Abstract
Mullerian Inhibiting Substance (MIS), a biological modifier that causes regression of Mullerian ducts in male embryos, is effective as a single agent in vitro and in vivo against human and mouse ovarian cancer cell lines expressing MIS type II receptor; however, little is known about how recombinant human MIS (rhMIS), now being scaled for preclinical trials, could be used in combination with cytotoxic or targeted chemotherapeutic agents. Mouse serous and endometrioid ovarian carcinoma cell lines were tested in vitro against rhMIS alone and with doxorubicin, paclitaxel, or cisplatin as agents in clinical use. Because MIS releases FK506 binding protein (FKBP12), which activates the mammalian target of rapamycin (mTOR) downstream of Akt, rhMIS and rapamycin combinations were tested. MIS increases p16 protein levels, and 5′-Aza-2′-deoxycytidine (AzadC) induces p16 mRNA; therefore, they were used in combination in vitro and in vivo with a human ovarian cancer cell line. A paclitaxel-resistant human ovarian cancer cell line and its parental line both respond to rhMIS in vitro. Additivity, synergy, or competition was observed with MIS and rapamycin, AzadC, doxorubicin, cisplatin, and paclitaxel, suggesting that MIS in combination with selective targeted therapies might achieve greater activity against ovarian cancer than the use of each individual agent alone. These assays and statistical analyses could be useful in selecting rhMIS and chemotherapeutic agent combinations that enhance clinical efficacy and reduce toxicity.
Keywords: combination treatment, Mullerian Inhibiting Substance type II receptor
Mullerian Inhibiting Substance (MIS) may have its most important clinical effect as a therapy for MIS type II receptor (MISRII)-expressing cancers because of its relative lack of toxicity. For example, granulosa cell tumors can produce serum MIS at concentrations 1,000-fold higher (1, 2), and normal baby boys can produce MIS concentrations at least 60-fold higher, than those found in normal females (3), without apparent adverse outcomes. It is anticipated that exogenous recombinant human MIS (rhMIS) will be nontoxic as a single agent and that it can be used effectively in combination with chemotherapy drugs to lower their toxicity. We originally hypothesized that cancers of Mullerian origin that expressed MISRII could be targets for MIS treatment (4–9), focusing first on ovarian cancers because they are derived from an MIS-sensitive surface or coelomic epithelium (10) and have the worst prognosis of all female reproductive tumors. Furthermore, ovarian ascites cells are also accessible ex vivo to examine biomarkers that may predict their response and mechanisms of biologic effect. The idea that MIS could be used to treat epithelial ovarian cancer is predicted by the fact that the histology of the embryonic Mullerian ducts is recapitulated in the common ovarian adenocarcinomas that arise from the surface or coelomic epithelium, which in the embryo invaginates to form the Mullerian duct (11–14).
The discovery that abdominal ascites cells from >50% of Stage III or IV ovarian cancer patients bound rhMIS, expressed MISRII mRNA, and were inhibited by rhMIS when treated in ex vivo proliferation assays (4), makes it compelling to study MIS as a potential therapeutic agent. The availability of highly purified rhMIS (15, 16), which inhibits human carcinoma cell lines of Mullerian origin in vitro (17) and in vivo in short-term experiments (18), and more recently in long-term (19) experiments, with no observable toxicity, now makes this feasible. These long-term experiments were done with transgenic mouse cell lines that recapitulate human ovarian cancers (5). MOVCAR7 serous cystoadenocarcinoma cells were derived from ascites of transgenic animals in which the MISRII promoter drives the expression of T antigen (T-ag), which inactivates the retinoblastoma (Rb) family of proteins (5). The 4306 cell line was derived from animals in which Kras was constitutively activated and Pten was inactivated to induce ovarian cancer formation (20).
Because some downstream actions of MIS are now known, and because MIS can inhibit cell growth and trigger apoptosis in normal Mullerian epithelium (10) with negligible toxicity, a series of in vitro studies of MIS were conducted in combination with commonly used cytotoxic drugs to examine for evidence of synergy, additivity, or competition. Indeed, recent experience with the biologic bevacizumab (Avastin) demonstrates that biologic agents not active as single agents can markedly increase response rates when combined with chemotherapy (21, 22). Therefore, the cytosine analogue 5′-Aza-2′-deoxycytidine (AzadC; decitabine), an inhibitor of DNA methyltransferase (23–25) that can induce p16 mRNA expression, was also used because MIS enhances p16 at the protein level in OVCAR8 human ovarian cancer cells (10). Rapamycin was studied because of the role discovered for FK506 binding protein (FKBP12) in posttranslational regulation of downstream activation by MIS and other members of the TGF-β family (26, 27) and the fact that rapamycin can act downstream of Pten-regulated Akt via the mammalian target of rapamycin (mTOR). Paclitaxel, cisplatin, and doxorubicin, as agents presently in use in the clinical treatment of ovarian cancer, were tested in proliferation assays in vitro with rhMIS. Cisplatin and paclitaxel are first-line agents and, because the development of multidrug resistance is a prominent characteristic of many ovarian cancers, MIS was tested against human ovarian cancer (IGROV-1) cells made resistant to paclitaxel. The results of these in vitro studies could be useful in directing combinations to be tested in more costly in vivo studies when clinical-grade MIS is more readily available.
Previous experiments elucidating downstream signaling pathways by which MIS inhibits cell proliferation will guide the selection of other therapeutics that have the potential to cause complementary interactions while avoiding potential negative interactions. Comprehensive studies in our laboratory, and in those of many others, have shown that MIS downstream signal transduction pathways include MISRII (28–30), the type I receptors Alk2 (31) and Alk3 (32), Smads 1/5/8 (33–35), cyclin-dependent kinase inhibitors (4, 9, 10), and cytokine-inducible pathways (36–39), suggesting mechanisms of action that are potentially different from those of most cytotoxic drugs.
Biological modifiers such as MIS and chemotherapeutic drugs can function in rationally selected combinations to achieve better tumor control at decreased doses of either agent, resulting in decreased toxicity, reduced morbidity, and most importantly a wider therapeutic window (36). This will be particularly important for those agents with a significant toxicity at the clinically efficacious dose range. Although this possibility can be examined in vivo in detail when clinical-grade rhMIS is available, at this point in time in vitro evaluations of the systems biology of such combinations can identify salutary combinations for use with this prototypical biological modifier for patients whose tumors express MISRII.
Results
MIS Acts Additively with AzadC in vitro and in Vivo.
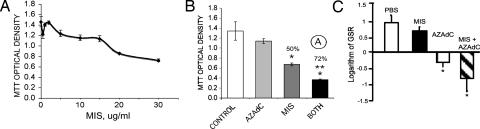
Proliferation of the human ovarian cancer cell line OVCAR8 is inhibited by rhMIS in a dose-dependent manner in vitro (Fig. 1A). AzadC at 15 μM and MIS at 20 μg/ml produced an additive effect on growth inhibition of OVCAR8 cells in vitro (Fig. 1B). These experiments served as a proof of principle for pilot animal studies. In initial in vivo studies, AzadC was toxic to nude mice at 1 mg/kg per day, whereas at 0.5 mg/kg per day, which is ≈10-fold lower (40) than the dose currently used in the clinic, the animals were viable and healthy before being killed at the end of the 3-week duration of the experiments. The dose of MIS was chosen as 10 μg per animal per day, the lower of two doses used in previous in vivo studies against OVCAR8 (18). AzadC alone, and MIS and AzadC in combination, showed a statistically significant difference from PBS (P = 0.003; Fig. 1C). MIS and AzadC appear to be additive in vivo (Fig. 1C), as predicted by the in vitro experimental data (Fig. 1B).
Fig. 1.
MIS inhibition of OVCAR8 cells in vitro. rhMIS in vitro dose–response plotted as OD in the MTT assay (± SD) (A) and in vivo is augmented by 15 μM AzadC (B and C, respectively). (B) A two-way factorial ANOVA was done to determine the nature of MIS/AzadC interaction in vitro. ○A, additive. MIS and the combined treatment were significantly different from the control (∗, P < 0.005 and 0.007, respectively), and the combination was different than MIS alone (∗∗, P < 2 ×10−5). (C) An analysis of the logarithm of graft size ratios (GSRs) (±SEM) reveals that the maximal effect with the combined treatment (MIS 10 μg per animal per day and AzadC 0.5 mg/kg per day) is additive (∗, P < 0.003, nonpaired t test vs. PBS control; n = 10 animals per group).
Predictors of Synergy or Additivity of MIS with Chemotherapeutic Agents.
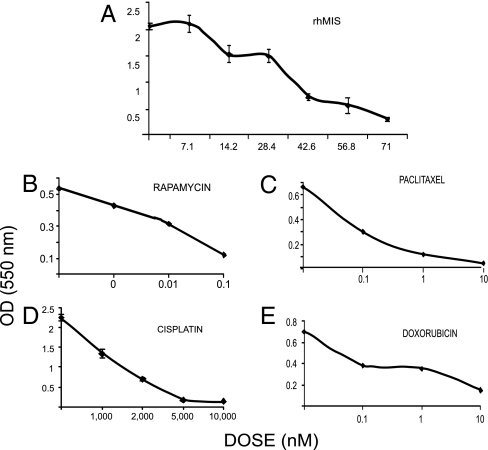
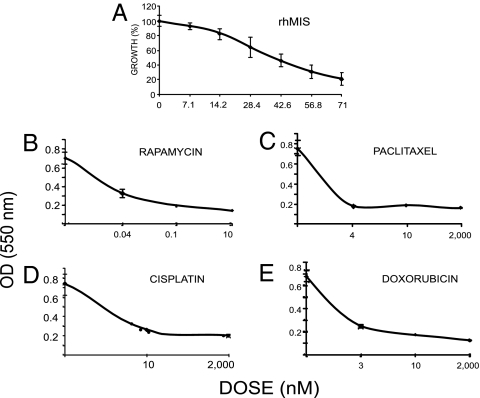
Dose–response curves were constructed for MIS and commonly used chemotherapeutic drugs, each with independent mechanisms of action. Specifically, rapamycin (an inhibitor of mTOR downstream in the Akt pathway) (41), paclitaxel (a tubulin-binding agent that inhibits microtubule depolymerization), cisplatin (DNA alkylation), and doxorubicin (topoisomerase II inhibition) were selected for evaluation with or without rhMIS, employing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The MIS dose selected (35 nM) produced ≈50% inhibition in MOVCAR7 cells (Fig. 2A). The drug doses chosen for rapamycin (0.02 nM), paclitaxel (4 nM), cisplatin (5 nM), and doxorubicin (3 nM) (Fig. 2 B–E) for the experiments (see below) were at or below the IC50 against MOVCAR cells and well below the doses used in vivo in animal studies (42) or in the clinic. MIS and drug dose responses were also established for the 4306 cells. The MIS dose selected (70 nM) produced between 50% and 87% inhibition against the less-sensitive 4306 cells (Fig. 3 A). The drug doses chosen for rapamycin (0.04 nM), paclitaxel (4 nM), cisplatin (10 nM), and doxorubicin (3 nM) (Fig. 3 B–E), for the experiments shown in Fig. 4, were at or below the IC50 against 4306 cells and well below the doses used in vivo in animal studies or in the clinic. The experimental design for the combination studies was to select doses of either MIS or a drug that would produce a partial response, so that the effect of their combinations could then be assessed. Because different batches of rhMIS were prepared weekly, rhMIS IC50 varied somewhat between experiments, as did the response of the cells to chemotherapeutic agents. However, each experimental result could be tested for synergy, additivity, or competition, even though the extent of the response to each drug or MIS could vary in replicate experiments.
Fig. 2.
Dose responses for MOVCAR7 cells. MOVCAR7 cell dose responses to rhMIS (A), rapamycin (B), paclitaxel (C), cisplatin (D), or doxorubicin (E) were established in MTT monolayer growth inhibition assays over 5–7 days in culture.
Fig. 3.
Dose responses for 4306 cells. Dose responses of 4306 cells to rhMIS (A), rapamycin (B), paclitaxel (C), cisplatin (D), or doxorubicin (E) were established in MTT monolayer growth inhibition assays over 5–7 days in culture.
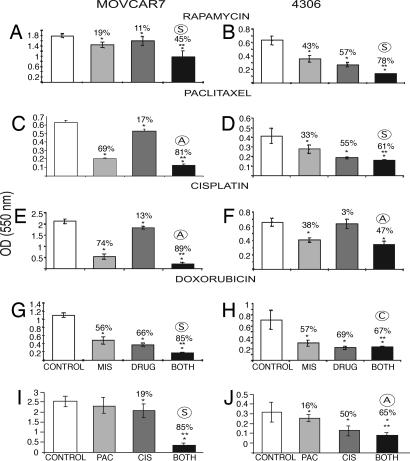
Fig. 4.
Representative data for combination treatment of MOVCAR7 and 4306 cells in MTT in vitro assays with MIS and chemotherapeutic drugs (OD ± SEM and percentage inhibition). MIS and the drug concentrations were held constant at or near their IC50 for each cell line. MIS doses were 35 nM for MOVCAR7 and 70 nM for 4306; the concentrations of chemotherapeutic drugs used are given below. (A) MOVCAR7 cells treated with vehicle as a control, MIS, 0.02 nM rapamycin, or both agents (n = 3; 7 days). (B) The same treatment of 4306 cells using 0.04 nM rapamycin (n = 3; 7 days). Rapamycin and MIS were synergistic ○S in both cases. (C and D) In MOVCAR cells, MIS and 3 nM paclitaxel were additive ○A (n = 4; 6 days) (C), whereas MIS and 4 nM paclitaxel were synergistic in 4306 cells (n = 3; 6 days) (D). (E and F) Cisplatin was additive with MIS in both MOVCAR (E) and 4306 cells (F) at 5 and 10 nM drug, respectively (n = 3; 6 days in both cases). (G and H) The combination of doxorubicin (3 nM) and MIS is synergistic in MOVCAR cells (G), but the same concentration of drugs is competitive ○C with MIS in 4306 cells (H) (n = 3; 6 days). Significant inhibition relative to controls is indicated by an asterisk. MIS alone was significantly different from combination treatment in all cases (∗∗), except for MIS and cisplatin in 4306 cells. (I and J) In combination experiments with 3 nM cisplatin (CIS) and 4 nM paclitaxel (PAC), these drugs were found to act synergistically in MOVCAR cells (I) but not in 4306 cells (J). One experiment showed that the effect was additive and two showed that the interaction was competitive. Two-way factorial ANOVA was done to determine the nature of the MIS/drug interactions.
RhMIS or rapamycin alone inhibited MOVCAR7 cell growth (P = 0.0001 and 0.0003, respectively) compared with controls. Inhibition induced by the combination of rapamycin and rhMIS in both cell lines was significantly different from rapamycin (P = 2 × 10−5) or MIS alone (P = 0.0003). The interaction term (MIS × rapamycin) indicated that the interaction was significant (P < 0.05) in MOVCAR7 cells and P = 0.008 in 4306 cells), consistent with synergy, as observed in four experiments (Fig. 4 A and B).
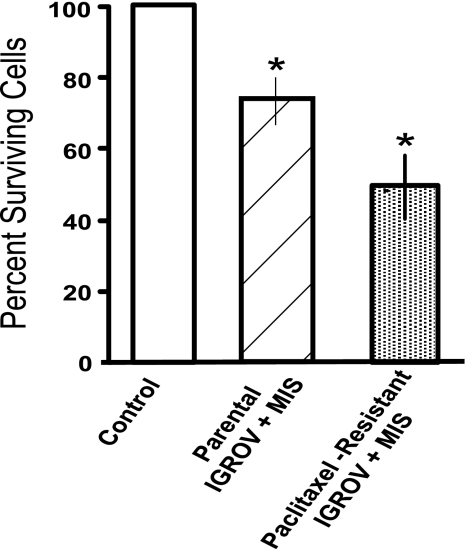
Inhibition by paclitaxel and rhMIS in combination was statistically significant when compared with rhMIS alone (P = 0.002) or paclitaxel alone (P < 0.05) against MOVCAR7 (Fig. 4C). However, when the paclitaxel data were tested by two-way factorial ANOVA with the interaction term (MIS × paclitaxel) in the model, results indicated that the interaction was not significant for synergy (P = 0.413); therefore, MIS and paclitaxel appear to be additive. This was true in four separate experiments. In 4306 cells when PBS, MIS alone, paclitaxel alone, or MIS plus the drug (Fig. 4D) were added, the interaction was significant for synergy (P = 0.02). To support this finding, the paclitaxel-resistant IGROV-1 cells responded significantly to rhMIS with a range of 40–70% inhibition with 15 μg/ml rhMIS in three separate experiments (P < 0.05) (Fig. 5), as was the case for the parental line (P < 0.05).
Fig. 5.
MIS inhibits paclitaxel-resistant cells in vitro. IGROV-1 cells made resistant to paclitaxel were tested in three separate experiments for their responsiveness to MIS. The stippled bar shows the average of three experiments. MIS also inhibited the parental IGROV-1 cell line in these monolayer cell assays (hatched bar). On each trial, 105 nM (15 μg/ml) MIS significantly inhibited proliferation relative to controls (open bar). ∗, P < 0.01 vs. PBS control (n = 10).
Cisplatin or rhMIS, when used alone, caused inhibition; when used together, the combination in four separate experiments for either cell line appeared to be additive by two-way factorial ANOVA (Fig. 4 E and F).
When the PBS buffer control was compared in MOVCAR7 cells with the combination of rhMIS and doxorubicin, a statistical significance was again noted (P = 10−10) (Fig. 4G). Importantly, the combination treatment was significantly different from MIS alone (P = 7 × 10−6). With the interaction term (MIS × doxorubicin) in this model, the results indicated that the interaction was significant (P = 0.023), consistent with synergy in two of three experiments; additivity was observed in the other experiment. The combination treatment produced the same level of inhibition as was seen by a higher dose of either drug alone (see Fig. 3). The combination of doxorubicin and rhMIS was neither synergistic nor additive against 4306 cells (n = 3) and therefore may be antagonistic (Fig. 4H). Fig. 4 I and J show the combination experiments with 3 nM cisplatin and 4 nM paclitaxel in MOVCAR7 and 4306 cells, respectively. These drugs acted synergistically in MOVCAR cells, but in 4306 cells the effects were additive in one experiment and competitive in the other two experiments, each done in triplicate.
Discussion
Although ovarian cancer is highly responsive to platinum-based therapy after initial cytoreductive surgery, there is a substantial risk of recurrence, which is often accompanied by the emergence of drug-resistant disease. Additional therapeutic agents and rational combination treatments are needed to prolong remissions, improve long-term outcomes, and reduce toxicity, thus improving the quality of life. We have found that the biological modifier MIS is effective against animal models recapitulating human ovarian cancer, with no apparent adverse affects when administered as a single agent for long courses (19) at concentrations below those normally observed in infant boys (3). Because MIS is now being prepared for clinical use, it is important to know whether it can be administrated in combinations that allow lower doses of paclitaxel, cisplatin, doxorubicin, AzadC, or rapamycin, to reduce the overall toxicity of these agents, and whether ovarian cancers resistant to standard therapies remain responsive to MIS. These studies provide a straightforward in vitro method to which statistics can be applied to predict which drug combinations can be used to advantage with the nontoxic MIS.
Given the fact that tumors may have any of several genetic alterations, providing multiple options to escape growth control, it is imperative to attack several molecular pathways simultaneously to increase tumor inhibition or to use two or more agents in combination to decrease the toxicity of an otherwise useful agent. Our extensive studies of the downstream signaling pathways of MIS, combined with the knowledge, albeit partial, of the mechanism of action of the effective chemotherapeutic agents now in clinical use, have made possible a more rational choice of therapies that could be used in combination with MIS. Furthermore, it is possible to predict whether the treatment modalities will act synergistically or additively, or, conversely and more importantly, whether competitive action on the same pathway generates antagonism that could preclude simultaneous use.
We would anticipate that combination therapies would be additive if the use of two substances in combination produces a total effect equal to the sum of the individual effects and could occur if two drugs act on the same pathway. Synergy implies that the effect of the interaction of two drugs is greater than the sum of their individual effects and might occur if the drugs act via different pathways. For example, drugs that lower apoptotic thresholds may be additive or synergistic with cytotoxic agents that typically block survival pathways. Either outcome may allow a lower dose of a cytotoxic agent that has a high toxicity profile to be used in combination with a nontoxic biological modifier such as MIS. How to approach an understandable reluctance to lower the effective doses of chemotherapeutic agents when used in combination with a biological modifier such as antiangiogenic drugs or MIS to reduce toxicity will have to be addressed, probably initially with patients who respond well to cancer drugs but exhibit toxicity at therapeutic levels.
Agents could be antagonistic if both affect the same growth-inhibitory pathway in opposite ways, and such combinations should be avoided. If the mechanisms of a drug's action are not fully understood, prior combination testing and use of the statistical interpretation, as done in these studies, could predict whether the combination would be additive, synergistic, or competitive. Similar studies of cytotoxic agents and a biologically targeted agent have been reported in preclinical studies for the her-2-neu binding agent trastuzumab (Herceptin) with taxanes which, in combination, demonstrate synergy (43), thus predicting an augmented clinical response and survival advantage that were confirmed in the clinic (44).
MIS is a new therapeutic agent whose production is being scaled up for clinical use against ovarian cancer. The present studies were undertaken to determine the agents with which MIS could be used most efficaciously. For example, MIS exerts its effect in the embryonic urogenital ridge by activating MISRII, Alk2 and Alk3, and the Smad 1/5/8 differentiation and growth inhibitory pathway (35). In addition, MIS up-regulates pocket proteins p130 and p107 in human ovarian (10) and endometrial cancer cell lines (9). These proteins inhibit proliferation and/or up-regulate cell cycle inhibitors in human ovarian and cervical carcinoma cell lines to cause cell cycle arrest. Our laboratory has reported that MIS caused G1 arrest during inhibition of OVCAR8 cells by up-regulating Ink4/p16 at the protein level (10, 45). Because AzadC, as a demethylating agent, up-regulates p16 gene transcription, it was anticipated that the combination of MIS and AzadC would be additive, which proved to be the case both in vitro and in vivo. Because AzadC has a high toxicity profile in patients, the clinical strategy might be to use AzadC in as low a dose as possible to enhance the antiproliferative effect of MIS. The recommended clinical dose for AzadC is 4 mg/kg, and the dose used in the animal experiment was 8-fold lower, at 0.5 mg/kg. Enhancement of p16 protein by MIS, and mRNA by AzadC, in ex vivo testing of a tumor specimen could identify a patient as a candidate for MIS and AzadC combination therapy.
Paclitaxel is a tubulin-binding agent that inhibits microtubule depolymerization, which can result in failure to transit mitosis or to traffic intracellular cytoplasmic proteins and organelles. Additive effects of this agent or doxorubicin with MIS may be due to a lowering of the apoptotic threshold through removal or alteration of antiapoptotic signals within the cells. Curiously, direct transfection of p16 in some model systems has generated resistance to paclitaxel and cisplatin, presumably due to G1 arrest, thus protecting cells from entering the S phase or M phase (required for maximal paclitaxel-associated toxicity). This result suggests that mechanisms of decreased proliferation seen with the paclitaxel and rhMIS combination are likely p16-independent.
Similarly, we would anticipate a synergistic effect with doxorubicin, which blocks cell proliferation by DNA intercalation, and increased DNA strand breaks due to inactivation of topoisomerase II function on a cell line such as MOVCAR7 in which MIS causes cell cycle arrest (19). It is also conceivable that prolonged cell cycle arrest might provide time to repair DNA strand breaks and thus reduce the toxicity of doxorubicin. The response of these cells in vitro to MIS with doxorubicin or paclitaxel is sufficiently encouraging to pursue in vivo combination studies, particularly because the combination protocol produces the same or greater inhibitory effect as a maximal effective dose of either MIS or drug alone. A wider dose range of cisplatin, MIS, and the combination should be examined in vitro prior to in vivo studies, to confirm the effects observed in our studies.
Rapamycin, in contrast, binds to and blocks the release of the FK506/rapamycin protein (FRAP) or mTOR from its complex with FKBP12 (41), which is released from type I receptor upon activation of MISRII by the MIS ligand (26, 27, 46). Hence, the sequestered mTOR is unavailable for activation by Akt. Therefore, one could predict that MIS and rapamycin would act synergistically, MIS to induce cell cycle arrest and rapamycin to inhibit regulators downstream from mTOR such as eukaryotic initiation factor 4E (eIF4E) and S6Kinase by PI3 kinase/Akt (47, 48). Further, we would anticipate, and did observe, a synergistic effect by combining MIS and rapamycin therapies in a cell line such as 4306 in which Pten is inactivated.
In ovarian cancer, the combination of paclitaxel with a platinum agent now defines the standard of care. The large majority of women with advanced disease characterized by peritoneal seeding and metastases will respond to aggressive surgical resection followed by these agents but will not be cured with this therapy (49). Maintenance therapy with paclitaxel after primary chemotherapy with paclitaxel and platinum has demonstrated improved progression-free survival but at the cost of significant neurotoxicity, which has limited the prolonged use of this agent. The experiments done in combination show that the drug effects are additive, thus consideration should be given to lowering the more toxic paclitaxel doses in this setting. A beneficial effect of MIS on motor neurons was recently observed (46), making it possible that MIS may also have advantages in ameliorating the neurotoxicity seen with taxanes that limits their use in maintenance therapy. Combination therapy with MIS may permit dose reduction of taxanes, whereas MIS may have a direct effect on motor neurons similar to that observed with glial-derived neurotrophic factor (GDNF), a well known neuroprotective agent (46). The present experiments also indicate that MIS may be effective in the face of Taxol-resistant tumors. Synergy with doxorubicin may be clinically useful in that women with platinum-resistant tumors typically receive liposomal doxorubicin. Although this drug is effective, survival in this group is ≈1 year (50).
Current clinical trials are exploring the potential efficacy of bevacizumab in the maintenance setting; however, concerns about hypertension, bleeding, and thrombosis raise questions about the therapeutic benefit of this strategy. Use of biologic agents such as MIS in maintenance, either alone or in combination with lower dose taxanes, on the other hand, may provide alternative strategies for improving overall clinical outcome with more favorable toxicity profiles. In the future, we envision that combination therapies including agents such as MIS will be pretested on patients' tumor cells to individualize treatment plans for each patient. Such a systems biology/bioinformatics approach will become the standard for choosing therapies for ovarian cancer, as it has in breast cancer.
Two other non-Mullerian organs, human breast and prostate, and their tumors, express MISRII and respond to MIS in growth inhibition assays (36, 37, 39, 51). Because MIS suppresses testosterone production (52–54), it might provide tumoricidial activity through dual mechanisms, namely direct growth inhibition and an indirect effect by lowering testosterone. Thus, prostate and breast cancer may be other target tumors for MIS, which expands the original target population of ovarian cancer to 212,920 new breast and 234,460 new prostate cancer patients per year in the United States (American Cancer Society; www.cancer.org). This larger target population also makes development of MIS as an anticancer therapeutic even more compelling because of the recent findings that MIS was effective against the progenitor cell population in transgenic mouse ovarian cancer cell lines (55) presumably responsible for tumor recurrence (56).
Materials and Methods
MIS Purification and Bioassay.
MIS was purified in our laboratory by either immunoaffinity (57) or carbohydrate affinity, followed by anion exchange chromatography as detailed previously in ref. 16. The MIS bioactivity of each preparation was assayed in an MIS-specific organ culture for its ability to cause Mullerian duct regression (58).
Cell Lines.
OVCAR8 cells were derived from ovarian cancer ascites cells from a cisplatin-resistant patient by Thomas Hamilton, Fox Chase Cancer Center (59). OVCAR8 expresses MISRII by Western analysis (8). The mouse ovarian cancer cell line MOVCAR7, which recapitulates human serous adenocarcinoma, was developed by D.C.C. (5) from ovarian ascites cells from transgenic animals in which the MISRII promoter drives the SV40 large T-ag to inactivate p53 and Rb. The 4306 cell line was developed by D.M.D. from conditional LSL-K-rasG12D/+; PtenloxP/loxP mice in which the ovarian surface epithelium was infected with adeno-Cre. The tumors produced by these mice mimic human endometrioid ovarian carcinoma (20). The MOVCAR7 and 4306 cell lines were maintained in DMEM supplemented with 4% female bovine calf serum/1× ITS (insulin-transferrin-selenium A)/1% penicillin/streptomycin/2 nM glutamine. Cells were grown at 37°C in a humidified chamber in 5% CO2 in air. Cultures were passed at 80% confluency 1:4 and not carried for more than 20 passages for OVCAR8 cells, 30–40 passages for MOVCAR7 cells, or 30 passages for 4306 cells. Both transgenic cell lines express the MISRII by Northern and Western analysis (ref. 19 and unpublished data). The human ovarian cancer cell line IGROV-1, originated from a human ovarian carcinoma (60); the daughter line, IGROV-1TR, was generated through a multimonth passage in incrementally increasing doses of paclitaxel to a final concentration of 0.01 μM, yielding a cell line that was 10-fold resistant to paclitaxel compared with the parental line.
Viable clones from the paclitaxel-exposed IGROV-1TR were maintained in DMEM with 10% female bovine calf serum/1% penicillin/streptomycin/1% l-glutamine/0.01 μM paclitaxel. The IGROV-1TR cells were treated with MIS and tested for growth inhibition in the MTT assay, as was the parental paclitaxel-sensitive IGROV-1 cell line.
MTT Growth Inhibition Assay.
Cells were harvested at 80% confluency and plated in 96-well plates at 1,000 cells per well in 200 μl of media per well. At 24 h after plating, 10 wells were treated at the desired dose of rhMIS, chemotherapeutic drug, a combination of rhMIS and drug, or appropriate control vehicle. At day 3, 5, and 7, depending on the growth rate of the controls, the MTT assay was used to quantify viable cells. Absorbance at 550 nm was measured in an ELISA plate reader (EL312e microplate reader; BioTek Instruments, Winooski, VT). The magnitude of absorbance is proportional to the number of viable cells in a given well. Each experiment was repeated at least three times. Statistical analyses of MIS effects were performed as described below.
The IC50s for MIS and the cytotoxic agents paclitaxel, cisplatin, doxorubicin, AzadC, and rapamycin were established for the cell lines. Each chemotherapeutic agent was then used at doses below the IC50 to test each agent in combination with MIS.
Cell responses to MIS and/or cytotoxic drugs varied quantitatively but not qualitatively from one experiment to another due to variations in MIS bioactivity from preparation to preparation and to the behavior of cells in culture. Data presented are representative of multiple replicate experiments.
in vivo Studies of rhMIS with AzadC.
In vivo studies were done using a nude mouse model in which OVCAR8 cells were implanted beneath the renal capsule. Animals were treated with PBS, MIS, AzadC, or MIS and AzadC. In this model (17, 61, 62), ≈250,000 cells in a 1-mm fragment of a fibrin/thrombin clot are placed under the kidney capsule. The clots were measured at the time of implantation and at the time of killing 3 weeks later, and each change in volume was calculated as a log of graft size ratios compared with the original volume. Thereby, each tumor is compared with itself, and the graft size ratios of the groups are compared.
Statistical Analysis.
For comparison of two sets of data having parametric characteristics (e.g., MIS treatment vs. no treatment), univariate two-tailed t tests were used. MIS, drug, and combinations were compared with vehicle as a control; each drug alone was then compared with treatment with the combination. In addition, two-way factorial ANOVA was used to test for an interaction between rhMIS and the drug being tested (where the interaction term in the model is MIS × doxorubicin, for example). If an interaction was found (P ≤ 0.05) and the growth inhibition was greater than for either treatment alone, the interaction was deemed synergistic (63). If an interaction was found (P ≤ 0.05) and the inhibition was less than for either treatment alone, the interaction was deemed antagonistic or competitive (63). If no interaction was found (P ≥ 0.05), but the effect was greater than for either treatment alone, then the effect of the combined treatment was considered additive.
Acknowledgments
We thank Dr. Jose Teixeira for insightful critiques and Ms. Debbie Freitas and Mr. Nima Pahlavan for their fine work in the production of rhMIS. D.M.D. is supported by a Burroughs–Wellcome Career Award for Biomedical Sciences, by the American Cancer Society, and by a National Institutes of Health (NIH) National Research Service Award; D.C.C. is supported by NIH Grants P50 CA083638 and U01 CA084242; M.V.S. is supported by NIH Mid-Career Development Award 1K24 CA109416; and P.K.D. and D.T.M. are supported by NIH Grants CA 17393 and HD 32212. This work was also supported by generous contributions to the Pediatric Surgical Research Laboratories from The McBride Family Fund, The Commons Development Company, The W. Gerald Austen Fund, and The Edmund C. Lynch, Jr., Cancer Research Fund.
Abbreviations
- AzadC
5′-Aza-2′-deoxycytidine (decitabine)
- MIS
Mullerian Inhibiting Substance
- MISRII
MIS type II receptor
- rhMIS
recombinant human MIS
- mTOR
mammalian target of rapamycin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gustafson ML, Lee MM, Scully RE, Moncure AC, Hirakawa T, Goodman A, Muntz HG, Donahoe PK, MacLaughlin DT, Fuller AF., Jr N Engl J Med. 1992;326:466–471. doi: 10.1056/NEJM199202133260707. [DOI] [PubMed] [Google Scholar]
- 2.Lane AH, Lee MM, Fuller AF, Jr, Kehas DJ, Donahoe PK, MacLaughlin DT. Gynecol Oncol. 1999;73:51–55. doi: 10.1006/gyno.1998.5290. [DOI] [PubMed] [Google Scholar]
- 3.Lee MM, Donahoe PK, Silverman BL, Hasegawa T, Hasegawa Y, Gustafson ML, Chang YC, MacLaughlin DT. N Engl J Med. 1997;336:1480–1486. doi: 10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 4.Barbie TU, Barbie DA, MacLaughlin DT, Maheswaran S, Donahoe PK. Proc Natl Acad Sci USA. 2003;100:15601–15606. doi: 10.1073/pnas.2636900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 6.Donahoe PK, Swann DA, Hayashi A, Sullivan MD. Science. 1979;205:913–915. doi: 10.1126/science.472712. [DOI] [PubMed] [Google Scholar]
- 7.Gouedard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massagué J, di Clemente N. J Biol Chem. 2000;275:27973–27978. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 8.Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF, Shah PC, Kehas DJ, Kenneally MK, Dombkowski DM, Ha TU, et al. Clin Cancer Res. 1999;5:3488–3499. [PubMed] [Google Scholar]
- 9.Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Proc Natl Acad Sci USA. 2005;102:111–116. doi: 10.1073/pnas.0407772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha TU, Segev DL, Barbie D, Masiakos PT, Tran TT, Dombkowski D, Glander M, Clarke TR, Lorenzo HK, Donahoe PK, Maheswaran S. J Biol Chem. 2000;275:37101–37109. doi: 10.1074/jbc.M005701200. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi A, Donahoe PK, Budzik GP, Trelstad RL. Dev Biol. 1982;92:16–26. doi: 10.1016/0012-1606(82)90146-4. [DOI] [PubMed] [Google Scholar]
- 12.Price JM, Donahoe PK, Ito Y. Am J Anat. 1979;156:265–284. doi: 10.1002/aja.1001560207. [DOI] [PubMed] [Google Scholar]
- 13.Price JM, Donahoe PK, Ito Y, Hendren WH., 3rd Am J Anat. 1977;149:353–375. doi: 10.1002/aja.1001490304. [DOI] [PubMed] [Google Scholar]
- 14.Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. Dev Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- 15.Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, et al. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzo HK, Teixeira J, Pahlavan N, Laurich VM, Donahoe PK, MacLaughlin DT. J Chromatogr B Biomed Sci Appl. 2002;766:89–98. doi: 10.1016/s0378-4347(01)00436-4. [DOI] [PubMed] [Google Scholar]
- 17.Chin TW, Parry RL, Donahoe PK. Cancer Res. 1991;51:2101–2106. [PubMed] [Google Scholar]
- 18.Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Clin Cancer Res. 2002;8:2640–2646. [PubMed] [Google Scholar]
- 19.Pieretti-Vanmarcke R, Donahoe PK, Szotek P, Manganaro T, Lorenzen MK, Lorenzen J, Connolly DC, Halpern EF, MacLaughlin DT. Clin Cancer Res. 2006;12:1593–1598. doi: 10.1158/1078-0432.CCR-05-2108. [DOI] [PubMed] [Google Scholar]
- 20.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 21.Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. J Clin Oncol. 2005;23:7889–7896. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 24.Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D. Nat Med. 1995;1:686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- 25.Otterson GA, Khleif SN, Chen W, Coxon AB, Kaye FJ. Oncogene. 1995;11:1211–1216. [PubMed] [Google Scholar]
- 26.Wang T, Donahoe PK. Front Biosci. 2004;9:619–631. doi: 10.2741/1095. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 28.Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels B, Wilming LG, Meijers JHC, Themmen AP. Development (Cambridge, UK) 1994;120:189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 29.di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard JY, Vigier B, Josso N, Cate R. Mol Endocrinol. 1994;8:1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira J, He WW, Shah PC, Morikawa N, Lee MM, Catlin EA, Hudson PL, Wing J, MacLaughlin DT, Donahoe PK. Endocrinology. 1996;137:160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- 31.Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. Development (Cambridge, UK) 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 32.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 33.Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Mol Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 34.Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Ingraham HA. Mol Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 35.Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, Teixeira J, Donahoe PK. Development. 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]
- 36.Hoshiya Y, Gupta V, Kawakubo H, Brachtel E, Carey JL, Sasur L, Scott A, Donahoe PK, Maheswaran S. J Biol Chem. 2003;278:51703–51712. doi: 10.1074/jbc.M307626200. [DOI] [PubMed] [Google Scholar]
- 37.Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, Tran TT, Ha TU, Maheswaran S. Mol Cell Endocrinol. 2003;211:43–49. doi: 10.1016/j.mce.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, MacLaughlin DT, Donahoe PK, Maheswaran S. J Biol Chem. 2000;275:28371–28379. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 39.Segev DL, Hoshiya Y, Hoshiya M, Tran TT, Carey JL, Stephen AE, MacLaughlin DT, Donahoe PK, Maheswaran S. Proc Natl Acad Sci USA. 2002;99:239–244. doi: 10.1073/pnas.221599298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S, Talpaz M, Garcia-Manero G, Kantarjian HM. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 41.Bustin SA, McKay IA. Br J Biomed Sci. 1994;51:147–157. [PubMed] [Google Scholar]
- 42.Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhl R, Kock M, Geiger KD, Gelperina SE. Int J Cancer. 2004;109:759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 43.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- 44.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez S, Klatt P, Delgado S, Conde E, Lopez-Rios F, Sanchez-Cespedes M, Mendez J, Antequera F, Serrano M. Nature. 2006;440:702–706. doi: 10.1038/nature04585. [DOI] [PubMed] [Google Scholar]
- 46.Wang PY, Koishi K, McGeachie AB, Kimber M, Maclaughlin DT, Donahoe PK, McLennan IS. Proc Natl Acad Sci USA. 2005;102:16421–16425. doi: 10.1073/pnas.0508304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Tan M, Stone Hawthorne V, Klos KS, Lan KH, Yang Y, Yang W, Smith TL, Shi D, Yu D. Clin Cancer Res. 2004;10:6779–6788. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 49.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 50.Gordon AN, Tonda M, Sun S, Rackoff W. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Segev DL, Hoshiya Y, Stephen AE, Hoshiya M, Tran TT, MacLaughlin DT, Donahoe PK, Maheswaran S. J Biol Chem. 2001;276:26799–26806. doi: 10.1074/jbc.M103092200. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira J, Fynn-Thompson E, Payne AH, Donahoe PK. Endocrinology. 1999;140:4732–4738. doi: 10.1210/endo.140.10.7075. [DOI] [PubMed] [Google Scholar]
- 53.Teixeira J, Kehas DJ, Antun R, Donahoe PK. Proc Natl Acad Sci USA. 1999;96:13831–13838. doi: 10.1073/pnas.96.24.13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trbovich AM, Sluss PM, Laurich VM, O'Neill FH, MacLaughlin DT, Donahoe PK, Teixeira J. Proc Natl Acad Sci USA. 2001;98:3393–3397. doi: 10.1073/pnas.051632298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, MacLaughlin DT, Donahoe PK. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reya T, Morrison SJ, Clarke MF, Weissman IL. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 57.Ragin RC, Donahoe PK, Kenneally MK, Ahmad MF, MacLaughlin DT. Protein Expression Purif. 1992;3:236–245. doi: 10.1016/1046-5928(92)90020-w. [DOI] [PubMed] [Google Scholar]
- 58.Donahoe PK, Ito Y, Hendren WH. J Surg Res. 1977;23:141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- 59.Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Cancer Res. 1997;57:850–856. [PubMed] [Google Scholar]
- 60.Benard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, Riou G. Cancer Res. 1985;45:4970–4979. [PubMed] [Google Scholar]
- 61.Parry RL, Chin TW, Epstein J, Hudson PL, Powell DM, Donahoe PK. Cancer Res. 1992;52:1182–1186. [PubMed] [Google Scholar]
- 62.Stephen AE, Masiakos PT, Segev DL, Vacanti JP, Donahoe PK, MacLaughlin DT. Proc Natl Acad Sci USA. 2001;98:3214–3219. doi: 10.1073/pnas.051625998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheffe H. The Analysis of Variance. Indianapolis, IN: Wiley; 1959. pp. 90–98. [Google Scholar]