Abstract
IKK (IκB kinase) α is essential for embryonic skin development in mice. Mice deficient in IKKα display markedly hyperplasic epidermis that lacks terminal differentiation, and they die because of this severely impaired skin. However, the function of IKKα in human skin diseases remains largely unknown. To shed light on the role of IKKα in human skin diseases, we examined IKKα expression and Ikkα mutations in human squamous cell carcinomas (SCCs). We found a marked reduction in IKKα expression in poorly differentiated human SCCs and identified Ikkα mutations in exon 15 of Ikkα in eight of nine human SCCs, implying that IKKα is involved in development of this human skin cancer. Furthermore, in a chemical carcinogen-induced skin carcinogenesis setting, mice overexpressing human IKKα in the epidermis under the control of a truncated loricrin promoter developed significantly fewer SCCs and metastases than did wild-type mice. The IKKα transgene altered the skin microenvironment conditions, leading to elevated terminal differentiation in the epidermis, reduced mitogenic activity in the epidermis, and decreased angiogenic activity in the skin stroma. Thus, overexpression of IKKα in the epidermis antagonized chemical carcinogen-induced mitogenic and angiogenic activities, repressing tumor progression and metastases.
Keywords: angiogenesis, mitogenesis, skin carcinogenesis, differentiation, tumor progression
Squamous cell carcinomas (SCCs) can be very aggressive and metastatic. Previous studies have shown that Pten mutations are found frequently in human SCCs; however, these Pten mutations are not detected in all cases (1, 2). p53 mutations have also been identified in a large proportion of human SCCs (3); however, these p53 mutations can be latent in skin cells for years before the onset of this disease. Clearly, additional pivotal factors in the development of this human skin cancer remain to be identified.
The IκB kinase (IKK) complex consists of IKKα, IKKβ, and IKKγ (4–7), which phosphorylates IκBα (S32/S36) and IκBβ (S19/S23) that are NF-κB inhibitors. This phosphorylation event triggers the degradation of IκB proteins via a 26S proteasome-ubiquitination pathway, leading to NF-κB translocation and activation. Previous findings have shown that IKKα is essential for embryonic skin development in mice (8–10). Mice lacking IKKα exhibit a strikingly hyperplastic epidermis, which lacks terminal differentiation, and they die of severely impaired skin soon after birth. The skin phenotypes of Ikkα−/− mice have not been observed in knockouts of any other NF-κB family members. In contrast, mice overexpressing IKKα in the basal epidermis develop normally (11). The ectopic IKKα is able to induce terminal differentiation and to repress hyperplasia in the skin of Ikkα−/− mice, implying that IKKα plays a role in maintaining skin homeostasis (11).
Although a role of IKKα in skin development in mice has been established, we still know little regarding the involvement of IKKα in human skin diseases. To shed light on the role of IKKα in human skin cancer, we examined IKKα expression and Ikkα mutations in human SCCs. We further evaluated the effect of overexpression of human IKKα cDNA in the epidermis on skin carcinogenesis induced by chemical carcinogens. Our results suggest that IKKα is involved in human SCC development and that overexpression of IKKα represses skin tumor progression and metastasis in mice.
Results
Alterations in IKKα Expression and in Ikkα in Human SCCs.
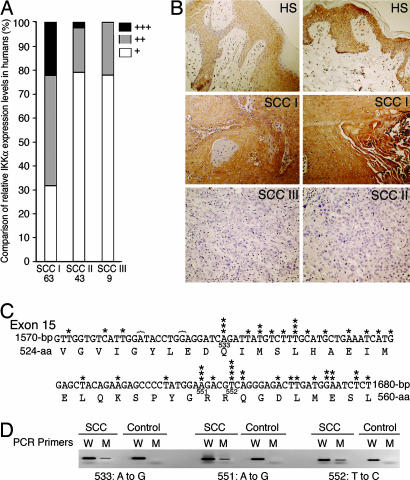
To determine the level of IKKα expression in human SCCs, we examined 114 human skin SCCs in two tissue arrays by using immunohistochemical staining. Fourteen (22.2%) of 63 grade I SCCs, 1 (2.3%) of 43 grade II SCCs, and 0 of 9 grade III SCCs showed strong positive reactivity to an anti-IKKα antibody, whereas 20 grade I SCCs (31.7%), 34 grade II SCCs (79.0%), and 7 grade III SCCs (77.7%) showed weak positive reactivity (Fig. 1A and B). The result strongly suggests that the aggressiveness of SCCs inversely correlates with the level of IKKα expression.
Fig. 1.
Alterations in IKKα expression and in Ikkα in human SCCs. (A) IKKα expression in tissue arrays containing human grade I, II, and III SCCs (SCC I, SCC II, and SCC III) immunostained with an anti-IKKα antibody. +++, strong positive reactivity; ++, medium positive reactivity; +, weak positive reactivity. (B) IKKα staining in human skin (HS; strong staining), grade I SCC (strong staining), grade II SCC (weak staining), and grade III SCC (weak staining). Dark brown color, IKKα-positive staining. (C) Nucleotide sequences of exon 15 of Ikkα and its protein-encoding sequences. 533, 551, and 552, amino acid numbers in IKKα; }, deletions; ∗, identified mutations. (D) Detection of Ikkα mutations by using PCR with specific mutation primers. 533, 551, and 552, amino acid numbers in IKKα; W, wild-type primers; M, mutation primers; control, DNA from normal human skin.
To determine whether Ikkα mutations occur in human SCCs, we examined exon 15 of Ikkα in nine human SCCs by using PCR and sequencing. Two normal human skin specimens were used as negative controls. We chose to examine exon 15 because the Ikkα mutations were detected in this region in carcinomas from mice (data not shown). We subcloned the PCR products into pGEM-T vectors and sequenced 20 clones for each sample. Somatic mutations of Ikkα were detected in eight of nine SCCs (Fig. 1C; see Fig. 7 and Table 1, which are published as supporting information on the PNAS web site). We verified three mutations in SCCs by using PCR with primers containing specific point mutations at their 3′ ends (Fig. 1D) (12). More transition mutations (T→C and A→G) than transversion mutations were detected. Because no mutations were highly repeated in a single tumor (Table 1 and Figs. 1D and 7), it is unlikely that these mutations were amplified. These nucleotide substitutions can cause missense and nonsense mutations in IKKα, and deletions can cause frameshifts for IKKα.
Expression of the IKKα Transgene in the Epidermis Enhances Terminal Differentiation.
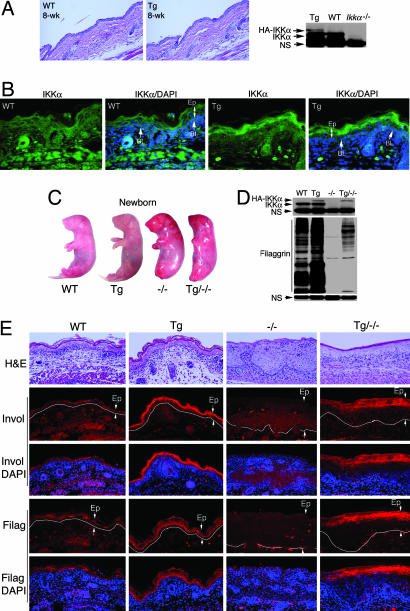
We generated Lori·IKKα-Tg mice overexpressing a human IKKα cDNA tagged with hemagglutinin A (HA) in the epidermis under the control of a truncated loricrin promoter (13). Lori·IKKα-Tg mice developed normally. On histologic examination, skins of newborn and 8-week-old wild-type (WT) and Lori·IKKα-Tg mice were identical (Fig. 2A and E). Western blot analysis showed that the level of HA-IKKα was substantially lower than the level of endogenous IKKα in full thickness skin (Fig. 2A). According to the report of DiSepio et al. (13), the expression level of this loricrin promoter is much higher in the suprabasal epidermis than in the basal epidermis. Endogenous IKKα is expressed ubiquitously. The tested proteins prepared from full thickness skin may dilute the transgene protein (Fig. 2A). Thus, we examined the expression of the IKKα transgene in the suprabasal and the basal epidermis by using immunofluorescent staining with the anti-IKKα antibody. The result showed that IKKα expression in the suprabasal epidermis of Lori·IKKα-Tg mice was significantly higher than in the suprabasal epidermis of WT mice (Fig. 2B). HA was highly expressed in the suprabasal epidermis of Lori·IKKα-Tg mice but not in the suprabasal epidermis of WT mice (Fig. 8, which is published as supporting information on the PNAS web site). Ikkα−/− skin was used as a negative control for IKKα staining (Fig. 8). These results suggested that the IKKα transgene was more highly expressed in the suprabasal epidermis compared with that in the basal epidermis.
Fig. 2.
Overexpression of IKKα in the epidermis enhances terminal differentiation in the skin. (A) Tissue specimens of skin of WT and Lori·IKKα-Tg (Tg) mice stained with H&E. Transgenic IKKα expression (HA) in skin of WT and Tg mice was analyzed by Western blotting. IKKα, endogenous IKKα; HA-IKKα, transgenic IKKα; NS, nonspecific band; 8-wk, 8-week-old. (B) Elevated IKKα expression in the suprabasal epidermis in Tg mice by immunofluorescent staining. Green, IKKα staining; blue, DAPI nuclear staining; BL, basal layer; Ep, epidermis. (C) Appearance of WT, Tg, Ikkα−/− (−/−), and Lori·IKKα-Tg/Ikkαα−/− (Tg/−/−) newborn mice. (D) Filaggrin levels in WT, Tg, −/−, and Tg/−/− skin. NS, nonspecific band. (E) Comparison of involucrin (Invol) and filaggrin (Filag) expression in skin of WT, Tg, −/−, and Tg/−/− newborn mice, analyzed by immunofluorescent staining. Red, involucrin or filaggrin staining; blue, DAPI nuclear staining; Ep, epidermis between two arrows. White line separates epidermis and dermis.
To determine whether expression of the IKKα transgene rescues Ikkα−/− mice, we crossed Lori·IKKα-Tg mice with Ikkα+/− mice to generate Lori·IKKα-Tg/Ikkα−/− mice. The IKKα transgene failed to rescue Ikkα−/− mice (Fig. 2C). It is known that the loricrin promoter is expressed at the late stage of embryonic development in mice (13). Thus, it is likely that IKKα expression at an earlier developmental stage is essential for rescuing Ikkα−/− mice (11). We then determined whether overexpression of IKKα affects terminal differentiation in skin. Western blot analysis showed that the level of the terminal differentiation marker filaggrin was higher in the skin of Lori·IKKα-Tg mice than in the skin of WT mice and higher in the skin of Lori·IKKα-Tg/Ikkα−/− mice than in the skin of Ikkα−/− mice (Fig. 2D). Immunofluorescent staining further showed that the levels of filaggrin and the intermediate differentiation marker involucrin were higher in the epidermis of Lori·IKKα-Tg mice than in the epidermis of WT mice and higher in the epidermis of Lori·IKKα-Tg/Ikkα−/− mice than in the epidermis of Ikkα−/− mice (Fig. 2E). Collectively, these results suggest that overexpression of IKKα in the epidermis enhances terminal differentiation in the skin.
Transgene IKKα Inhibits Development of Carcinomas and Metastases.
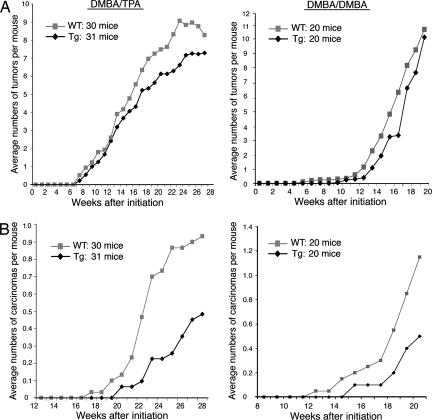
To evaluate the effect of overexpression of IKKα on skin tumor development, we conducted two-stage skin carcinogenesis experiments by using topical treatment with the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA), and complete skin carcinogenesis experiments by using treatment with DMBA/DMBA in WT and Lori·IKKα-Tg mice. In both settings, DMBA mediates Ras mutations to initiate skin carcinogenesis, and subsequent treatment with TPA or DMBA, both of which are irritants in mouse skin, can promote the expansion of initiated cells (14). WT and Lori·IKKα-Tg mice on a FVB background were topically treated with a single dose of DMBA (25 μg) and then treated with TPA (2.5 μg) twice a week for 28 weeks or DMBA (50 μg) weekly for 20 weeks. Although the Lori·IKKα-Tg mice developed slightly fewer tumors than did the WT mice after treatments with DMBA/TPA or with DMBA/DMBA (Fig. 3A), tumor multiplicity was not statistically significantly different between WT and Lori·IKKα-Tg mice (DMBA/TPA, 8.00 versus 7.29; DMBA/DMBA, 10.25 versus 8.85). Controls did not develop any tumors. These results suggest that overexpression of IKKα in the suprabasal epidermis does not significantly affect tumor numbers.
Fig. 3.
Lori·IKKα-Tg mice developed significantly fewer carcinomas than WT mice. Shown are the average number of tumors (A) and average number of carcinomas (B) induced by DMBA/TPA (Left) or DMBA/DMBA (Right) in WT and Lori·IKKα-Tg (Tg) mice.
We histologically examined all of the tumors with a carcinomatous appearance and some of the papillomas. In the DMBA/TPA group, 28 (11.7%) of 240 tumors in WT mice and 15 (6.6%) of 226 tumors in Lori·IKKα-Tg mice were carcinomas (P = 0.04, Poisson regression), and in the DMBA/DMBA group, 25 (12.2%) of 205 tumors in WT mice and 10 (5.6%) of 177 tumors in Lori·IKKα-Tg mice were carcinomas (P = 0.01, Poisson regression) (Fig. 3B). Moreover, WT mice developed carcinomas earlier than did Lori·IKKα-Tg mice both with DMBA/TPA treatment (P = 0.030, Wilcoxon–Breslow test) and with DMBA/DMBA treatment (P = 0.044, Wilcoxon–Breslow test). Most of the carcinomas were SCCs (Figs. 9A and 10, which are published as supporting information on the PNAS web site). Collectively, these results indicate that overexpression of IKKα in the suprabasal epidermis inhibits carcinoma development.
Moreover, we found that, with DMBA/TPA treatment, three WT mice developed multiple metastatic carcinomas in their lungs (Fig. 9B), whereas none of the Lori·IKKα-Tg mice did. With DMBA/DMBA treatment, one WT mouse had metastatic carcinomas in the lungs, and two had carcinomas in the lymph nodes, whereas no Lori·IKKα-Tg mice had carcinomas in the lungs, and only one had carcinomas in the lymph nodes. These results suggest that carcinomas induced by DMBA/TPA tend to metastasize to the lungs, whereas carcinomas induced by DMBA/DMBA tend to metastasize to lymph nodes. One possible explanation for this apparent trend is that DMBA and TPA have different targets, which may differentially affect tumors and skin environments (14). All of the metastatic carcinomas expressed K5 (Fig. 9B) and pan keratins (data not shown), suggesting that these carcinomas originally derived from skin. These results indicate that the overexpression of IKKα in the suprabasal epidermis inhibits tumor metastases.
Expression of the IKKα Transgene Affects Endogenous IKKα Levels in Carcinomas.
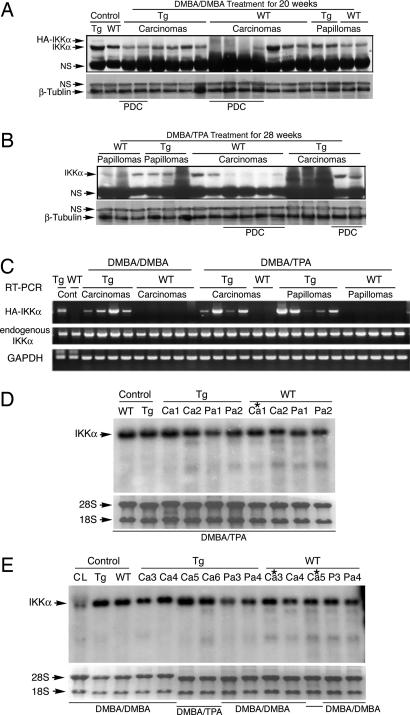
To determine how overexpression of IKKα affects skin tumor development, we examined IKKα levels in tumors by Western blotting with the anti-IKKα antibody. After treatment with DMBA/DMBA or DMBA/TPA, IKKα levels were markedly decreased in the poorly differentiated WT carcinomas, but not in the poorly differentiated Lori·IKKα-Tg carcinomas (Fig. 4 A and B), suggesting that overexpression of IKKα in the epidermis may affect endogenous IKKα expression in tumors.
Fig. 4.
IKKα protein and mRNA levels in papillomas and carcinomas induced by treatment with DMBA/TPA and DMBA/DMBA in WT and Lori·IKKα-Tg (Tg) mice. (A and B) IKKα protein levels in WT and Tg papillomas and carcinomas. Control, normal skin; NS, nonspecific band; β-Tubulin, loading controls; PDC, poorly differentiated carcinomas. (C) IKKα mRNA levels measured by RT-PCR. HA-IKKα, transgenic IKKα; GAPDH, loading control. (D and E) IKKα mRNA levels in papillomas and carcinomas detected by Northern blotting. Control, normal skin; Tg, transgene; Ca, carcinoma; Pa, papilloma; 28S and 18S, RNA loading controls; CL, Ikkα−/− immortalized cell line; ∗, poorly differentiated carcinomas.
Using Western blotting, we did not detect HA-IKKα in tumors (Fig. 4 A and B). To determine whether tumors from transgenic mice express the IKKα transgene, we examined its transcript by RT-PCR with primers to the human IKKα cDNA and HA (5′-gccatgtacccatacgatgttcc-3′ and 5′-gctccaataatcaacagtggctg-3′). The transgenic transcripts were detected only in the tumors from transgenic mice, not in those from WT mice (Fig. 4C). Thus, Lori·IKKα-Tg tumors expressed a low level of the IKKα transgene.
Because there were differences in expression of endogenous IKKα in the poorly differentiated carcinomas from WT and transgenic mice after treatment with DMBA/TPA or DMBA/DMBA, we used RT-PCR with primers to the mouse IKKα cDNA to examine whether the IKKα transgene affected transcription of the endogenous IKKα in Lori·IKKα-Tg tumors. No significant alterations in the endogenous IKKα mRNA in WT and Lori·IKKα-Tg tumors were detected (Fig. 4C), and these results were confirmed by Northern blot analysis (Fig. 4 D and E). The IKKα transgene mRNA contained a HA tag and a loricrin polyA tail, and the endogenous IKKα mRNA contained untranslated regions. We thus were not able to distinguish the Lori·IKKα mRNA from the endogenous IKKα mRNA by using Northern blotting. Taken together, these results suggest that the transgene IKKα did not affect transcription of the endogenous IKKα in Lori·IKKα-Tg tumors and also that the IKKα proteins in poorly differentiated WT carcinomas were not down-regulated at the transcription level.
Overexpression of IKKα in the Epidermis Represses Mitogenic Activity in the Skin.
According to the study by Sil et al. (11), reintroduction of IKKα induces filaggrin expression and represses ERK activity in the skin of Ikkα−/− mice. To determine whether overexpression of IKKα in the suprabasal epidermis affects mitogenic activity in the epidermis, we compared the filaggrin levels, and ERK and Raf activities in the skin between WT and Lori·IKKα-Tg mice treated with DMBA/TPA at week 19. The filaggrin levels were higher in the skin of Lori·IKKα-Tg mice than in the skin of WT mice (Fig. 5A). In contrast, the activities of ERK and Raf were lower in the skin of Lori·IKKα-Tg than in the skin of WT mice as assessed by Western blotting (Fig. 5B). Consistent with these results, there were significantly more Ki67-positive cells in the epidermis of WT mice than in the epidermis of Lori·IKKα-Tg mice (P = 0.002, Student's t test) (Fig. 5 C and D). These results suggest that overexpression of IKKα in the suprabasal epidermis enhances terminal differentiation and inhibits the mitogenic activity in the epidermis after challenge by DMBA/TPA.
Fig. 5.
Expression of IKKα transgene enhances terminal differentiation and reduces mitogenic activity. (A) Filaggrin levels in WT and Lori·IKKα-Tg (Tg) skin treated with DMBA/TPA for 19 weeks. Relative ratio, densities of filaggin signals normalized by controls (β-actin); β-actin, loading control. (B) Levels of Raf and ERK activity in WT and Tg skin treated with DMBA/TPA for 19 weeks. β-actin, loading control. (C) Ki67-positive cell numbers in epidermis from WT and Tg mice treated with DMBA/TPA for 28 weeks. Seven slides (15 views in each slide) from WT mice and seven slides from Tg mice were counted. (D) Skin of WT and Tg mice stained with an anti-Ki67 antibody. Dark brown color, Ki67-positive cells. (Original magnification: ×200.)
Overexpression of IKKα in the Epidermis Represses Angiogenic Activity in the Skin Stroma.
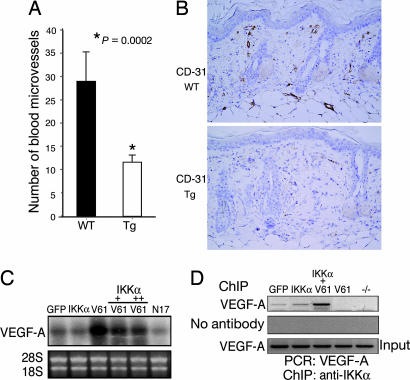
Elevation of neovascularization induced by VEGF-A, a major keratinocyte-derived skin angiogenesis factor, promotes tumor progression and metastases (15, 16). To gain insight into the mechanism of how overexpressed IKKα represses tumor progression and metastasis, we examined the effect of the IKKα transgene on blood microvessel formation in the skin stroma. We found significantly more blood microvessels in the WT stroma than in the Lori·IKKα-Tg stroma (P = 0.0002, Student's t test) (Fig. 6A and B), suggesting that overexpressed IKKα may repress VEGF-A expression in keratinocytes, so that less of this factor is released into the skin stroma. Ras has been shown to elevate VEGF-A expression, and DMBA/TPA promotes Ras-mitogenic activity in skin carcinogenesis (17). To determine the effect of overexpressed IKKα on VEGF-A expression, we performed Northern blot analysis, which showed that RasV61 dramatically increased VEGF-A expression and that overexpressed IKKα repressed RasV61-mediated VEGF-A expression in keratinocytes (Fig. 6C). We further tested whether IKKα regulated VEGF-A expression by using ChIP assays and PCR with primers to a distal VEGF-A promoter (5′-tgggttagaggtgggggttttg-3′ and 5′-aactgaagccagggtgccaatg-3′, region from −2414 bp to −2065 bp). We found that IKKα bound to the distal VEGF-A promoter (Fig. 6D), whereas RasV61 inhibited this binding. Interestingly, overexpression of IKKα and RasV61 enhanced the binding. We did not observe binding of IKKα to a proximal VEGF-A promoter region (PCR primers, 5′-gtgttcctgagcccagtttgaag-3′ and 5′-agtccgctgaatagtctgccttg-3′, located at a VEGF-A promoter region from −903 bp to −1233 bp) (data not shown). The binding of IKKα to the distal VEGF-A promoter correlated with a decrease in VEGF-A expression, suggesting that IKKα negatively regulates VEGF-A expression. According to the report of Kishimoto et al. (18), TPA up-regulates VEGF-A expression in the suprabasal epidermis. Immunofluorescent staining showed that VEGF-A expression was lower in the epidermis of the transgenic mice than in the epidermis of WT mice (Fig. 11, which is published as supporting information on the PNAS web site). Thus, overexpression of IKKα in the suprabasal epidermis antagonized DMBA/TPA-enhanced-VEGF-A expression in the epidermis.
Fig. 6.
Expression of IKKα transgene represses angiogenic activity in the skin stroma. (A) Average numbers of blood microvessels in the stroma of WT and Lori·IKKα-Tg (Tg) mice treated with DMBA/TPA for 28 weeks. (B) Blood microvessels in the skin stroma of WT and Tg mice. Eight slides (15 views in each slide) from WT mice and eight slides from Tg mice were counted. (Original magnification, ×200.) (C) VEGF-A mRNA levels in primary cultured WT keratinocytes infected with adenoviruses expressing GFP, IKKα, RasV61 (V61, a GTP active form), and RasN17 (N17, a dominant-negative form) measured by Northern blotting. (D) Binding of IKKα to the VEGF-A promoter detected by ChIP assay.
Discussion
In summary, we identified an inverse correlation between IKKα expression levels and human SCC aggressiveness. Also we identified Ikkα mutations in exon 15 in human SCCs. Thus, IKKα may play an important role in development of this human skin cancer. Furthermore, we showed that overexpression of IKKα in the epidermis repressed tumor progression and metastases through repressing mitogenic and angiogenic activities in mice. These results reveal a protective function of IKKα in skin carcinogenesis.
Human Ikkα is located at 10q24.31, close to Pten (10q23). It has been reported that alterations in more than one gene within the 10q22–10q26 region may be involved in human cancer development (1). In the present study, mutations in exon 15 of Ikkα were detected in human SCCs. These mutations appeared to occur randomly at any nucleotide in one of the Ikkα DNA strands (Fig. 1C). Some mutations were detected from several tumors and may represent hot spots. Furthermore, the frequency of these repeated mutations from the same tumor was low (Figs. 1D and 7 and Table 1), suggesting that the mutations did not occur at an early stage of cancer development. Nucleotide substitutions can cause missense and nonsense mutations, and deletions can cause frameshifts. Therefore, some of these mutations may be involved in destabilization or inactivation of IKKα or interfere with IKKα synthesis. In addition, we observed that most poorly differentiated human and mouse SCCs expressed reduced IKKα protein (Figs. 1A and 4 A and B). It is possible that genetic alterations in Ikkα affect the level of IKKα protein in these tumors.
Microenvironmental conditions have been shown to be pivotal factors in controlling tumor development (19). Lori·IKKα-Tg mice developed significantly fewer carcinomas and metastases than did WT mice (Fig. 3B). Because the basal cells expressed low levels of the IKKα transgene (Fig. 2B), the overexpressed IKKα in the suprabasal epidermis may account for most of its function. We found that Raf and ERK activities were lower in the Lori·IKKα-Tg skin than in the WT skin (Fig. 5B). The number of proliferating cells in the epidermis was dramatically reduced in Lori·IKKα-Tg mice compared with WT mice (Fig. 5C). Moreover, the number of blood microvessels was significantly lower in the skin stroma of Lori·IKKα-Tg mice than in the skin stroma of WT mice. Our results first demonstrated that IKKα negatively regulated VEGF-A expression at the transcription level (Fig. 6 C and D). Overexpression of IKKα repressed DMBA/TPA-enhanced VEGF-A expression in the suprabasal epidermis, where VEGF-A is normally induced (18). As a result, keratinocytes in the suprabasal epidermis of Lori·IKKα-Tg mice released less VEGF-A into skin than did WT mice. Collectively, our findings reveal that overexpression of IKKα in the epidermis represses mitogenesis and angiogenesis, preventing tumor progression and metastasis.
Previous studies have demonstrated that IKKα is able to induce keratinocyte terminal differentiation in in vitro and in vivo systems independently of its kinase activity (11, 20, 21). In the present study, we showed that overexpression of IKKα in the suprabasal epidermis elevated terminal differentiation in the skin (Fig. 2 D and E). However, Nakayama et al. (22) reported that overexpression of IKKα in human SCC cell lines repressed calcium-induced terminal differentiation and that this activity likely required IKKα kinase activity, suggesting that transformed keratinocytes do not respond to IKKα-induced terminal differentiation signals. Thus, it is likely that primary cultured keratinocytes and normal skin retain a normal program that regulates keratinocyte differentiation in response to differentiation inducers, whereas this program must be interrupted in cells undergoing transformation, such that the cells no longer respond to these inducers. Taken together, these findings indicate that overexpression of IKKα in normal keratinocytes surrounding tumors is an important mechanism for IKKα-mediated suppressive activity in skin carcinogenesis.
Materials and Methods
Human Tissue Arrays.
Two paraffin sections (SK801-107 and SK802-111) containing 114 human skin SCC specimens and eight human skin arrays were purchased from US Biomax (Rockville, MD) and were immunohistochemically stained with anti-IKKα antibody (Santa Cruz Biotechnology, Santa Cruz, CA) by the Histology Core at the Science Park.
DNA Preparation of Human SCCs.
Paraffin sections (5 μm) of human SCCs from archived tissue blocks from the Department of Pathology at M. D. Anderson Cancer Center (IRB-approved protocol Lab01-037) were isolated under a microscope, dissolved in xylene, and centrifuged for 10 min at 16,000 × g. The pellets were dissolved in xylene, incubated with 100% ethanol for 30 min, and centrifuged. The pellets were air-dried, lysed in a 500-μl solution containing 50 mM Tris·Cl, 10 mM EDTA, 1% Tween 20, and 100 μg of fresh proteinase K at 55°C overnight, incubated at 95°C for 10 min, and centrifuged. The supernatant contained genomic DNA.
Detection of Mutations of Ikkα.
PCR primers (5′-tcagaatctgaagaatagaaag-3′ and 5′-tatatttggggaaaggaaaac-3′) located in intron 14 and intron 15 of the human Ikkα gene were used to generate a genomic DNA fragment. An expanded high-fidelityplus PCR system (Roche, Indianapolis, IN) was used for PCR. The PCR products were subcloned into pGEM-T vectors (Promega, Madison, WI) and sequenced. The mutation specific PCR primers included: 533 (A to G, 228-bp), forward (exon 15): 5′-tggatacctggaggatca-3′ (WT) and 5′-tggatacctggaggatcg-3′ (M), and reverse (intron 15–16): 5′-tatatttggggaaaggaaaac-3′; 551 (A to G, 175-bp), forward (exon 15): 5′-agaagagcccctatggaa-3′ (WT), 5′-agaagagcccctatggag-3′ (M), and reverse (intron 15–16): 5′-tatatttggggaaaggaaaac-3′; 552 (T to C, 104-bp), forward (exon 15): 5′-gttggtgtcattggatacctg-3′, and reverse (intron 15–16): 5′-tccatcaagtctccctga-3′ (WT) and 5′-tccatcaagtctccctgg-3′ (M).
Generation of Lori·IKKα-Tg Mice.
Human IKKα cDNA fragment tagged with HA was generated by using PCR (primers, 5′-atcgatttggccatgtacccatacgatgtt-3′ and 5′-atcgattcattctgttaaccaactccaatc-3′) from a vector expressing IKKα (4). The PCR fragment was subcloned into the ClaI site of a pG11-ML vector that contained a truncated loricrin promoter (13), which was used to generate Lori·IKKα-Tg mice at the Transgenic Core at the Science Park. Three founders were obtained that were then crossed with WT mice on an FVB background. Two lines, Lori·IKKα-Tg-8 and Lori·IKKα-Tg-5, expressed the IKKα transgene but one line did not. Lori·IKKα-Tg-8 line was used for this skin carcinogenesis study. Lori·IKKα-Tg-5 mice had an activity similar to that of Lori·IKKα-Tg-8 mice in skin carcinogenesis (data not shown). The PCR primers 5′-aaagtgtgggctgaagcagtg-3′ and 5′-gcccaacaacttgctcaaatg-3′, which generated a 546-bp fragment of the human IKKα cDNA, were used for genotyping of these mice.
Skin Carcinogenesis Experiments and Histologic Examinations.
All of the mice were cared for in accordance with the guidelines of the M. D. Anderson Institutional Animal Care and Use Committee (protocol 04–01-05732). Female 6- to 8-week-old heterozygous Lori·IKKα-Tg and WT mice were bred from the same parents for this study. Mice were treated once with 25 μg of DMBA in 200 μl of acetone and 2 weeks later with 2.5 μg of TPA in 200 μl of acetone twice a week for 28 weeks or 50 μg of DMBA in 200 μl of acetone once a week for 20 weeks. Control mice were treated with only 2.5 μg of TPA in 200 μl of acetone twice a week for 28 weeks or 25 μg of DMBA in 200 μl of acetone once and then with acetone for 28 weeks. Tumors were counted weekly. The preparation of paraffin sections, H&E staining, and immunohistochemical staining were performed by the Histology Core at the Science Park Research Division. Antibodies against K5 (BAbCO, Richmond, CA), CD-31 (Pharmingen, San Diego, CA), and Ki67 (DAKO, Carpinteria, CA) were used. Blood microvessels were counted in the stroma, and staining with an anti-Ki67 antibody in the epidermis was performed as described in refs. 23 and 24.
Western Blotting.
A protein lysate (40 μg) was subjected to 10% SDS/PAGE (20). The separated proteins were transferred to an immobilon-P transfer membrane (Millipore, Bedford, MA) and blotted with anti-IKKα (Imgenex, San Diego, CA), filaggrin (BAbCO), p-Raf (Cell Signaling Technology, Beverly, MA), Raf (Santa Cruz Biotechnology), p-ERK (Cell Signaling), ERK1/2 (Santa Cruz Biotechnology), or β-actin (Sigma, St. Louis, MO) antibodies.
Northern Blotting.
Total RNA was isolated from cultured cells, skin, and tumors by using TRI Reagent (Molecular Research Center, Inc. Cincinatti, OH). Total RNA (10 μg) from each sample was separated and transferred to a membrane (Bio-Rad, Hercules, CA) and hybridized with a mouse IKKα cDNA probe (1–575 nucleotides) or a mouse VEGF-A cDNA probe (79–569 nucleotides), according to the manufacturer's instructions. The VEGF-A probe was generated by using PCR with the primers 5′-gcacccacgacagaaggagagcaga-3′ and 5′-cgccttggcttgtcacatctgcaa-3′. Keratinocytes were isolated and cultured as described in ref. 20.
ChIP Assay.
ChIP assay was performed by using a kit according to the manufacturer's instructions (Upstate Cell Signaling Solutions, Temecula, CA). Primary cultured keratinocytes were infected with adenoviruses expressing RasV61, RasN17, IKKα, and GFP as described previously in ref. 20. The chromatin DNA was precipitated by using an anti-IKKα antibody (Imgenex).
Supplementary Material
Acknowledgments
We thank Dennis Roop (Baylor College of Medicine, Houston, TX), for providing the loricrin promoter vector, Debra Hollowell for generating the IKKα transgenic mice, Howard Thames and Kevin Lin for statistical analyses, and Vanessa Edwards for assisting with manuscript preparation. This work was supported by National Cancer Institute Grants CA102510 and CA117314 (to Y.H.), CA105345 (to S.M.F.), and CA16672.
Abbreviations
- DMBA
7,12-dimethylbenz[a]anthracene
- HA
hemagglutinin A
- IKK
IκB kinase
- SCC
squamous cell carcinoma
- TPA
12-O-tetradecanoylphorbol-13-acetate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Petersen S, Rudolf J, Bockmuhl U, Gellert K, Wolf G, Dietel M, Petersen I. Oncogene. 1998;17:449–454. doi: 10.1038/sj.onc.1201949. [DOI] [PubMed] [Google Scholar]
- 2.Kubo Y, Urano Y, Hida Y, Arase S. J Dermatol Sci. 1999;19:199–201. doi: 10.1016/s0923-1811(98)00058-9. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, Halperin AJ, Baden HP, Shapiro PE, Bale AE, et al. Proc Natl Acad Sci USA. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 5.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 6.Rothwarf DM, Zandi E, Natoli G, Karin M. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 7.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC, Verma IM. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 11.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 12.Bottema CDK, Sommer SS. In: Mutation Detection, A Practical Approach. Cotton RGH, Edkins E, Forrest S, editors. Oxford: Oxford Univ Press; 1998. pp. 161–187. [Google Scholar]
- 13.DiSepio D, Bickenbach JR, Longley MA, Bundman DS, Rothnagel JA, Roop DR. Differentiation. 1999;64:225–235. doi: 10.1046/j.1432-0436.1999.6440225.x. [DOI] [PubMed] [Google Scholar]
- 14.Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 16.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Nat Med. 1997;3:1222–1227. doi: 10.1038/nm1197-1222. [DOI] [PubMed] [Google Scholar]
- 17.Larcher F, Franco M, Bolontrade M, Rodriguez-Puebla M, Casanova L, Navarro M, Yancopoulos G, Jorcano JL, Conti CJ. Mol Carcinog. 2003;37:83–90. doi: 10.1002/mc.10126. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto J, Ehama R, Ge Y, Kobayashi T, Nishiyama T, Detmar M, Burgeson RE. Am J Pathol. 2000;157:103–110. doi: 10.1016/S0002-9440(10)64522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill R, Song Y, Cardiff RD, Van Dyke T. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama H, Ikebe T, Shirasuna K. Oral Oncol. 2005;41:729–737. doi: 10.1016/j.oraloncology.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Giatromanolaki A, Koukourakis MI, Theodossiou D, Barbatis K, O'Byrne K, Harris AL, Gatter KC. Clin Cancer Res. 1997;3:2485–2492. [PubMed] [Google Scholar]
- 24.Sung YM, He G, Hwang DH, Fischer SM. Oncogene. 2006;25:5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.