyl determinant in hyperacute rejection. J Immunol. 1995 May 15;154(10):5500–5510. [PubMed] [Google Scholar]
- Cooper D. K., Human P. A., Lexer G., Rose A. G., Rees J., Keraan M., Du Toit E. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988 May-Jun;7(3):238–246. [PubMed] [Google Scholar]
- Cooper N. R. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Fischel R. J., Bolman R. M., Bach F. H., Platt J. L. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol. 1992 May;140(5):1157–1166. [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M. Manipulating the immune system with immune globulin. N Engl J Med. 1992 Jan 9;326(2):107–116. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischel R. J., Matas A. J., Platt J. L., Perry E., Noreen H., Shumway S. J., Bolman R. M., 3rd Cardiac xenografting in the pig-to-rhesus monkey model: manipulation of antiendothelial antibody prolongs survival. J Heart Lung Transplant. 1992 Sep-Oct;11(5):965–974. [PubMed] [Google Scholar]
- Frank M. M., Basta M., Fries L. F. The effects of intravenous immune globulin on complement-dependent immune damage of cells and tissues. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S82–S86. doi: 10.1016/0090-1229(92)90045-p. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Gaither T. A., Hammer C. H., Frank M. M. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984 Dec 1;160(6):1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Clark M. R., Shohet S. B., Buehler J., Macher B. A. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993 Oct;14(10):480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- Galili U., Macher B. A., Buehler J., Shohet S. B. Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha (1----3)-linked galactose residues. J Exp Med. 1985 Aug 1;162(2):573–582. doi: 10.1084/jem.162.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Rachmilewitz E. A., Peleg A., Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotz D., Haymann J. P., Sansonetti N., Francois A., Menoyo-Calonge V., Bariety J., Druet P. Suppression of HLA-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg). A potential tool for transplantation of immunized patients. Transplantation. 1993 Aug;56(2):335–337. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- Holzknecht Z. E., Platt J. L. Identification of porcine endothelial cell membrane antigens recognized by human xenoreactive natural antibodies. J Immunol. 1995 May 1;154(9):4565–4575. [PubMed] [Google Scholar]
- Joiner K. A., Fries L. F., Schmetz M. A., Frank M. M. IgG bearing covalently bound C3b has enhanced bactericidal activity for Escherichia coli 0111. J Exp Med. 1985 Sep 1;162(3):877–889. doi: 10.1084/jem.162.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal J. R., Dalmasso A. P., Cromwell J. W., Platt J. L., Manivel C. J., Bolman R. M., 3rd, Matas A. J. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993 Apr;55(4):857–866. doi: 10.1097/00007890-199304000-00033. [DOI] [PubMed] [Google Scholar]
- Magee J. C., Platt J. L. Xenograft rejection--molecular mechanisms and therapeutic implications. Ther Immunol. 1994 Jan;1(1):45–58. [PubMed] [Google Scholar]
- McCurry K. R., Kooyman D. L., Alvarado C. G., Cotterell A. H., Martin M. J., Logan J. S., Platt J. L. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995 May;1(5):423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- Michler R. E., McManus R. P., Smith C. R., Sadeghi A. N., Rose E. A. Technique for primate heterotopic cardiac xenotransplantation. J Med Primatol. 1985;14(6):357–362. [PubMed] [Google Scholar]
- Moore F. D., Jr, Austen K. F., Fearon D. T. Antibody restores human alternative complement pathway activation by mouse erythrocytes rendered functionally deficient by pretreatment with pronase. J Immunol. 1982 Mar;128(3):1302–1306. [PubMed] [Google Scholar]
- Parker W., Bruno D., Holzknecht Z. E., Platt J. L. Characterization and affinity isolation of xenoreactive human natural antibodies. J Immunol. 1994 Oct 15;153(8):3791–3803. [PubMed] [Google Scholar]
- Platt J. L., Dalmasso A. P., Lindman B. J., Ihrcke N. S., Bach F. H. The role of C5a and antibody in the release of heparan sulfate from endothelial cells. Eur J Immunol. 1991 Nov;21(11):2887–2890. doi: 10.1002/eji.1830211135. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Fischel R. J., Matas A. J., Reif S. A., Bolman R. M., Bach F. H. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991 Aug;52(2):214–220. doi: 10.1097/00007890-199108000-00006. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Grant B. W., Eddy A. A., Michael A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983 Oct 1;158(4):1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Lindman B. J., Chen H., Spitalnik S. L., Bach F. H. Endothelial cell antigens recognized by xenoreactive human natural antibodies. Transplantation. 1990 Nov;50(5):817–822. doi: 10.1097/00007890-199011000-00015. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Lindman B. J., Geller R. L., Noreen H. J., Swanson J. L., Dalmasso A. P., Bach F. H. The role of natural antibodies in the activation of xenogenic endothelial cells. Transplantation. 1991 Dec;52(6):1037–1043. doi: 10.1097/00007890-199112000-00019. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Turman M. A., Noreen H. J., Fischel R. J., Bolman R. M., 3rd, Bach F. H. An ELISA assay for xenoreactive natural antibodies. Transplantation. 1990 May;49(5):1000–1001. doi: 10.1097/00007890-199005000-00033. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Vercellotti G. M., Dalmasso A. P., Matas A. J., Bolman R. M., Najarian J. S., Bach F. H. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990 Dec;11(12):450–457. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Vercellotti G. M., Lindman B. J., Oegema T. R., Jr, Bach F. H., Dalmasso A. P. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990 Apr 1;171(4):1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt S. K., Kirk A. D., Bollinger R. R., Marsh H. C., Jr, Collins B. H., Levin J. L., Mault J. R., Heinle J. S., Ibrahim S., Rudolph A. R. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation. 1994 Feb;57(3):363–370. doi: 10.1097/00007890-199402150-00009. [DOI] [PubMed] [Google Scholar]
- Ratnoff W. D., Fearon D. T., Austen K. F. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6(4):361–371. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- Ronda N., Hurez V., Kazatchkine M. D. Intravenous immunoglobulin therapy of autoimmune and systemic inflammatory diseases. Vox Sang. 1993;64(2):65–72. doi: 10.1111/j.1423-0410.1993.tb02521.x. [DOI] [PubMed] [Google Scholar]
- Saadi S., Platt J. L. Transient perturbation of endothelial integrity induced by natural antibodies and complement. J Exp Med. 1995 Jan 1;181(1):21–31. doi: 10.1084/jem.181.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi A. M., Robbins R. C., Smith C. R., Kurlansky P. A., Michler R. E., Reemtsma K., Rose E. A. Cardiac xenotransplantation in primates. J Thorac Cardiovasc Surg. 1987 Jun;93(6):809–814. [PubMed] [Google Scholar]
- Sadeghi A. M., Robbins R. C., Smith C. R., Kurlansky R. A., Michler R. E., Reemtsma K., Rose E. A. Cardiac xenograft survival in baboons treated with cyclosporine in combination with conventional immunosuppression. Transplant Proc. 1987 Feb;19(1 Pt 2):1149–1152. [PubMed] [Google Scholar]
- Sim R. B., Reid K. B. C1: molecular interactions with activating systems. Immunol Today. 1991 Sep;12(9):307–311. doi: 10.1016/0167-5699(91)90004-D. [DOI] [PubMed] [Google Scholar]




