Abstract
Exposure to UV radiation induces skin cancer and suppresses the immune response. To induce immune suppression, the electromagnetic energy of UV radiation must be absorbed by an epidermal photoreceptor and converted into a biologically recognizable signal. Two photoreceptors have been recognized: DNA and trans-urocanic acid (UCA). Trans-UCA is normally found in the outermost layer of skin and isomerizes to the cis isomer upon exposure to UV radiation. Although UCA was identified as a UV photoreceptor years ago, and many have documented its ability to induce immune suppression, its exact mode of action remains elusive. Particularly vexing has been the identity of the molecular pathway by which cis-UCA mediates immune suppression. Here we provide evidence that cis-UCA binds to the serotonin [5-hydroxytryptamine (5-HT)] receptor with relatively high affinity (Kd = 4.6 nM). Anti-cis-UCA antibody precipitates radiolabeled 5-HT, and the binding is inhibited by excess 5-HT and/or excess cis-UCA. Similarly, anti-5-HT antibody precipitates radiolabeled cis-UCA, and the binding is inhibited by excess 5-HT or excess cis-UCA. Calcium mobilization was activated when a mouse fibroblast line, stably transfected with the human 5-HT2A receptor, was treated with cis-UCA. Cis-UCA-induced calcium mobilization was blocked with a selective 5-HT2A receptor antagonist. UV- and cis-UCA-induced immune suppression was blocked by antiserotonin antibodies or by treating the mice with 5-HT2A receptor antagonists. Our findings identify cis-UCA as a serotonin receptor ligand and indicate that the immunosuppressive effects of cis-UCA and UV radiation are mediated by activation of the 5-HT2A receptor.
Keywords: immune regulation, inflammation, serotonin, UV radiation
The UV radiation found in sunlight is the primary cause of nonmelanoma skin cancer and is implicated in the induction of malignant melanoma (1). Skin cancer is the most prevalent form of human cancer. The American Cancer Society estimates that over one-half of all cancers diagnosed in the United States are skin cancer. Approximately 1 million cases of skin cancer were diagnosed last year, and ≈10,000 deaths were attributed to skin cancer (www.cancer.org/statistics). In addition to its carcinogenic potential, UV exposure is also immune suppressive. Data from studies with experimental animals and with biopsy-proven skin cancer patients indicate that the immune suppression induced by UV radiation is a major risk factor for skin cancer induction (2, 3). Moreover, UV exposure suppresses the immune response to infectious agents. After a single exposure to doses of UV radiation equivalent to those received during normal human occupational or recreational activities, the immune response of experimental animals (4, 5) or human volunteers (6) to microbial antigens is suppressed. The immune suppression caused by sunlight exposure also plays a significant role in herpes virus recrudescence (7). Because of the association between UV-induced immune suppression and carcinogenesis, and in light of the fact that exposure to UV radiation occurs daily and may be increasing due to the effects of atmospheric pollution on the ozone layer, it is critically important to understand the mechanisms underlying UV-induced immune suppression.
To induce immune suppression, the electromagnetic energy of UV radiation must first be absorbed by an epidermal photoreceptor and then converted into a biologically recognizable signal. Two such epidermal photoreceptors have been identified: DNA (8) and urocanic acid [3-(1H-imidazol-4-yl)-2-propenoic acid; UCA] (9). UCA is located superficially in the stratum corneum. Metabolism of epidermal UCA does not occur in situ due to the absence of epidermal urocanase, resulting in the accumulation of UCA in the epidermis. Upon UV exposure, naturally occurring trans-UCA converts to the cis isomer, in a dose-dependent manner, until the photostationary state is reached (10). Although UCA was first recognized as a UV-photoreceptor for immune suppression 20 years ago (9), and many have documented its ability to influence immune suppression (11) and carcinogenesis (12), its exact mode of action remains elusive. Particularly vexing has been the identity of the molecular pathway and the cellular receptor by which cis-UCA mediates immune suppression.
Once the physical energy of UV radiation is converted into a biologically recognizable signal, that signal must be transmitted to the immune system in order to induce immune suppression. Considerable evidence supports a role for UV-induced biological response modifiers and immune modulatory cytokines in activating immune suppression after UV exposure (13). Although the interplay between these various UV-induced immune modulatory factors is complex and not completely understood, it appears that a cytokine cascade is activated that ultimately induces immune suppression (14). One of the earliest biochemical events in the cascade of events leading to immune suppression is the secretion of platelet-activating factor (PAF) by UV-irradiated keratinocytes. PAF is secreted by epidermal cells almost immediately following UV radiation (15), and injecting PAF in lieu of UV exposure activates immune suppression (16). Similarly, blocking PAF receptor binding with a variety of PAF receptor antagonists blocked cytokine gene transcription and UV-induced immune suppression (16).
During our investigation into the immunosuppressive properties of PAF, we asked whether PAF receptor binding plays a role in cis-UCA-induced immune suppression. We treated mice with cis-UCA in the presence or absence of PAF receptor antagonists and measured the immune response (16). In these series of experiments, we included what we thought was an appropriate control, the selective serotonin (5-HT) receptor antagonist, ketanserin (17). Much to our surprise, ketanserin blocked cis-UCA-induced immune suppression. This observation suggested that cis-UCA and serotonin share the same receptor.
Here we provide data indicating that cis-UCA binds to the serotonin receptor. In addition, we report that UV-induced and cis-UCA-induced immune suppression were blocked by selective serotonin 5-HT2A receptor antagonists. Our findings identity the cellular receptor by which cis-UCA mediates immune suppression and provide insights into the role of serotonin in immune regulation.
Results
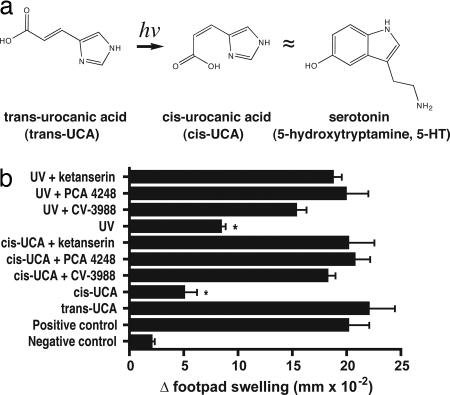
Several clues led us to hypothesize that cis-UCA might be a serotonin receptor agonist. The work of Ash et al. (18) revealed structural clues to the behavior of cis-UCA in aqueous solution. Upon isomerization, cis-UCA forms a ring-like structure. Analysis by NMR indicates that the ring structure of cis-UCA is stabilized by strong intramolecular hydrogen bonding between the inner nitrogen of the imidazole ring and the carboxylic acid moiety. This ring-like structure resembles the structure of serotonin (Fig. 1a). Another clue came from our continuing studies on the role of PAF receptor binding in UV-induced immune suppression. Because of the important role cis-UCA plays in UV-induced immune suppression, we wished to determine whether blocking of PAF receptor binding would interfere with cis-UCA-induced immune suppression. Mice were injected with 500 nmol of the selective PAF antagonists PCA-4248 or CV-3988, as described previously (16). Another group of mice were injected with an equimolar amount of ketanserin, a 5-HT2A receptor antagonist. Thirty minutes later, the mice were injected with an immunosuppressive (5 μmol) dose of cis-UCA (19). Five days later, the mice in both groups were immunized with Candida albicans and the effect that cis-UCA treatment had on the induction of a delayed-type hypersensitivity (DTH) reaction was measured (Fig. 1b). As expected, PAF receptor antagonists blocked cis-UCA and UV-induced immune suppression. Ketanserin, a selective serotonin receptor antagonist that we used as a negative control in these experiments, unexpectedly also blocked cis-UCA and UV-induced immune suppression. Previous experiments indicated that injecting the receptor antagonists by themselves did not affect DTH (20). This observation suggested that cis-UCA and serotonin share the same receptor, prompting a direct test of the hypothesis.
Fig. 1.
PAF and serotonin receptor antagonists block cis-UCA-induced immune suppression. (a) Structural comparison of 5-HT and cis-UCA. (b) Treating mice with either 500 nmol of PAF or 500 nmol of serotonin receptor antagonists before injecting 5 μmol cis-UCA or exposing them to 10 kJ/m2 of UV radiation blocks the induction of immune suppression. The asterisk denotes a significant difference (P < 0.01) from the positive control.
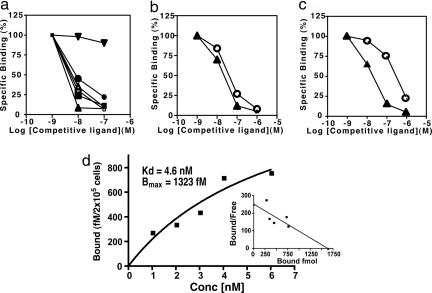
Competitive binding experiments were used to determine the ability of radiolabeled cis-UCA to bind to the serotonin receptor. Membrane preparations, isolated from Sf9 insect cells transfected with the human serotonin receptor, were incubated with 0.1 μM cis-[14C]UCA in the presence or absence of 10−6 to 10−9 M nonradioactive trans-UCA, cis-UCA, or 5-HT (Fig. 2a). Excess cis-UCA and serotonin, but not trans-UCA, displaced the binding of cis-[14C]UCA to the 5-HT-positive membranes. Treating the membrane preparations with PCA-4248, a selective serotonin receptor antagonist (21), also blocked the binding of radiolabeled cis-[14C]UCA to the 5-HT positive membranes. Similarly, when 0.1 μM [14C]5-HT was incubated with the membrane preparations in the presence of nonradioactive cis-UCA or 5-HT, the binding of radiolabeled 5-HT to the 5-HT receptor was blocked. Here also, PCA-4248 blocked the binding of [14C]5-HT to its receptor (Fig. 2a). These observations indicate that serotonin and cis-UCA bind to the same receptor.
Fig. 2.
5-HT and cis-UCA share binding sites. (a) Membrane preparations isolated from insect cells expressing the human serotonin receptor were incubated with radiolabeled cis-UCA (0.1 μM) or radiolabeled 5-HT (0.1 μM) in the presence of different amounts of nonradioactive ligand. Filled inverted triangles, [14C]5-HT + trans-UCA; filled circles, [14C]5-HT + cis-UCA; filled squares, [14C]5-HT + 5-HT; open triangles, [14C]5-HT + PCA-4248; M, cis-[14C]UCA + PCA-4248; filled triangles, cis-[14C]UCA + 5-HT. (b) cis-[14C]UCA was immunoprecipitated with cis-UCA mAb in the presence of increasing amounts of nonradioactive cis-UCA (open circles) or nonradioactive 5-HT (filled triangles). (c) cis-[14C]UCA was immunoprecipitated with anti-5-HT antibody in the presence of increasing amounts of nonradioactive cis-UCA (open circles) or nonradioactive 5-HT (filled triangles). (d) Saturation binding of cis-[3H]UCA to L-NCG-5HT2A cells. Data points represent specific binding; Kd and Bmax were determined from the best-fit nonlinear regression curve of the saturation isotherm. The saturation binding curve was converted to a Scatchard plot.
Immunoprecipitation studies confirmed the structural similarity of cis-UCA and serotonin. cis-[14C]UCA (0.1 μM) was incubated with 5 μg/ml anti-cis-UCA antibody in the presence or absence of 10−6 to 10−9 M nonradioactive cis-UCA or 5-HT. Precipitation of the radiolabeled ligand was blocked by excess cis-UCA and 5-HT (Fig. 2b). Similarly, [14C]5-HT was incubated with antiserotonin antibody, and excess amounts of unlabeled cis-UCA or serotonin blocked the precipitation of the radiolabeled ligand (Fig. 2c). These observations indicate that the ring-like structure formed by trans-to-cis isomerization of UCA (Fig. 1a) is immunologically similar to the epitope recognized by the antiserotonin antibody. Saturation binding studies (Fig. 2d) indicated that cis-UCA binds to the human 5-HT2A receptor with relatively high affinity (Kd = 4.6 nM). This result compares favorably with the binding of serotonin to the 5-HT2A receptor (22).
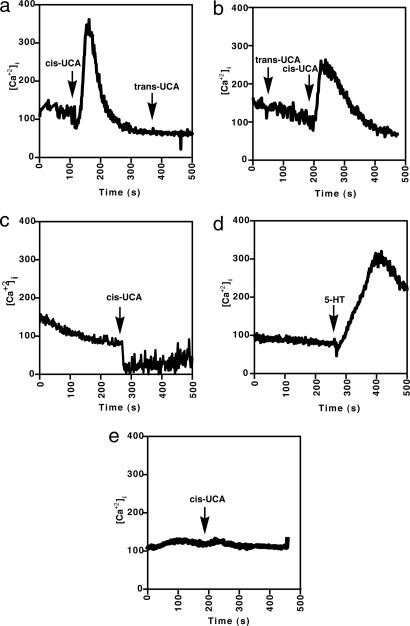
To determine whether cis-UCA could activate cells by means of the serotonin receptor, we measured Ca2+ mobilization. L-NCG-5-HT2A cells, or the untransfected control cells, LM(TK−), were loaded with fura-2 acetoxymethyl ester and then treated with 10 μM serotonin, 10 μM cis-UCA or 10 μM trans-UCA in the presence or absence of a serotonin receptor antagonist. Mobilization of intracellular calcium stores was then monitored. Calcium was released rapidly when the serotonin receptor-positive cells were treated with cis-UCA but not after subsequent treatment with trans-UCA (Fig. 3a). Similarly, L-NCG-5-HT2A cells first treated with trans-UCA did not react but did release calcium upon subsequent cis-UCA treatment (Fig. 3b). Pretreating the L-NCG-5-HT2A cells with 500 nmol of ketanserin completely prevented cis-UCA-induced calcium mobilization (Fig. 3c). As a positive control, L-NCG-5HT2A cells were treated with 5-HT, which clearly induced calcium mobilization (Fig. 3d). No calcium flux was observed when the serotonin receptor-negative cell line LM(TK−) was treated with cis-UCA (Fig. 3e). These data indicate that cis-UCA and serotonin bind to and activate cells by engaging the 5-HT2A receptor.
Fig. 3.
cis-UCA activates intracellular calcium flux via the serotonin receptor. (a and b) Calcium flux was induced in L-NGC-5HT2A by adding cis-UCA but not trans-UCA. (c) Pretreating the cells with ketanserin, a 5-HT2A receptor antagonist, blocks cis-UCA-induced calcium flux. (d) Positive control, L-NGC-5HT2A cells treated with 5-HT. (e) Negative control, LM(TK−) cells treated with cis-UCA.
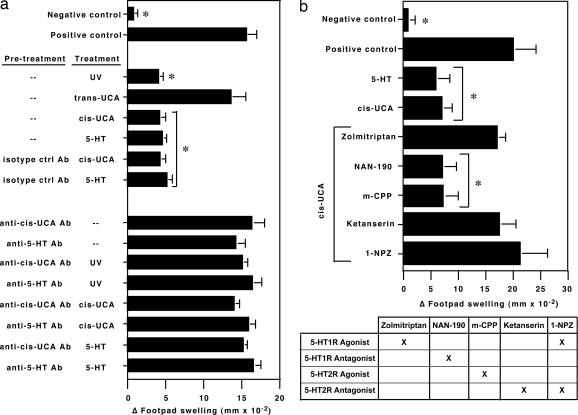
Next we examined the effects of a serotonin-specific antibody, or antibody specific for cis-UCA, on UV-, cis-UCA-, or 5-HT-induced immune suppression (Fig. 4a). Mice were injected with 5 μg of anti-cis-UCA or 5 μg of anti-5-HT antibody, or an equivalent amount of isotype-matched control antibody, 30 min before UV exposure (10 kJ/m2 UVB radiation), treatment with cis-UCA (5 μmol, i.p.), or treatment with serotonin (250 pmol, i.p.). Five days later, the mice were immunized with C. albicans, and DTH in vivo was used to measure the effect that each of these treatments had on the immune response. As expected, exposing the mice to UV or injecting them with cis-UCA suppressed immunity. Similarly, DTH was suppressed when mice were injected with 250 pmol serotonin, a physiological dose encountered during inflammation (23) (Fig. 4a). Confirming previous findings, we found that UV- or cis-UCA-induced immune suppression was blocked in mice treated with monoclonal anti-cis-UCA antibody (19). Similarly, the immune suppression induced by injecting 5-HT into mice was blocked when the mice were treated with anti-5-HT or anti-cis-UCA. The antibodies by themselves, when injected in mice that were not treated with UV, 5-HT, or cis-UCA, had no effect on DTH (Fig. 4a).
Fig. 4.
Inhibition of cis-UCA- and UV-induced immune suppression with selective 5-HT receptor antagonists, anti-cis-UCA antibody, or anti-5-HT antibody. (a) Treating mice with 5 μmol cis-UCA, 10 kJ/m2 of UV radiation, or 250 pmol 5-HT induces immune suppression. Pretreating the mice with anti-cis-UCA mAbs or with anti-5-HT mAb before UV exposure, 5-HT-treatment, or cis-UCA treatment blocks the induction of immune suppression. (b) Treating mice with the 5-HT2A receptor antagonists (ketanserin and 1-NZP) and with a 5-HT1 receptor agonist, zolmitriptan, blocks cis-UCA-induced immune suppression. The 5-HT1 receptor antagonist NAN-190 and the 5-HT2C receptor agonist m-CPP were ineffective in blocking cis-UCA-induced immune suppression. An asterisk indicates a significant difference (P < 0.01) from the positive control.
Because there are 14 known 5-HT receptor subtypes, and because at least 8 are expressed on immune tissues (24), we wanted to determine whether blocking/activating different classes of serotonin receptors had an effect on cis-UCA-induced immune suppression (Fig. 4b). In this experiment, mice were injected with 500 nmol of the various serotonin receptor agonists or antagonists 30 min before they were injected with an immunosuppressive dose of cis-UCA. As expected, ketanserin blocked cis-UCA-induced immune suppression. Interestingly, pretreating the cis-UCA-injected mice with the antimigraine drug zolmitriptan, a 5-HT1B/D receptor agonist (25), reversed cis-UCA-induced immune suppression. Similarly, the dual 5-HT1 agonist/5-HT2 antagonist (1-NPZ) (26) prevented cis-UCA-induced immune suppression. On the other hand, pretreating the cis-UCA-injected mice with m-CPP, a 5-HT2C receptor agonist (27), or with NAN-190, a 5-HT1 receptor antagonist (28), was ineffective in blocking cis-UCA-induced immune suppression. These data support our hypothesis that in vivo activation of the 5-HT2A receptor suppresses DTH.
Discussion
The observation that cis-UCA, an immune modulator produced in the stratum corneum, induces immunosuppression by binding to the 5-HT2A receptor provides insight into sunlight-induced immune suppression, sunlight-induced carcinogenesis, and the immunomodulatory role of serotonin. Our data indicate that serotonin receptor engagement can suppresses the immune response. This appears to contradict data published by Matsuda et al. (29), who showed that the vasoactive effects of serotonin were critically important for the efflux of immune cells into the site of antigen deposition during the challenge phase of DTH. A major difference in the way the experiments were designed may help to explain this discrepancy. In our experiments, serotonin/cis-UCA/UV exposure was given 5 days before immunization and 15 days before challenge. We propose that serotonin or cis-UCA given well before challenge activates processes known to be critical for UV- and cis-UCA-induced immunosuppression, such as mast cell activation (30, 31) and the secretion of antiinflammatory cytokines (14) that normally serve to dampen hypersensitivity reactions. UV exposure maybe acting in vivo to accelerate these normal feedback mechanisms, thereby promoting immune suppression. Alternatively, serotonin receptors may be desensitized (32), or serotonin transporters activated (33), when UV or cis-UCA is given 15 days before antigenic challenge.
Although our analysis was limited, our findings suggest that the activation/inhibition of different serotonin receptor subtypes may have different effects on the induction of immune suppression. This observation was not too surprising because it is known that activation of different 5-HT subtypes has diverse immunological consequences. For example, 5-HT1 receptor binding stimulates T and B cell proliferation (24). Activation of the 5-HT3, 5-HT4, and 5-HT7 receptors activates monocytes to secrete cytokines (34). Our findings are consistent with the concept that differential 5-HT receptor activation may induce different immunological consequences. We found that 5-HT2A receptor antagonism blocked UV- and cis-UCA-induced immune suppression and that treating cis-UCA-treated mice with a 5-HT1 receptor agonist (zolmitriptan) also blocked immune suppression. Similarly, 1-NZP, which is both a 5-HT2A receptor antagonist and a 5-HT1 receptor agonist, blocked immune suppression. Whether the reversal of immune suppression by 1-NZP is simply due to the inhibition of cis-UCA binding to the 5-HT2A receptor or is due to an activation of immune cells via the 5-HT1 receptor remains to be seen.
The downstream immunologic targets of cis-UCA are not entirely clear, but the observation that cis-UCA binds to the 5-HT2A receptor may shed some light on strategies to address this problem. The 5-HT2A receptors are found on dendritic cells (35). In view of the fact that antigen-presenting cell function is down-regulated after cis-UCA treatment and UV exposure (36), it may be worthwhile to determine whether 5-HT2A receptor antagonists can block depressed antigen-presenting cell function. Alternatively, T and B cells express 5-HT2A receptors (37). It is interesting to note that others have demonstrated that treating activated T cells with cis-UCA causes the secretion of IL-10 (38), a cytokine previously found to play a critical role in the immune suppression induced by UV radiation (39). Similarly, a recent report in the literature indicates that total-body UV exposure induces regulatory B cells to secrete IL-10 (40). Whether cis-UCA binding to 5-HT2A receptors expressed on T and B cells is responsible for IL-10 production remains to be seen. It is also well known that mast cell activation is critical for UV- and/or cis-UCA-induced immune suppression (30, 31). Kahlil et al. (41) report that cis-UCA does not directly cause degranulation of mast cells, but rather stimulates neuropeptide release from peripheral nerves, which in turn activates mast cells. It is interesting to note that peripheral nerves express 5-HT2A receptors (42). Here again, it is not clear whether cis-UCA binding to 5-HT2A receptors on nerve cells is causing the release of neuropeptides and subsequent mast cell activation. Alternatively, cis-UCA may be binding to mast cells, stimulating the release of PAF, which then activates the cytokine cascade responsible for UV-induced immune suppression.
Previously, Laihia and colleagues (43, 44) presented data indicating that cis-UCA binds to the GABAA receptor. Although predominately found in the brain, functional GABAA receptors are also found on T cells, and GABAA receptor agonists and antagonists have been shown to modulate immune function (45). Although the evidence for cis-UCA binding to the GABA receptor is clear, it is not readily apparent that cis-UCA is mediating immune suppression through the GABA receptor. Because GABA receptor engagement mediates an inhibitory event in the brain, some have suggested that cis-UCA may act as a competitive inhibitor in the periphery to reverse the effects of GABA and allow the production of the prostaglandins and cytokines necessary for UV-induced immune suppression (44). Although injecting GABA into normal mice suppresses DTH to a degree (≈30% reduction) (45), the threshold dose for GABA-mediated inhibition of T cell function (300 μM) is well above the levels of GABA (3 μM) (45) or cis-UCA (217 nM) (46) found in the periphery. Moreover, no information is available to indicate whether treating UV-irradiated mice with specific GABAA receptor antagonists or agonists will modulate UV- or cis-UCA-induced immune suppression, unlike the direct evidence we present here indicating a role for 5-HT2A receptor binding in UV- and cis-UCA-induced immune suppression.
The role of histamine in UV- and cis-UCA-induced immune suppression is well documented (47). Given the structural similarities between histamine and serotonin, a question that arises is whether cis-UCA binding to histamine receptors is responsible for immune suppression. Two pieces of data suggest that this is not the case. First, Laihia et al. (43) found that cis-UCA does not bind to histamine receptors. Second, the binding of ketanserin to the histamine receptor is negligible (17), suggesting that reversal of cis-UCA- and/or UV-induced immune suppression by ketanserin cannot be attributed to antagonism of histamine receptor binding.
Recently, Woodward et al. (48) suggested that serotonin is not the receptor for cis-UCA. Unfortunately, unlike the studies described here, Woodward et al. failed to directly measure immune suppression after treatment with cis-UCA, but rather used monocyte prostaglandin E2 (PGE2) secretion as a surrogate endpoint. They report that activation of monocytes with a 5-HT2A/2C receptor agonist fails to induce PGE2 secretion. Although it is clear that PGE2 plays a role in UV-induced immune suppression (14), it is not clear that PGE2 secretion by monocytes is, by itself, solely responsible for inducing immune suppression. Nor should the failure of 5-HT2A activation to stimulate cytokine secretion by monocytes be too surprising because others have shown that monocyte cytokine secretion is activated by 5-HT3, 5-HT4, and 5-HT7 receptor binding (34) and suppressed by 5-HT2A receptor binding (49). Based on these observations, we stand by our conclusion that the receptor involved in cis-UCA-induced immune suppression is 5-HT2A.
Methods
Reagents.
UCA was purchased from Acros Organics (Geel, Belgium). Ketanserin, NAN-190, 1-(1-naphthyl) piperazine hydrochloride, 1-(m-chlorophenyl)piperazine, serotonin, antiserotonin antibody, histidase, and l-histidine were purchased from Sigma-Aldrich (St. Louis, MO). CV-3988 and PCA-4248 were purchased from Biomol (Plymouth Meeting, PA). Radiolabeled 14C, [14C]serotonin, and [3H]l-histidine were purchased from Amersham Biosciences (Piscataway, NJ). L-NCG-5-HT2 and the nontransfected parental cell line LM(TK−) were purchased from American Type Culture Collection (Manassas, VA).
Preparation of Radiolabeled cis-UCA.
trans-[3H]UCA or trans-[14C]UCA was prepared by the enzymatic deamination of radiolabeled histamine, as described previously (50). Radiolabeled trans-UCA was irradiated with 500 J/m2 of UVB radiation (FS-40 sunlamp; National Biological, Twinsberg, OH). The solution was spotted on a prerun silica gel 60 TLC plate (Merck, Darmstadt, Germany) and developed with 40% methanol/60% chloroform. After chromatography, the position of the trans and cis isomers was determined by UV-illumination of the TLC plate and comparison of the migration of the radiolabeled material vs. known standards. The radiolabeled cis-UCA was eluted from the silica gel powder by several rounds of chloroform/methanol solvent extraction. The concentration of cis-UCA was determined spectrophotometrically, as described by Morrison (10).
Radioligand Binding Assays.
Membrane preparations isolated from Sf9 cells that express the human 5-HT receptor were purchased from Sigma-Aldrich. The membrane suspension (500 μl), diluted 1:50 in 50 mM Tris·HCL (pH 7.4) containing 10 mM MgSO4, 0.5 mM EDTA, and 0.1% ascorbic acid was incubated with 0.1 μM cis-[14C]UCA or [14C]5-HT in the presence of 10−6 to 10−9 M nonradioactive ligand. After 3 h at 37°, the receptor–ligand complexes were captured on 3% polyethyleneimine-soaked glass fiber membranes. After washing, the radioactivity captured on the membranes was measured by liquid scintillation.
Alternatively, 0.1 μM cis-[14C]UCA or 0.1 μM [14C]5-HT was precipitated with 5 μg/ml anti-cis-UCA or 5 μg/ml anti-5-HT antibody in the presence or absence of 10−6 to 10−9 M nonradioactive ligand. After 3 h at 37°, the receptor–ligand complexes were captured on 3% polyethyleneimine-soaked glass fiber filters, and the signal captured on the filters was measured by liquid scintillation.
To determine the affinity of cis-UCA binding to the 5-HT2A receptor, saturation binding curves were constructed. Briefly, 2 × 105 L-NGC-5-HT2A cells were plated in 96-well tissue culture dishes the evening before the experiment. On the day of the experiment, the medium was removed and various dilutions of cis-[3H]UCA (0.001–6 nM in cRPMI medium 1640) were added to the confluent monolayers and incubated for 3 h in the cold. After incubation, the cells were washed three times with ice-cold PBS and then lysed with 1 M NaOH. The radioactivity in the washes and in the cell lysate was determined by liquid scintillation counting. Nonspecific binding was determined by measuring binding of the radiolabeled ligand to LM(TK−) cells, which are devoid of 5-HT receptors and do not bind serotonin (51). The saturation binding curve, Kd, and Bmax were determined by using Prism Statistical Software (GraphPad, San Diego, CA).
Intracellular Calcium Flux.
LM(TK−) and L-NGC-5-HT2A cells, plated on 22 × 30-mm glass coverslips, were loaded with 10 μM fura-2 acetoxymethyl ester (Molecular Probes, Eugene, OR) for 1 h at 37°C. The coverslips were washed thoroughly with PBS and then mounted on a 1.5-ml volume chamber and bathed in Hanks' balanced salt solution (HBSS) with Ca2+ at room temperature. The chamber was placed on an epifluorescence/phase-contrast microscope for Ca2+ imaging and quantitation. After a baseline [Ca2+]i was established, cells were then treated with 10 μM serotonin and/or cis-UCA in the presence or absence of ketanserin. An INCA workstation (Intracellular Imaging, Cincinnati, OH) was used to quantify [Ca2+]i levels based on fura-2 fluorescence. Fluorescence was monitored using a ×20 fluorescence objective. Cells were illuminated alternately at excitation wavelengths of 340 and 380 nm by using a xenon arc lamp. The emitted fluorescence was monitored at 511 nm with a video camera, and the calculated free [Ca2+]i was determined using the cell-free calibration curve. Calcium concentrations were determined using a cell-free calibration curve and INCA software (Win 3.1 version; Intracellular Imaging), as described previously (52). Results are given as mean ± SD from at least 10–20 cells.
DTH.
The dorsal hair of C3H/HeNCr mice (National Cancer Institute, Frederick, MD) was removed with electric clippers, and the mice were exposed to 10 kJ/m2 of UVB radiation from sunlamps (FS 40; National Biological). Alternatively, 5 μmol cis-UCA or 250 pmol serotonin were injected into the peritoneal cavity. Anti-cis-UCA antibody or antiserotonin antibody (5 μg of protein per mouse, i.p.) was given 30 min before UV exposure or cis-UCA injection. Similarly, serotonin receptor antagonists (500 nmol per mouse) were injected 30 min before UV exposure or cis-UCA or serotonin injection. Five days later, the animals were immunized by injecting 107 formalin-fixed C. albicans into each flank. Nine days later, the mice were challenged with antigen, and the immune response was measured 18–24 h later. The data are expressed as the mean change in footpad swelling ± SD (n = 5). Statistical differences between the controls and experimental groups were determined by one-way ANOVA, followed by the Dunnett's multiple comparison test. Probabilities <0.05 were considered significant (Prism Statistical Software; GraphPad). All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
Acknowledgments
We thank Drs. Margaret L. Kripke and Konrad Muller for critical reading of the manuscript. This work was supported by National Cancer Institute (NCI) Grants SEU-CA112660, SEU-CA75575, and DJM-CA69676. The animal facilities at the University of Texas M. D. Anderson Cancer Center are supported in part by NCI Core Grant CA16672.
Abbreviations
- DTH
delayed-type hypersensitivity
- 5-HT
5-hydroxytryptamine (serotonin)
- PAF
platelet activating factor
- PGE2
prostaglandin E2
- UCA
urocanic acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Boring CC, Squires TS, Tong T. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Fisher MS, Kripke ML. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 4.Nghiem DX, Kazimi N, Clydesdale G, Ananthaswamy HN, Kripke ML, Ullrich SE. J Invest Dermatol. 2001;117:1193–1199. doi: 10.1046/j.0022-202x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 5.Jeevan A, Kripke ML. J Immunol. 1989;143:2837–2843. [PubMed] [Google Scholar]
- 6.Garssen J, Goettsch W, de Gruijl F, Slob W, van Loveren H. Photochem Photobiol. 1996;64:269–274. doi: 10.1111/j.1751-1097.1996.tb02457.x. [DOI] [PubMed] [Google Scholar]
- 7.Norval M, Garssen J, Van Loveren H, el-Ghorr AA. J Epidemiol. 1999;9:S84–S92. doi: 10.2188/jea.9.6sup_84. [DOI] [PubMed] [Google Scholar]
- 8.Kripke ML, Cox PA, Alas LG, Yarosh DB. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Fabo EC, Noonan FP. J Exp Med. 1983;157:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison H. Photodermatol. 1985;2:158–165. [PubMed] [Google Scholar]
- 11.Norval M, Gibbs NK, Gilmour J. Photochem Photobiol. 1995;62:209–217. doi: 10.1111/j.1751-1097.1995.tb05261.x. [DOI] [PubMed] [Google Scholar]
- 12.Beissert S, Ruhlemann D, Mohammad T, Grabbe S, El-Ghorr A, Norval M, Morrison H, Granstein RD, Schwarz T. J Immunol. 2001;167:6232–6238. doi: 10.4049/jimmunol.167.11.6232. [DOI] [PubMed] [Google Scholar]
- 13.Ullrich SE. Front Biosci. 2002;7:D684–D703. doi: 10.2741/A804. [DOI] [PubMed] [Google Scholar]
- 14.Shreedhar V, Giese T, Sung VW, Ullrich SE. J Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- 15.Barber LA, Spandau DF, Rathman SC, Murphy RC, Johnson CA, Kelley SW, Hurwitz SA, Travers JB. J Biol Chem. 1998;273:18891–18897. doi: 10.1074/jbc.273.30.18891. [DOI] [PubMed] [Google Scholar]
- 16.Walterscheid JP, Ullrich SE, Nghiem DX. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- 18.Ash EL, Sudmeier JL, De Fabo EC, Bachovchin WW. Science. 1997;278:1128–1132. doi: 10.1126/science.278.5340.1128. [DOI] [PubMed] [Google Scholar]
- 19.Moodycliffe AM, Bucana CD, Kripke ML, Norval M, Ullrich SE. J Immunol. 1996;157:2891–2899. [PubMed] [Google Scholar]
- 20.Ramos G, Kazimi N, Nghiem DX, Walterscheid JP, Ullrich SE. Toxicol Appl Pharmacol. 2004;195:331–338. doi: 10.1016/j.taap.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Martins MA, Lima MC, Bozza PT, Faria Neto HC, Silva PM, Sunkel CE, Cordeiro RS. Eur J Pharmacol. 1993;237:17–22. doi: 10.1016/0014-2999(93)90087-x. [DOI] [PubMed] [Google Scholar]
- 22.Bonaventure P, Nepomuceno D, Miller K, Chen J, Kuei C, Kamme F, Tran DT, Lovenberg TW, Liu C. Eur J Pharmacol. 2005;513:181–192. doi: 10.1016/j.ejphar.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Mossner R, Lesch KP. Brain Behav Immun. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 24.Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J. Immunology. 2005;115:289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deleu D, Hanssens Y. J Clin Pharmacol. 2000;40:687–700. doi: 10.1177/00912700022009431. [DOI] [PubMed] [Google Scholar]
- 26.Fuller RW, Mason NR, Snoddy HD, Perry KW. Res Commun Chem Pathol Pharmacol. 1986;51:37–45. [PubMed] [Google Scholar]
- 27.Steardo L, Monteleone P, Trabace L, Cannizzaro C, Maj M, Cuomo V. J Pharmacol Exp Ther. 2000;295:266–273. [PubMed] [Google Scholar]
- 28.Rydelek-Fitzgerald L, Teitler M, Fletcher PW, Ismaiel AM, Glennon RA. Brain Res. 1990;532:191–196. doi: 10.1016/0006-8993(90)91759-a. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda H, Ushio H, Geba GP, Askenase PW. J Immunol. 1997;158:2891–2897. [PubMed] [Google Scholar]
- 30.Niizeki H, Alard P, Streilein JW. J Immunol. 1997;159:5183–5186. [PubMed] [Google Scholar]
- 31.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesch KP, Mossner R. Biol Psychiatry. 1998;44:179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 34.Durk T, Panther E, Muller T, Sorichter S, Ferrari D, Pizzirani C, Di Virgilio F, Myrtek D, Norgauer J, Idzko M. Int Immunol. 2005;17:599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 35.Idzko M, Panther E, Stratz C, Muller T, Bayer H, Zissel G, Durk T, Sorichter S, Di Virgilio F, Geissler M, et al. J Immunol. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 36.Noonan FP, De Fabo EC, Morrison H. J Invest Dermatol. 1988;90:92–99. doi: 10.1111/1523-1747.ep12462045. [DOI] [PubMed] [Google Scholar]
- 37.Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. Brain Behav Immun. 2000;14:219–224. doi: 10.1006/brbi.1999.0579. [DOI] [PubMed] [Google Scholar]
- 38.Holan V, Kuffová L, Zajícová A, Krulová M, Filipec M, Holler P, Janacárek A. J Immunol. 1998;161:3237–3241. [PubMed] [Google Scholar]
- 39.Rivas JM, Ullrich SE. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 40.Byrne SN, Halliday GM. J Invest Dermatol. 2005;124:570–578. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- 41.Khalil Z, Townley SL, Grimbaldeston MA, Finlay-Jones JJ, Hart PH. J Invest Dermatol. 2001;117:886–891. doi: 10.1046/j.0022-202x.2001.01466.x. [DOI] [PubMed] [Google Scholar]
- 42.Gaietta GM, Yoder EJ, Deerinck T, Kinder K, Hanono A, Han A, Wu C, Ellisman MH. J Neurocytol. 2003;32:373–380. doi: 10.1023/B:NEUR.0000011331.58835.fd. [DOI] [PubMed] [Google Scholar]
- 43.Laihia JK, Attila M, Neuvonen K, Pasanen P, Tuomisto L, Jansen CT. J Invest Dermatol. 1998;111:705–706. doi: 10.1046/j.1523-1747.1998.00351.x. [DOI] [PubMed] [Google Scholar]
- 44.Uusi-Oukari M, Soini SL, Heikkila J, Koivisto A, Neuvonen K, Pasanen P, Sinkkonen ST, Laihia JK, Jansen CT, Korpi ER. Eur J Pharmacol. 2000;400:11–17. doi: 10.1016/s0014-2999(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 45.Tian J, Chau C, Hales TG, Kaufman DL. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- 46.Moodycliffe AM, Norval M, Kimber I, Simpson TJ. Immunology. 1993;79:667–672. [PMC free article] [PubMed] [Google Scholar]
- 47.Hart PH, Townley SL, Grimbaldeston MA, Khalil Z, Finlay-Jones JJ. Methods. 2002;28:79–89. doi: 10.1016/s1046-2023(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 48.Woodward EA, Prele CM, Finlay-Jones JJ, Hart PH. J Invest Dermatol. 2006;126:1191–1193. doi: 10.1038/sj.jid.5700249. [DOI] [PubMed] [Google Scholar]
- 49.Cloez-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM. Int Immunol. 2003;15:233–240. doi: 10.1093/intimm/dxg027. [DOI] [PubMed] [Google Scholar]
- 50.Farrow SJ, Mohammad T, Baird W, Morrison H. Chem Biol Interact. 1990;75:105–118. doi: 10.1016/0009-2797(90)90026-j. [DOI] [PubMed] [Google Scholar]
- 51.Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, Durkin M, Hartig PR, Weinshank RL, Branchek TA. Proc Natl Acad Sci USA. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nutt LK, Chandra J, Pataer A, Fang B, Roth JA, Swisher SG, O'Neil RG, McConkey DJ. J Biol Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]