Abstract
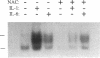
We have recently discovered bovine and human vascular smooth muscle cells (SMCs) express a novel constitutive Nuclear Factor-kappa B (NF-kappa B)/Rel-like activity (Lawrence, R., L.-J. Chang, U. Siebenlist, P. Bressler, and G.E. Sonenshein. 1994. J. Biol. Chem. 269:28913-28918), here termed SMC-Rel. Since cytomegalovirus (CMV) infection of human vascular SMCs has been implicated in aberrant SMC proliferation during post-angioplasty restenosis, we tested the role of NF-kappa B/Rel activity in transactivation of the CMV immediate early (ie) promoter. The basal CMV ie promoter linked to three wild-type, but not mutant, copies of its NF-kappa B element was active in bovine aortic SMCs. The anti-oxidants N-acetyl cysteine (NAC) or pentoxifylline (PTX), which are used clinically to reduce NF-kappa B/Rel activity, inhibited NF-kappa B driven promoter transactivation, and SMC-Rel binding activity. Treatment with either NAC or PTX was observed to slow the growth of the SMCs in a dose dependent fashion. Microinjection of either purified I kappa B-alpha, a naturally occurring specific inhibitor of NF-kappa B/Rel activity, or double-stranded oligonucleotides harboring wild type, but not non-binding mutants of NF-kappa B elements selectively inhibited SMC proliferation. Thus constitutive NF-kappa B/Rel activity appears essential for proliferation of vascular SMCs and might be a novel target for therapeutic intervention for restenosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Biswas D. K., Dezube B. J., Ahlers C. M., Pardee A. B. Pentoxifylline inhibits HIV-1 LTR-driven gene expression by blocking NF-kappa B action. J Acquir Immune Defic Syndr. 1993 Jul;6(7):778–786. [PubMed] [Google Scholar]
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Brown K. E., Kindy M. S., Sonenshein G. E. Expression of the c-myb proto-oncogene in bovine vascular smooth muscle cells. J Biol Chem. 1992 Mar 5;267(7):4625–4630. [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington J. M., Mocarski E. S. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989 Mar;63(3):1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Forster L., Stewart-Lee A., Konneh M., Nourooz-Zadeh J., Anggård E. E. Probucol inhibits neointimal thickening and macrophage accumulation after balloon injury in the cholesterol-fed rabbit. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11312–11316. doi: 10.1073/pnas.89.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyschuss A., Stiko-Rahm A., Swedenborg J., Henriksson P., Björkhem I., Berglund L., Nilsson J. Antioxidant treatment inhibits the development of intimal thickening after balloon injury of the aorta in hypercholesterolemic rabbits. J Clin Invest. 1993 Apr;91(4):1282–1288. doi: 10.1172/JCI116326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Ji L., Arcinas M., Boxer L. M. NF-kappa B sites function as positive regulators of expression of the translocated c-myc allele in Burkitt's lymphoma. Mol Cell Biol. 1994 Dec;14(12):7967–7974. doi: 10.1128/mcb.14.12.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa F. A., Pierce J. W., Sonenshein G. E. Differential regulation of the c-myc oncogene promoter by the NF-kappa B rel family of transcription factors. Mol Cell Biol. 1994 Feb;14(2):1039–1044. doi: 10.1128/mcb.14.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Tourkine N., Jeanteur P., Blanchard J. M. Demonstration in living cells of an intragenic negative regulatory element within the rodent c-fos gene. Cell. 1990 May 4;61(3):485–496. doi: 10.1016/0092-8674(90)90530-r. [DOI] [PubMed] [Google Scholar]
- Lawrence R., Chang L. J., Siebenlist U., Bressler P., Sonenshein G. E. Vascular smooth muscle cells express a constitutive NF-kappa B-like activity. J Biol Chem. 1994 Nov 18;269(46):28913–28918. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. E., Berk B. C., Gravanis M. B., Santoian E. C., Cipolla G. D., Tarazona N., Lassegue B., King S. B., 3rd Probucol decreases neointimal formation in a swine model of coronary artery balloon injury. A possible role for antioxidants in restenosis. Circulation. 1993 Aug;88(2):628–637. doi: 10.1161/01.cir.88.2.628. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Simons M., Rosenberg R. D. Antisense nonmuscle myosin heavy chain and c-myb oligonucleotides suppress smooth muscle cell proliferation in vitro. Circ Res. 1992 Apr;70(4):835–843. doi: 10.1161/01.res.70.4.835. [DOI] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]