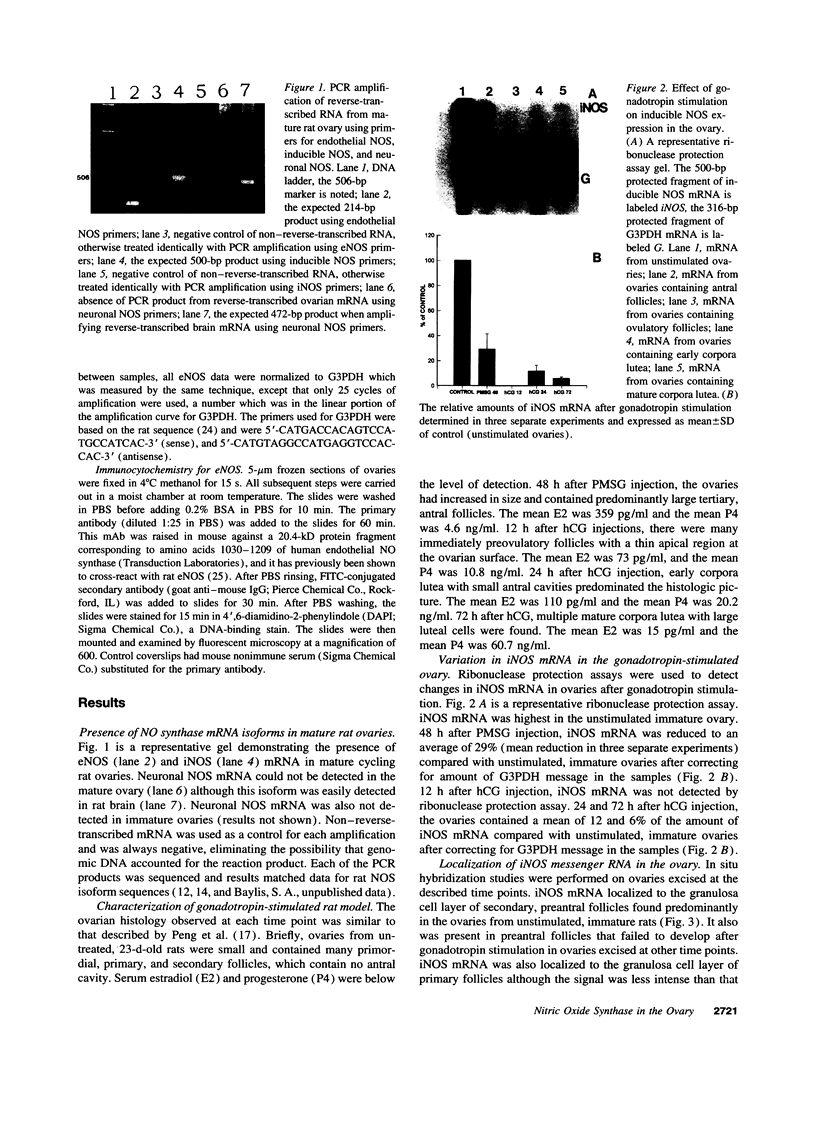
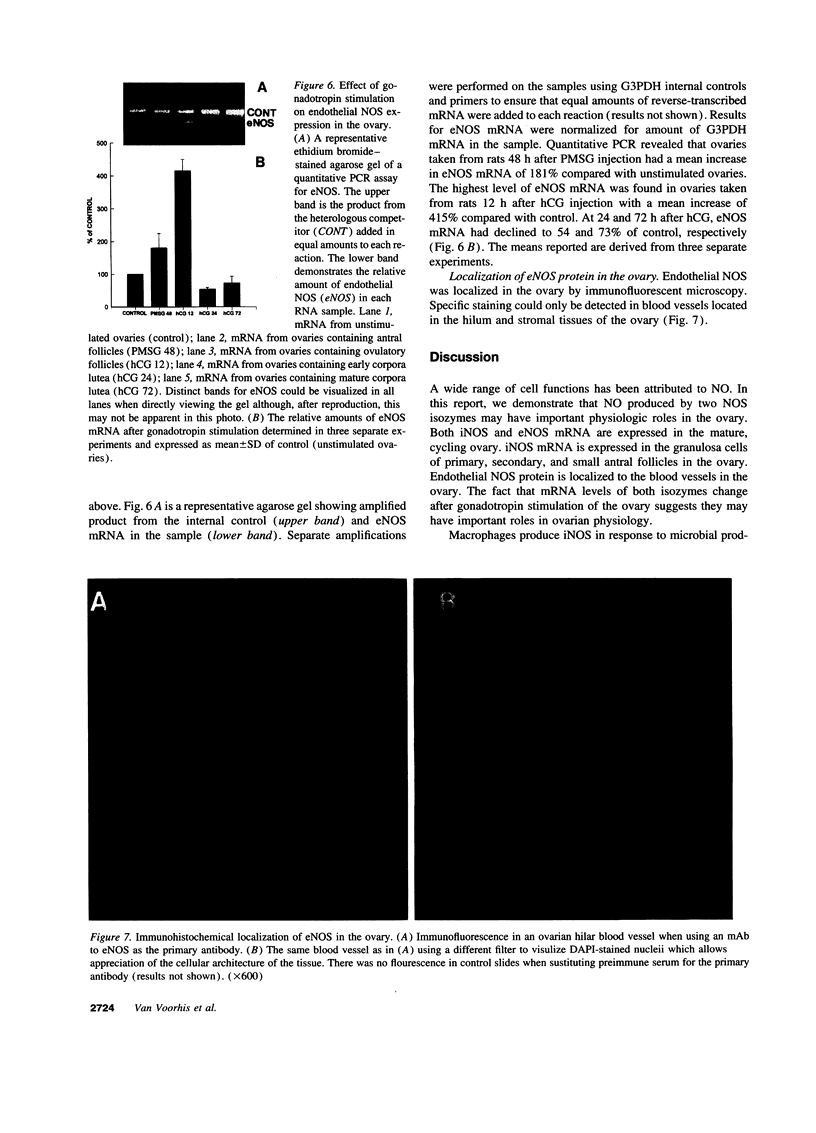
Abstract
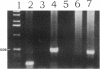
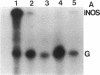
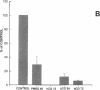
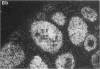
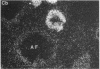
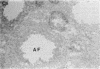
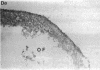
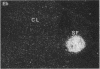
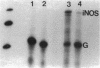
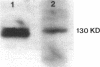
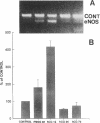
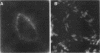
Nitric oxide is reportedly involved in the regulation of several ovarian processes, yet the isoforms of nitric oxide synthase (NOS) expressed in the ovary are unknown. Our purpose was to identify and localize NOS isoenzymes in the rat ovary and to examine++ if mRNA expression of NOS isoenzymes change after gonadotropin stimulation. Using reverse transcriptase-PCR, we demonstrated that inducible (iNOS) and endothelial (eNOS), but not neuronal, NOS mRNAs are expressed in the ovary. In a gonadotropin-stimulated rat model, unstimulated ovaries had the highest levels of iNOS mRNA as quantified by ribonuclease protection assay. After gonadotropin injection, iNOS mRNA declined to undetectable levels in ovaries containing ovulatory follicles before increasing slighty in ovaries containing copora lutea. In situ hybridization studies localized iNOS to granulosa cells of secondary follicles and small antral follicles. Western blots of unstimulated ovaries demonstrated iNOS protein. In contrast to iNOS, eNOS mRNA levels, determined by quantitative PCR, increased after gonadotropin stimulation and peaked in ovaries containing ovulatory follicles before declining in the luteal phase. eNOS protein was localized to blood vessels in the ovary by immunohistochemistry. We conclude that two isoforms of NOS are expressed in the ovary and the mRNA levels for these isozymes are differentially regulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Shlomo I., Adashi E. Y., Payne D. W. The morphogenic/cytotoxic and prostaglandin-stimulating activities of interleukin-1 beta in the rat ovary are nitric oxide independent. J Clin Invest. 1994 Oct;94(4):1463–1469. doi: 10.1172/JCI117484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo I., Kokia E., Jackson M. J., Adashi E. Y., Payne D. W. Interleukin-1 beta stimulates nitrite production in the rat ovary: evidence for heterologous cell-cell interaction and for insulin-mediated regulation of the inducible isoform of nitric oxide synthase. Biol Reprod. 1994 Aug;51(2):310–318. doi: 10.1095/biolreprod51.2.310. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Chun S. Y., Eisenhauer K. M., Kubo M., Hsueh A. J. Interleukin-1 beta suppresses apoptosis in rat ovarian follicles by increasing nitric oxide production. Endocrinology. 1995 Jul;136(7):3120–3127. doi: 10.1210/endo.136.7.7540548. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Dawson T. M., Schell M. J., Snowman A., Snyder S. H. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman C., Corbett J. A., Misko T. P., McDaniel M., Beckerman K. P. Nitric oxide mediates interleukin-1-induced cellular cytotoxicity in the rat ovary. A potential role for nitric oxide in the ovulatory process. J Clin Invest. 1993 Dec;92(6):3053–3056. doi: 10.1172/JCI116930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Roby K. F., Pace J. L., Russell S. W., Hunt J. S. Cellular localization and hormonal regulation of inducible nitric oxide synthase in cycling mouse uterus. J Leukoc Biol. 1995 Jan;57(1):27–35. doi: 10.1002/jlb.57.1.27. [DOI] [PubMed] [Google Scholar]
- Hurwitz A., Loukides J., Ricciarelli E., Botero L., Katz E., McAllister J. M., Garcia J. E., Rohan R., Adashi E. Y., Hernandez E. R. Human intraovarian interleukin-1 (IL-1) system: highly compartmentalized and hormonally dependent regulation of the genes encoding IL-1, its receptor, and its receptor antagonist. J Clin Invest. 1992 Jun;89(6):1746–1754. doi: 10.1172/JCI115777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S. P., Shimouchi A., Quertermous T., Bloch D. B., Bloch K. D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992 Jul 25;267(21):14519–14522. [PubMed] [Google Scholar]
- Liu S., Adcock I. M., Old R. W., Barnes P. J., Evans T. W. Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1208–1213. doi: 10.1006/bbrc.1993.2380. [DOI] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994 May 13;269(19):13725–13728. [PubMed] [Google Scholar]
- Peng X. R., Hsueh A. J., LaPolt P. S., Bjersing L., Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991 Dec;129(6):3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Pratt G. D., Kokaia M., Bengzon J., Kokaia Z., Fritschy J. M., Möhler H., Lindvall O. Differential regulation of N-methyl-D-aspartate receptor subunit messenger RNAs in kindling-induced epileptogenesis. Neuroscience. 1993 Nov;57(2):307–318. doi: 10.1016/0306-4522(93)90064-m. [DOI] [PubMed] [Google Scholar]
- Reich R., Daphna-Iken D., Chun S. Y., Popliker M., Slager R., Adelmann-Grill B. C., Tsafriri A. Preovulatory changes in ovarian expression of collagenases and tissue metalloproteinase inhibitor messenger ribonucleic acid: role of eicosanoids. Endocrinology. 1991 Oct;129(4):1869–1875. doi: 10.1210/endo-129-4-1869. [DOI] [PubMed] [Google Scholar]
- Shukovski L., Tsafriri A. The involvement of nitric oxide in the ovulatory process in the rat. Endocrinology. 1994 Nov;135(5):2287–2290. doi: 10.1210/endo.135.5.7525265. [DOI] [PubMed] [Google Scholar]
- Sladek S. M., Regenstein A. C., Lykins D., Roberts J. M. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. Am J Obstet Gynecol. 1993 Nov;169(5):1285–1291. doi: 10.1016/0002-9378(93)90295-t. [DOI] [PubMed] [Google Scholar]
- Takagi K., Isobe Y., Yasukawa K., Okouchi E., Suketa Y. Nitric oxide blocks the cell cycle of mouse macrophage-like cells in the early G2+M phase. FEBS Lett. 1994 Mar 7;340(3):159–162. doi: 10.1016/0014-5793(94)80128-2. [DOI] [PubMed] [Google Scholar]
- Van Voorhis B. J., Dunn M. S., Falck J. R., Bhatt R. K., VanRollins M., Snyder G. D. Metabolism of arachidonic acid to epoxyeicosatrienoic acids by human granulosa cells may mediate steroidogenesis. J Clin Endocrinol Metab. 1993 Jun;76(6):1555–1559. doi: 10.1210/jcem.76.6.8388882. [DOI] [PubMed] [Google Scholar]
- Van Voorhis B. J., Dunn M. S., Snyder G. D., Weiner C. P. Nitric oxide: an autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology. 1994 Nov;135(5):1799–1806. doi: 10.1210/endo.135.5.7525252. [DOI] [PubMed] [Google Scholar]
- Weiner C. P., Lizasoain I., Baylis S. A., Knowles R. G., Charles I. G., Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. R., Berger H., Jr, Sherman P. A., Lapetina E. G. Hepatocytes and macrophages express an identical cytokine inducible nitric oxide synthase gene. Biochem Biophys Res Commun. 1993 Mar 31;191(3):767–774. doi: 10.1006/bbrc.1993.1283. [DOI] [PubMed] [Google Scholar]