Abstract
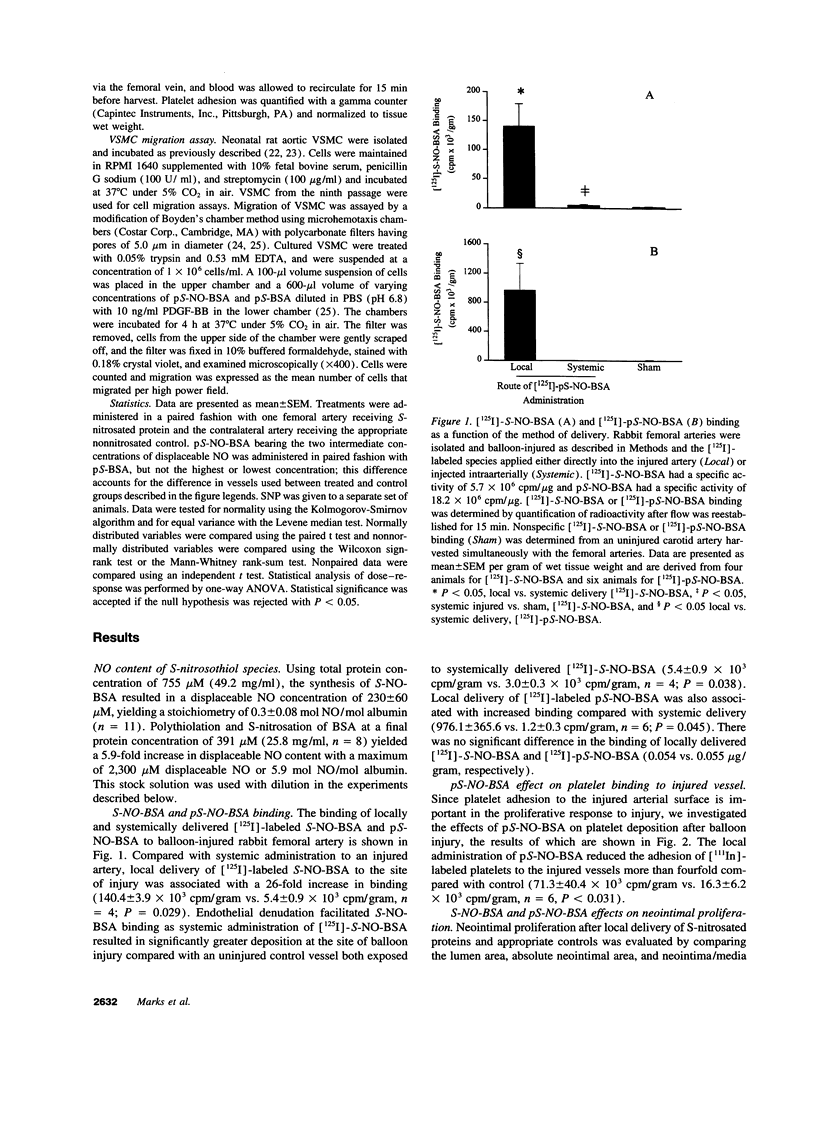
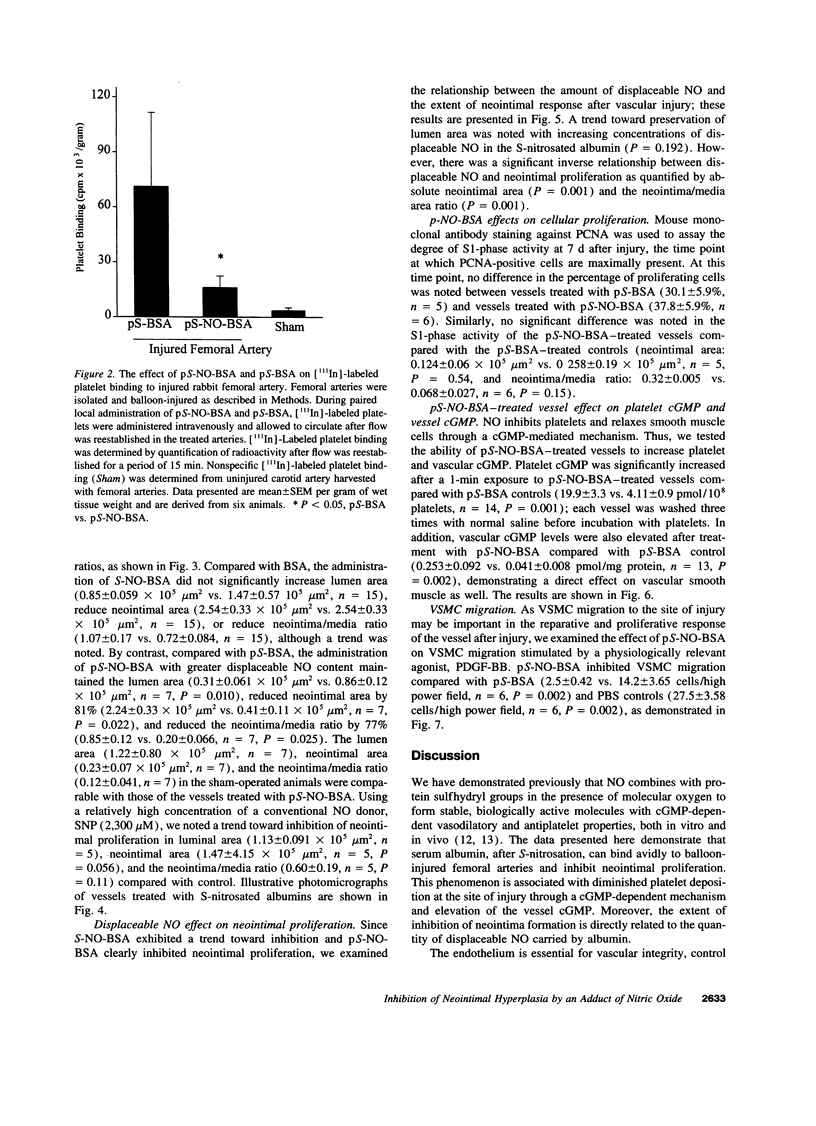
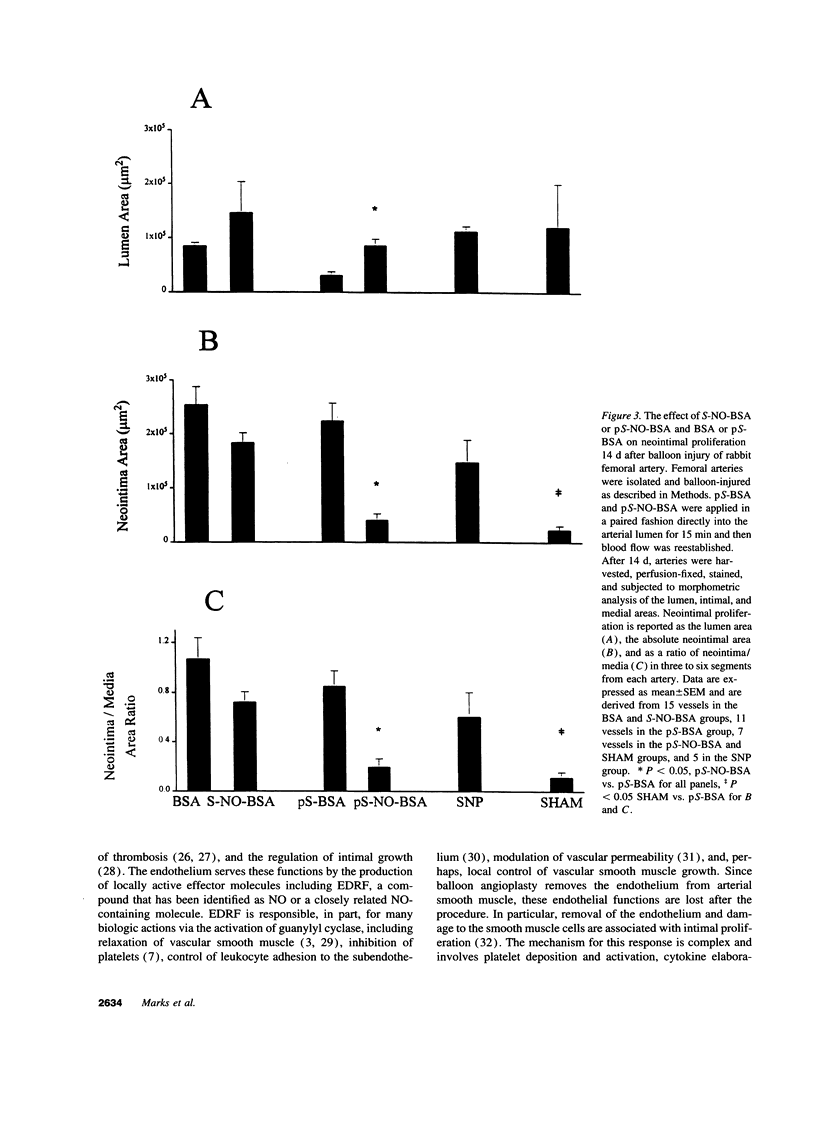
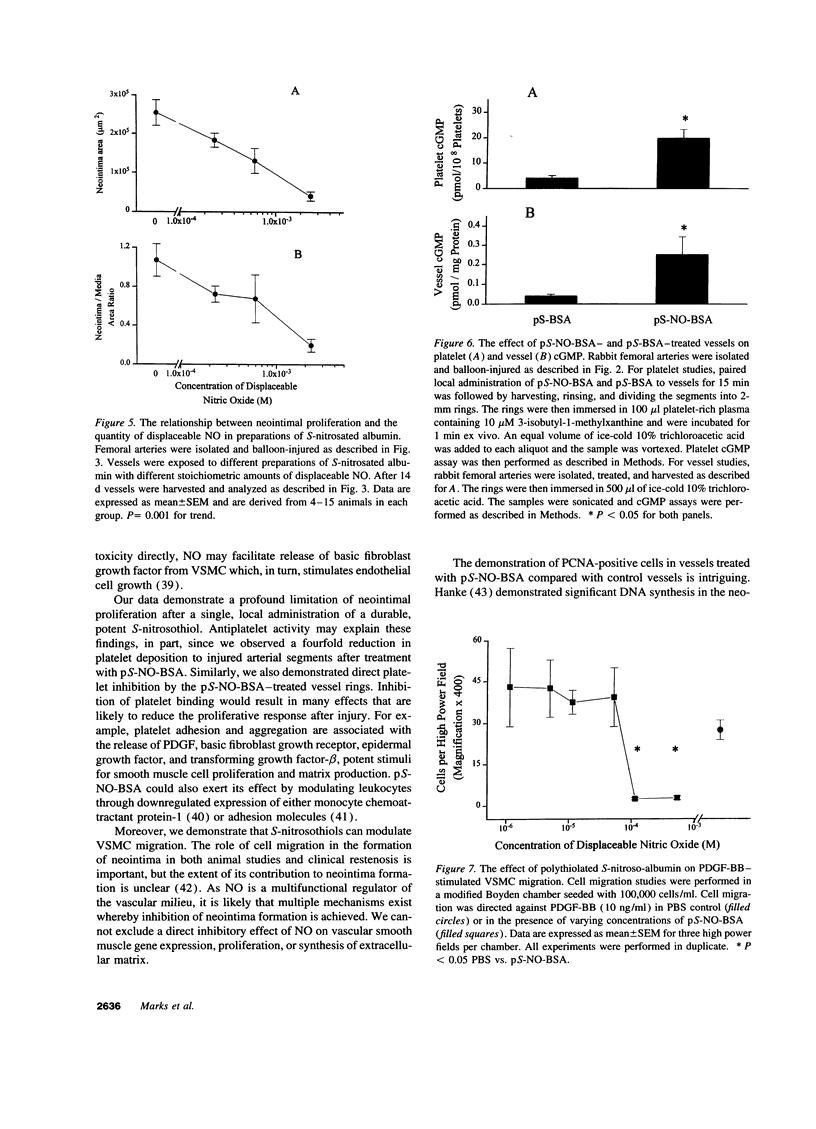
Endothelium-derived relaxing factor is important for vascular homeostasis and possesses qualities that may modulate vascular injury, including vasodilation, platelet inhibition, and inhibition of smooth muscle proliferation. S-nitrososerum albumin is a naturally occurring adduct of nitric oxide (NO) with a prolonged biologic half-life and is a potent vasodilator and platelet inhibitor. Given the avidity of serum albumin for subendothelial matrix and the antiproliferative effects of NO, we investigated the effects of locally delivered S-nitroso-bovine serum albumin (S-NO-BSA) and a polythiolated form of bovine serum albumin (pS-BSA) modified to carry several S-nitrosothiol groups (pS-NO-BSA) on neointimal responses in an animal model of vascular injury. Locally delivered S-NO-BSA bound preferentially to denuded rabbit femoral vessels producing a 26-fold increase in local concentration compared with uninjured vessels (P = 0.029). pS-NO-BSA significantly reduced the intimal/medial ratio (P = 0.038) and did so in conjunction with elevations in platelet (P < 0.001) and vascular cGMP content (P < or = 0.001). pS-NO-BSA treatment also inhibited platelet deposition (P = 0.031) after denuding injury. Comparison of BSA, S-NO-BSA, pS-NO-BSA, and control revealed a dose-response relationship between the amount of displaceable NO delivered and the extent of inhibition of neointimal proliferation at 2 wk (P < or = 0.001). Local administration of a stable protein S-nitrosothiol inhibits intimal proliferation and platelet deposition after vascular arterial balloon injury. This strategy for the local delivery of a long-lived NO adduct has potential for preventing restenosis after angioplasty.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barone L. M., Faris B., Chipman S. D., Toselli P., Oakes B. W., Franzblau C. Alteration of the extracellular matrix of smooth muscle cells by ascorbate treatment. Biochim Biophys Acta. 1985 Jun 18;840(2):245–254. doi: 10.1016/0304-4165(85)90125-4. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. THIOLATION OF PROTEINS. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):848–853. doi: 10.1073/pnas.44.9.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Cayatte A. J., Palacino J. J., Horten K., Cohen R. A. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb. 1994 May;14(5):753–759. doi: 10.1161/01.atv.14.5.753. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fukuo K., Inoue T., Morimoto S., Nakahashi T., Yasuda O., Kitano S., Sasada R., Ogihara T. Nitric oxide mediates cytotoxicity and basic fibroblast growth factor release in cultured vascular smooth muscle cells. A possible mechanism of neovascularization in atherosclerotic plaques. J Clin Invest. 1995 Feb;95(2):669–676. doi: 10.1172/JCI117712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves P. H., Lewis M. J., Cheadle H. A., Penny W. J. SIN-1 reduces platelet adhesion and platelet thrombus formation in a porcine model of balloon angioplasty. Circulation. 1993 Feb;87(2):590–597. doi: 10.1161/01.cir.87.2.590. [DOI] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Keaney J. F., Jr, Simon D. I., Stamler J. S., Jaraki O., Scharfstein J., Vita J. A., Loscalzo J. NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Invest. 1993 Apr;91(4):1582–1589. doi: 10.1172/JCI116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama N., Hart C. E., Clowes A. W. Different functions of the platelet-derived growth factor-alpha and -beta receptors for the migration and proliferation of cultured baboon smooth muscle cells. Circ Res. 1994 Oct;75(4):682–691. doi: 10.1161/01.res.75.4.682. [DOI] [PubMed] [Google Scholar]
- Koyama N., Morisaki N., Saito Y., Yoshida S. Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22806–22812. [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Loscalzo J., Inbal A., Handin R. I. von Willebrand protein facilitates platelet incorporation in polymerizing fibrin. J Clin Invest. 1986 Oct;78(4):1112–1119. doi: 10.1172/JCI112668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J. N-Acetylcysteine potentiates inhibition of platelet aggregation by nitroglycerin. J Clin Invest. 1985 Aug;76(2):703–708. doi: 10.1172/JCI112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B. Thromboregulation: multicellular modulation of platelet reactivity in hemostasis and thrombosis. FASEB J. 1993 Apr 1;7(6):516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- McNamara D. B., Bedi B., Aurora H., Tena L., Ignarro L. J., Kadowitz P. J., Akers D. L. L-arginine inhibits balloon catheter-induced intimal hyperplasia. Biochem Biophys Res Commun. 1993 May 28;193(1):291–296. doi: 10.1006/bbrc.1993.1622. [DOI] [PubMed] [Google Scholar]
- Mendelsohn M. E., O'Neill S., George D., Loscalzo J. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J Biol Chem. 1990 Nov 5;265(31):19028–19034. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Oakes B. W., Batty A. C., Handley C. J., Sandberg L. B. The synthesis of elastin, collagen, and glycosaminoglycans by high density primary cultures of neonatal rat aortic smooth muscle. An ultrastructural and biochemical study. Eur J Cell Biol. 1982 Apr;27(1):34–46. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A. A reassessment of endothelial injury and arterial lesion formation. Lab Invest. 1985 Nov;53(5):513–520. [PubMed] [Google Scholar]
- Scharfstein J. S., Keaney J. F., Jr, Slivka A., Welch G. N., Vita J. A., Stamler J. S., Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994 Oct;94(4):1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J., Mendelsohn M. E., Amarante P., Smick D., Andon N., Davies P. F., Cooke J. P., Loscalzo J. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ Res. 1989 Sep;65(3):789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- Steele P. M., Chesebro J. H., Stanson A. W., Holmes D. R., Jr, Dewanjee M. K., Badimon L., Fuster V. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985 Jul;57(1):105–112. doi: 10.1161/01.res.57.1.105. [DOI] [PubMed] [Google Scholar]
- Taubman M. B. Gene induction in vessel wall injury. Thromb Haemost. 1993 Jul 1;70(1):180–183. [PubMed] [Google Scholar]
- Uchida Y., Hasegawa K., Kawamura K., Shibuya I. Angioscopic observation of the coronary luminal changes induced by percutaneous transluminal coronary angioplasty. Am Heart J. 1989 Apr;117(4):769–776. doi: 10.1016/0002-8703(89)90611-x. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Weidinger F. F., McLenachan J. M., Cybulsky M. I., Gordon J. B., Rennke H. G., Hollenberg N. K., Fallon J. T., Ganz P., Cooke J. P. Persistent dysfunction of regenerated endothelium after balloon angioplasty of rabbit iliac artery. Circulation. 1990 May;81(5):1667–1679. doi: 10.1161/01.cir.81.5.1667. [DOI] [PubMed] [Google Scholar]
- Wistow B. W., Grossman Z. D., McAfee J. G., Subramanian G., Henderson R. W., Roskopf M. L. Labeling of platelets with oxine complexes of Tc-99m and In-111. Part 1. In vitro studies and survival in the rabbit. J Nucl Med. 1978 May;19(5):483–487. [PubMed] [Google Scholar]
- Yao S. K., Ober J. C., Krishnaswami A., Ferguson J. J., Anderson H. V., Golino P., Buja L. M., Willerson J. T. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation. 1992 Oct;86(4):1302–1309. doi: 10.1161/01.cir.86.4.1302. [DOI] [PubMed] [Google Scholar]