Abstract
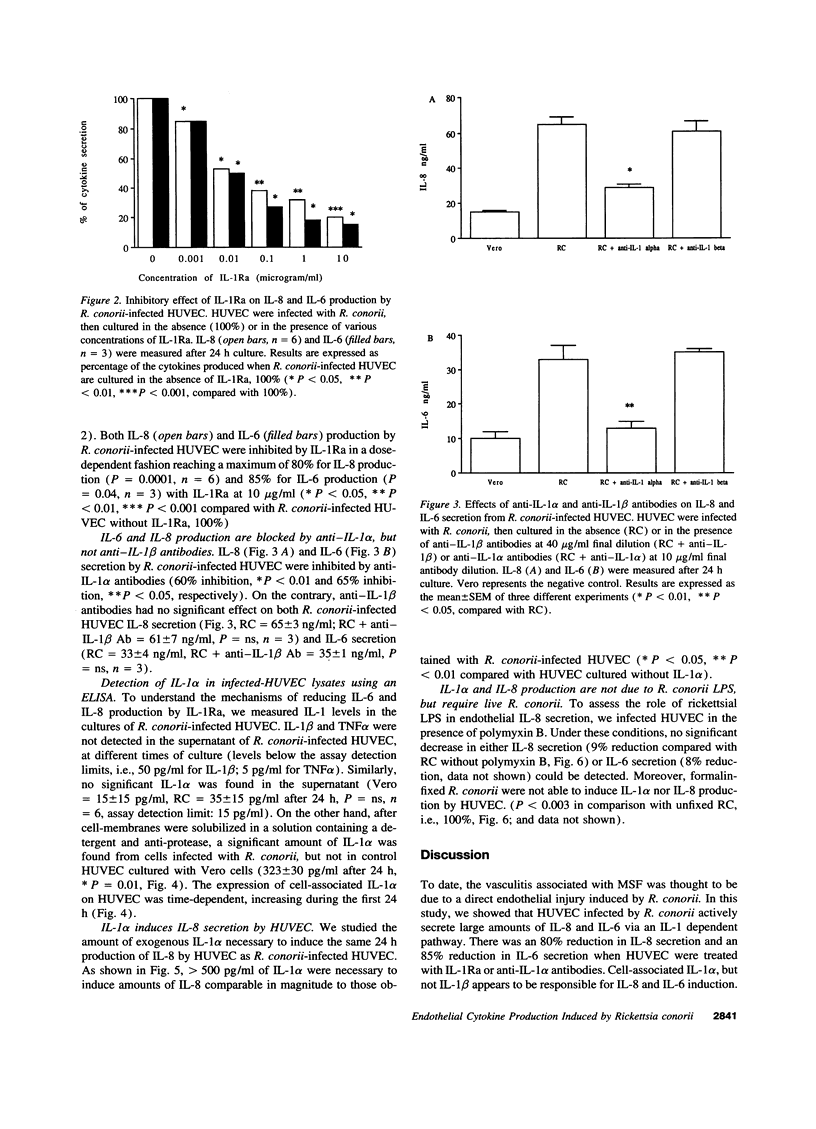
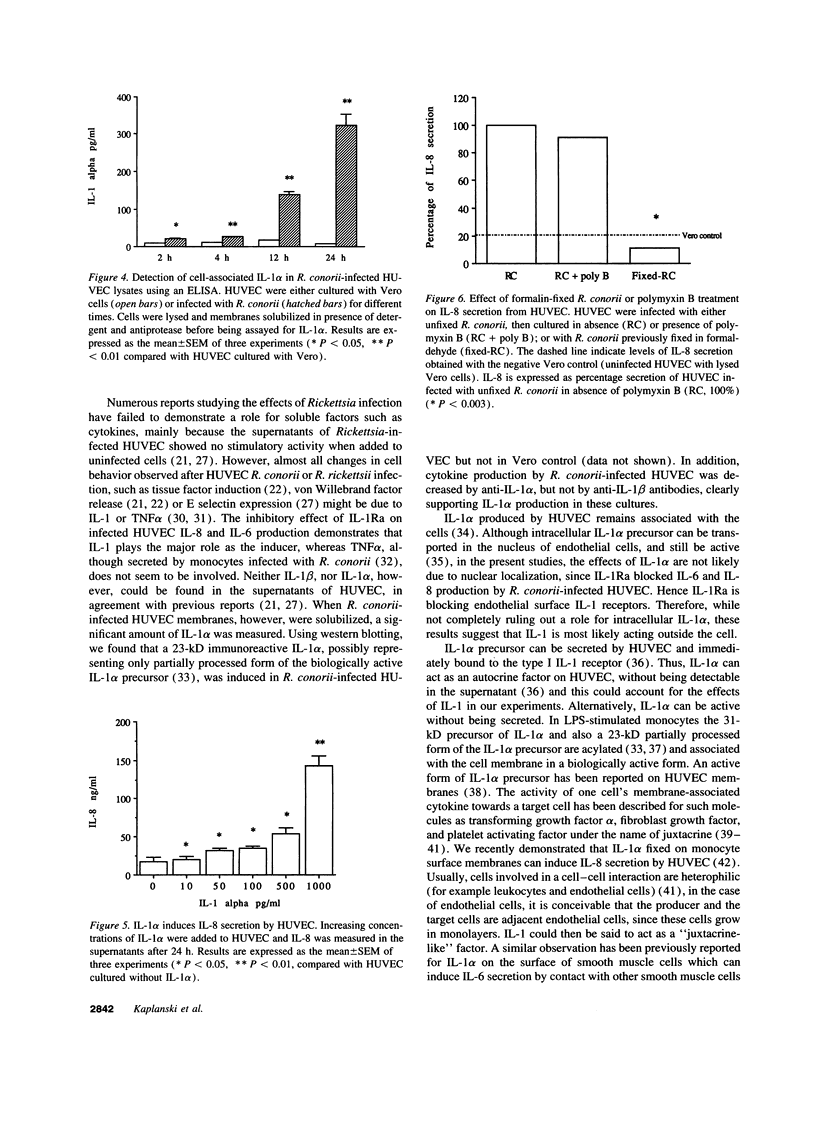
Mediterranean spotted fever due to infection by Rickettsia conorii, is characterized by a general vasculitis. This vasculitis is thought to be due to a direct injury to endothelial cells induced by R. conorii. However, production and activity of cytokines on endothelial cells is an important pathway in inflammation, and part of the underlying mechanism of vasculitis. In the present studies, human umbilical vein endothelial cells (HUVEC) infected with R. conorii actively secrete high levels of IL-8 and IL-6 (P < 0.002, and P < 0.03, respectively, compared with uninfected cells). IL-1alpha, IL-1beta, or TNFalpha were not detected in the culture supernates. Nevertheless, IL-6 and IL-8 production was due, in a large part, to a cell-associated form of IL-1 alpha expressed on R. conorii-infected HUVEC, since production of these cytokines was suppressed by 80% (P = 0.0001) and 85% (P < 0.04) by the addition of IL-1 receptor antagonist, or anti-IL-1alpha antibodies (60% inhibition, P < 0.01 and 65% inhibition, P < 0.05, respectively) and IL-1alpha was measured after lysis of R. conorii-infected HUVEC but not in uninfected cells (P < 0.01). Rickettsial lipopolysaccharide does not seem to be involved, since polymyxin B did not reduce cytokine secretion. On the contrary, infection by intracellular R. conorii appears to be necessary to induce IL-1alpha and subsequently IL-8, since formalin-fixed R. conorii did not induce cytokine production. These observations demonstrate that R. conorii-infected HUVEC secrete IL-6 and IL-8 via the induction of cell-associated IL-1alpha, providing a possible mechanism for the vasculitis observed in Mediterranean spotted fever.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anklesaria P., Teixidó J., Laiho M., Pierce J. H., Greenberger J. S., Massagué J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci U S A. 1990 May;87(9):3289–3293. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Bakouche O., Moreau J. L., Lachman L. B. Acylation of cell-associated IL-1 by palmitic acid. J Immunol. 1991 Oct 1;147(7):2164–2169. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carveth H. J., Bohnsack J. F., McIntyre T. M., Baggiolini M., Prescott S. M., Zimmerman G. A. Neutrophil activating factor (NAF) induces polymorphonuclear leukocyte adherence to endothelial cells and to subendothelial matrix proteins. Biochem Biophys Res Commun. 1989 Jul 14;162(1):387–393. doi: 10.1016/0006-291x(89)92009-3. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Drancourt M., Alessi M. C., Levy P. Y., Juhan-Vague I., Raoult D. Secretion of tissue-type plasminogen activator and plasminogen activator inhibitor by Rickettsia conorii- and Rickettsia rickettsii-infected cultured endothelial cells. Infect Immun. 1990 Aug;58(8):2459–2463. doi: 10.1128/iai.58.8.2459-2463.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler J. S., Wisseman C. L., Jr, Fiset P., Clements M. L. Cell-mediated immune responses of adults to vaccination, challenge with Rickettsia rickettsii, or both. Am J Trop Med Hyg. 1992 Feb;46(2):105–115. doi: 10.4269/ajtmh.1992.46.105. [DOI] [PubMed] [Google Scholar]
- Dumler J. S., Wisseman C. L., Jr Preliminary characterization of inflammatory infiltrates in response to Rickettsia prowazekii reinfection in man: immunohistology. Acta Virol. 1992 Jan;36(1):45–51. [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Kaplanski G., Farnarier C., Kaplanski S., Porat R., Shapiro L., Bongrand P., Dinarello C. A. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994 Dec 15;84(12):4242–4248. [PubMed] [Google Scholar]
- Kaplanski G., Porat R., Aiura K., Erban J. K., Gelfand J. A., Dinarello C. A. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993 May 15;81(10):2492–2495. [PubMed] [Google Scholar]
- Kuijpers T. W., Hakkert B. C., Hart M. H., Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992 May;117(3):565–572. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Fiers W., Pober J. S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987 Oct 1;139(7):2317–2324. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P. Functional significance of human vascular smooth muscle cell-derived interleukin 1 in paracrine and autocrine regulation pathways. Exp Cell Res. 1992 Feb;198(2):283–290. doi: 10.1016/0014-4827(92)90381-h. [DOI] [PubMed] [Google Scholar]
- Lorant D. E., Patel K. D., McIntyre T. M., McEver R. P., Prescott S. M., Zimmerman G. A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991 Oct;115(1):223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Kiely J. M., Ding H., Obin M. S., Hébert C. A., Baker J. B., Gimbrone M. A., Jr In vitro inhibitory effect of IL-8 and other chemoattractants on neutrophil-endothelial adhesive interactions. J Immunol. 1992 Sep 15;149(6):2163–2171. [PubMed] [Google Scholar]
- Maier J. A., Statuto M., Ragnotti G. Endogenous interleukin 1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol Cell Biol. 1994 Mar;14(3):1845–1851. doi: 10.1128/mcb.14.3.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor E., Sarov I. Tumor necrosis factor alpha and prostaglandin E2 production by human monocyte-derived macrophages infected with spotted fever group rickettsiae. Ann N Y Acad Sci. 1990;590:157–167. doi: 10.1111/j.1749-6632.1990.tb42218.x. [DOI] [PubMed] [Google Scholar]
- Massagué J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990 Dec 15;265(35):21393–21396. [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Poubelle P. E., Grassi J., Pradelles P., Marceau F. Pharmacological modulation of interleukin 1 production by cultured endothelial cells from human umbilical veins. Immunopharmacology. 1990 Mar-Apr;19(2):121–130. doi: 10.1016/0162-3109(90)90047-i. [DOI] [PubMed] [Google Scholar]
- Rao A. K., Schapira M., Clements M. L., Niewiarowski S., Budzynski A. Z., Schmaier A. H., Harpel P. C., Blackwelder W. C., Scherrer J. R., Sobel E. A prospective study of platelets and plasma proteolytic systems during the early stages of Rocky Mountain spotted fever. N Engl J Med. 1988 Apr 21;318(16):1021–1028. doi: 10.1056/NEJM198804213181603. [DOI] [PubMed] [Google Scholar]
- Raoult D., Drancourt A. M., Ardissone J. P. Enzyme secretion by human endothelial cells infected with Rickettsia conorii. Acta Virol. 1987 Aug;31(4):352–356. [PubMed] [Google Scholar]
- Renauld J. C., Vink A., Van Snick J. Accessory signals in murine cytolytic T cell responses. Dual requirement for IL-1 and IL-6. J Immunol. 1989 Sep 15;143(6):1894–1898. [PubMed] [Google Scholar]
- Ruiz R., Herrero J. I., Martin A. M., Sanz F., Mateos A., Hernandez A., Querol R., Portugal J. Vascular permeability in boutonneuse fever. J Infect Dis. 1984 Jun;149(6):1036–1036. doi: 10.1093/infdis/149.6.1036. [DOI] [PubMed] [Google Scholar]
- Schorer A. E., Moldow C. F., Rick M. E. Interleukin 1 or endotoxin increases the release of von Willebrand factor from human endothelial cells. Br J Haematol. 1987 Oct;67(2):193–197. doi: 10.1111/j.1365-2141.1987.tb02326.x. [DOI] [PubMed] [Google Scholar]
- Silverman D. J. Adherence of platelets to human endothelial cells infected by Rickettsia rickettsii. J Infect Dis. 1986 Apr;153(4):694–700. doi: 10.1093/infdis/153.4.694. [DOI] [PubMed] [Google Scholar]
- Silverman D. J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984 Jun;44(3):545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. B., Gamble J. R., Clark-Lewis I., Vadas M. A. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991 Jan;72(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- Sporn L. A., Lawrence S. O., Silverman D. J., Marder V. J. E-selectin-dependent neutrophil adhesion to Rickettsia rickettsii-infected endothelial cells. Blood. 1993 May 1;81(9):2406–2412. [PubMed] [Google Scholar]
- Sporn L. A., Shi R. J., Lawrence S. O., Silverman D. J., Marder V. J. Rickettsia rickettsii infection of cultured endothelial cells induces release of large von Willebrand factor multimers from Weibel-Palade bodies. Blood. 1991 Nov 15;78(10):2595–2602. [PubMed] [Google Scholar]
- Stevenson F. T., Bursten S. L., Fanton C., Locksley R. M., Lovett D. H. The 31-kDa precursor of interleukin 1 alpha is myristoylated on specific lysines within the 16-kDa N-terminal propiece. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teysseire N., Arnoux D., George F., Sampol J., Raoult D. von Willebrand factor release and thrombomodulin and tissue factor expression in Rickettsia conorii-infected endothelial cells. Infect Immun. 1992 Oct;60(10):4388–4393. doi: 10.1128/iai.60.10.4388-4393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Vicente V., Alberca I., Ruiz R., Herrero I., Gonzalez R., Portugal J. Coagulation abnormalities in patients with Mediterranean spotted fever. J Infect Dis. 1986 Jan;153(1):128–131. doi: 10.1093/infdis/153.1.128. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Occhino C., Tringali G. R., Di Rosa S., Mansueto S. Pathogenesis of rickettsial eschars: the tache noire of boutonneuse fever. Hum Pathol. 1988 Dec;19(12):1449–1454. doi: 10.1016/s0046-8177(88)80238-7. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Brown J. S., Hoover C. S., Morgan D. A. Endothelial prostaglandin secretion: effects of typhus rickettsiae. J Infect Dis. 1990 Nov;162(5):1136–1144. doi: 10.1093/infdis/162.5.1136. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsia species (as organisms). Annu Rev Microbiol. 1990;44:131–153. doi: 10.1146/annurev.mi.44.100190.001023. [DOI] [PubMed] [Google Scholar]



