1. Executive summary
Purpose of report
This document has been commissioned by the British Society of Gastroenterology. It is intended to draw together the evidence needed to fill the void created by the absence of a national framework or guidance for service provision for the management of patients with gastrointestinal and hepatic disorders. It sets out the service, economic and personal burden of such disorders in the UK, describes current service provision, and draws conclusions about the effectiveness of current models, based on available evidence. It does not seek to replicate existing guidance, which has been produced for upper and lower gastrointestinal cancers, hepatobiliary and pancreatic disorders, and many chronic disorders of the gut. It does, however, draw on evidence contained in these documents. It is intended to be of value to patient groups, clinicians, managers, civil servants, and politicians, particularly those responsible for developing or delivering services for patients with gastrointestinal disorders.
Methods used
A systematic review of the literature was undertaken to document the burden of disease and to identify new methods of service delivery in gastroenterology. This systematic review was supplemented by additional papers, identified when the literature on incidence, mortality, morbidity, and costs was assessed.
Routine data sources were interrogated to obtain additional data on burden of disease, the activity of the NHS, and costs, in relation to gastrointestinal disorders.
The views of users of the service were sought, through discussions with the voluntary sector and through a workshop held at the Royal College of Physicians in December 2004.
The views of professionals were obtained by wide dissemination of the document in a draft form, seeking feedback on the content and additional material.
Main findings
The burden of gastrointestinal and liver disease is heavy for patients, the NHS, and the economy, with gastrointestinal disease the third most common cause of death, the leading cause of cancer death, and the most common cause of hospital admission. There have been increases in the incidence of most gastrointestinal diseases which have major implications for future healthcare needs. These diseases include hepatitis C infections, acute and chronic pancreatitis, alcoholic liver disease, gallstone disease, upper gastrointestinal haemorrhage, diverticular disease, Barrett's oesophagus, and oesophageal and colorectal cancers. Socioeconomic deprivation is linked to a number of gastrointestinal diseases, such as gastric and oesophageal cancers, hepatitis B and C infections, peptic ulcer, upper gastrointestinal haemorrhage, as well as poorer prognosis for colorectal, gastric, and oesophageal cancers.
The burden on patients' health related quality of life has been found to be substantial for symptoms, activities of daily living, and employment, with conditions with a high level of disruption to sufferers' lives found to include: gastro‐oesophageal reflux disease, dyspepsia, irritable bowel syndrome, anorectal disorders, gastrointestinal cancers, and chronic liver disease. However, impact on patients is neither fully nor accurately reflected in routine mortality and activity statistics and although overall, the burden of gastrointestinal disease on health related quality of life in the general population appears to be high, the burden is neither systematically nor comprehensively described.
An overwhelming finding concerning evidence related to service delivery is the lack of high quality health technology assessment and evaluation. In particular, evidence of cost effectiveness from multicentre studies is lacking, with more research needed to establish a robust evidence base for models of service delivery.
Waiting times form the bulk of patients' concerns, with great difficulty in meeting government standards for referral and treatment. An extensive and systematic study of the problem of access for the delivery of gastrointestinal services has yet to be carried out and significant publications reporting inequalities in the delivery of gastrointestinal services are lacking. There is also a need to increase awareness and the implementation of initiatives aimed at improving the information flow between patients and practitioners.
Strong evidence exists, however, for a shift in care towards greater patient self management for chronic disease. The development of general practitioners with a special interest in gastroenterology is supported in primary care, but their clinical and cost effectiveness need to be researched. Indeed, emphasis needs to be given to developing interventions to increase preventative activities in primary care, and more research is required to determine their effectiveness and cost effectiveness.
Despite strong support for the development and use of widespread screening programmes for a wide variety of gastrointestinal diseases, there is a lack of evidence about how they are managed, their effectiveness, and their cost effectiveness. In contrast, a strong body of evidence exists on diagnostic services, and the need to develop and implement appropriate training and stringent assessment to ensure patient safety. There is also a substantial amount of work detailing guidelines for care.
In hospital, patients with gastrointestinal disorders should be looked after by those with specialist training, and more diagnostic endoscopies could be undertaken by trained nurses. Importantly, for service reconfiguration, there is currently insufficient evidence to support greater concentration of specialists in tertiary centres. More research is needed especially on the impact on secondary services before further changes are implemented.
Consultant gastroenterologist numbers need to increase to meet a rising burden of gastrointestinal disease. Gastroenterology teams should be led by consultants, but include appropriate non‐consultant career grade staff, specialist nurses, and other staff with integrated specialist training, where appropriate.
More research is needed into the delivery and organisation of services for patients with gastrointestinal and liver disorders, in particular to assess the clinical and cost effectiveness of general practitioners with a special interest in gastroenterology and endoscopy; the clinical and cost effectiveness of undertaking endoscopy or minor gastrointestinal surgery in diagnosis and treatment centres; and the reconfiguration of specialist services and the potential impact on secondary and primary care and on patients.
2. Introduction
2.0 Background including policy drivers
This document has been commissioned by the British Society of Gastroenterology. It is intended to draw together the evidence needed to fill the void created by the absence of a national framework or guidance for service provision for the management of patients with gastrointestinal (GI) and hepatic disorders. It sets out the service, economic and personal burden of such disorders in the UK, describes current service provision, and draws conclusions about the effectiveness of current models, based on the presently available evidence. It does not seek to replicate existing guidance, which has been produced for upper and lower gastrointestinal cancers, hepatobiliary and pancreatic disorders, and many chronic disorders of the gut. It does, however, draw on evidence contained in these documents.
The document takes into account recent strategies for the NHS in the UK, and recommendations for quality and service improvement, new information strategies in England and Wales. In particular, it builds on the recommendations of three reports from Derek Wanless, which have significantly influenced the strategic direction of the NHS.
In July 2000 the Government published the NHS plan which set out the core principles for the NHS and a framework for delivering these principles over the next decade. Following on from this the Chancellor of the Exchequer commissioned the first Wanless Report1 to examine future health trends and resources required over the next two decades. The report welcomed the Government's intention to extend the National Service Framework (NSF) approach to other disease areas and recommended that the NSFs and their equivalents in the developed administrations are rolled out in a similar way to the diseases already covered. It also recommended that a more effective partnership between health professionals and the public should be facilitated in a number of ways. These include setting standards for the service to help give people a clearer understanding of what the health service will and will not provide for them. Other factors include improving health information, reducing key health risk factors, and reinforcing patient involvement in NHS activities.
These recommendations were repeated and reinforced in a report on the NHS in Wales advised by Sir Derek Wanless.2 The report re‐emphasised the need for sustainable change: a shift in delivery from secondary care towards greater care in the community and more self management by patients; and significant investment in improving information and information technology. The report also emphasised the importance of change based on evidence. The third Wanless report3 emphasised the need for improvements in public health and the need for greater investment in prevention and risk reduction.
2.1 Aims and objectives
This review aims at describing how best to provide services for patients with gastrointestinal disorders from a professional and patient perspective, based on available evidence on disease burden and service provision.
Its objectives are to:
-
Review and synthesise published research evidence and routine data concerning the burden of GI diseases on
-
-
Patients—their mortality, morbidity, and quality of life
-
-
The NHS—its volume and cost
-
-
The economy of the UK.
-
-
Systematically review and synthesise research findings concerning the effectiveness of models of service provision for GI diseases and the cost effectiveness of GI services.
Describe the patients perspective on emerging issues of service delivery highlighted through the literature review as undergoing change.
Draw conclusions about optimal service provision based on evidence of burden and effectiveness, patients' view and in the current policy and service context.
The report covers the broad spectrum of GI and liver conditions. It does not examine disorders of nutrition, both malnutrition and obesity, as these have been dealt with in detail elsewhere.4,5,6
2.2 Overview of methods
Four methods were used in the generation of this document:
A systematic review of the literature was undertaken to identify research papers concerning the effectiveness of methods of service delivery in gastroenterology. This systematic review was supplemented by additional papers identified when the publications on incidence, mortality, morbidity, service activity, and costs were assessed. Some further papers were identified and included from consultation feedback.
Routine data sources were interrogated to identify additional data on burden of disease and the activity of the NHS in relation to GI disorders.
The views of users of the service were sought, through discussions with the voluntary sector and through a workshop held at the Royal College of Physicians in December 2004.
The views of professionals were obtained by wide dissemination of the document in a draft form, seeking feedback on the content and additional material. The full draft report was presented at the BSG annual conference in March 2005, alongside a strategy document outlined by the BSG president, based on the review findings. After this meeting, comments were invited, and the online report was made available to the BSG membership through a web link. In addition, patient representative groups and other GI specialist organisations were contacted to gain feedback. Comments were received over a 6 month period after release of the first draft, and these were incorporated where they were supported by evidence from well designed and reported research studies. Table A.13 summarises and appraises these papers.
More detail of the methods used is given in the appropriate sections of the document.
3. Burden of gastrointestinal and liver disease in the UK
3.0 Methods and data limitations
Figure 1 The digestive system. Source: Department of Gastroenterology, University of Miami, 2005.7
Gastrointestinal and liver disorders affect people of all ages. Some disorders are acute and life threatening, others are more chronic, less dangerous to life, but severely debilitating. Gastrointestinal cancers are common—some are curable, others are almost invariably fatal. Bowel problems cause considerable distress in the elderly. The care and management of such diverse problems requires contributions from a wide variety of professions.
The main methods used in this chapter involved extensive and comprehensive searches of the literature on incidence, prevalence, mortality, and patients' quality of life for the various gastrointestinal diseases in the UK and, for comparative purposes, for those in other European or Western countries. Part of the literature had been already compiled through reviews undertaken during the course of previous studies of the incidence and mortality of gastrointestinal diseases such as inflammatory bowel disease, liver cirrhosis, and acute pancreatitis.
The literature searches were primarily undertaken on the Medline and Embase databases with “incidence”, “prevalence”, “case fatality”, “mortality”, “quality of life”, “death rate”, “hospital”, “admission”, “gastrointestinal”, “review”, “epidemiology”, “aetiology”, “trend”, “population”, “rate”, “100 000”, “10 000”, “million”, “UK”, “England”, “Scotland”, “Wales”, other countries, and the various gastrointestinal diseases as the main search terms.
The literature reviews were supplemented with extensive searches of routine data sources in the UK to provide additional information on the burden of gastrointestinal disease in the UK. The main routine data sources used in this chapter were: firstly, the cancer surveillance and registry units in England, Wales, Scotland, and northern Ireland for publications and data on the incidence, mortality, survival, and socioeconomic aspects of gastrointestinal cancers. Secondly, data and reports published by the Office of National Statistics (ONS) and its predecessor, the Office of Population Censuses and Surveys (OPCS), were obtained for information on the causes of gastrointestinal and other mortality in England and Wales. Thirdly, information on hepatitis B and C infections was obtained from publications involving communicable disease surveillance units in the UK.
The main categories of gastrointestinal disease with corresponding ICD‐9 and ICD‐10 codes used are as follows: diseases of the digestive system (ICD‐9 = 520–579; ICD‐10 = K00‐K93), malignant neoplasms of the digestive system (150–159; K15‐K26), benign and other neoplasms of the digestive system (210, 211, 230, 235.2–235.5; D00, D01, D12, D13, D37), intestinal infectious diseases (001–009; A00‐A09), and viral hepatitis (070; B15‐B19).
Some of the main limitations of available data in the UK for investigating the burden of gastrointestinal diseases are: firstly, that incidence and prevalence data are routinely compiled for gastrointestinal cancers and communicable diseases only. Fairly complete incidence data for a few acute gastrointestinal disorders such as acute pancreatitis and acute appendicitis can be traced from hospital admissions, although there have been major concerns about the accuracy of routine hospital data.8,9,10,11 Secondly, different criteria for measuring incidence, case mix variation, and different methods used for age standardising population based incidence and mortality rates can also affect comparability across studies; while case fatality from follow‐up studies is affected by factors such as the length of follow‐up and the inclusion of deaths after discharge with in‐hospital deaths, as well as case mix. Trends in hospital admissions for many gastrointestinal disorders, such as gallstone operations and liver replacements, are also strongly affected by factors such as the availability of hospital facilities, as well as the prevailing clinical practice at the time.
People with other gastrointestinal diseases such as functional disorders are mainly managed in primary care; and so incidence or prevalence data for these diseases can usually only be determined through national primary care surveys, costly databases compiled by pharmaceutical companies, or through intensive local or regional surveys of general practices.
For other gastrointestinal disorders, many people remain undiagnosed. Incidence or prevalence data for some of these diseases, such as gastro‐oesophageal reflux disease, irritable bowel syndrome, and dyspepsia, can often be obtained at a regional level only, through diagnostic questionnaires or interviews; while differences in diagnostic criteria often affect comparability across studies.
For some gastrointestinal disorders, it is not possible to distinguish functional disorders from more serious diseases without the use of special investigation or tests. The growing sophistication of gastrointestinal diagnostic methods has probably resulted in increased diagnosis of milder forms of what would have been traditionally regarded as serious digestive diseases, and caution is therefore required when making comparisons longitudinally over time.12 In other words, increases in reported incidence over time may be attributable to improvements in diagnostic methods rather than real increases.
Routine mortality data are usually available for underlying cause of death only, while patterns of certification of the underlying cause of death vary according to the type of disease or condition. People who die soon after a hospital admission for myocardial infarction, stroke or lung cancer are almost always certified with these diseases as their underlying cause of death. In contrast, the certified underlying causes of death for those who die soon after admission for most gastrointestinal disorders are typically much less likely to be these gastrointestinal diseases.13 Therefore, mortality statistics, based on underlying cause of death often underreport true mortality from gastrointestinal diseases.
In summary, for many gastrointestinal diseases, other than cancers, burden of disease data are often patchy, collected at a local or regional level, have variation in case ascertainment and in comparability between studies and longitudinally over time, and can underreport the true burden of disease. Even for cancers that have been allocated specialist surveillance and registration units, despite improvements over time, there are sometimes differences between cancer registries in case ascertainment and completeness of registrations, so that some degree of caution is required when making comparisons longitudinally and between registry regions.
3.1 Spectrum of gastrointestinal disorders
Gastrointestinal disorders cover disease of the alimentary canal (from oesophagus to anus) and its associated organs (liver, gallbladder, and pancreas). They affect a significant proportion of the population. Of the cancers, those of the gastrointestinal tract are among the most common, with colorectal cancer being the second most common cancer in England and Wales as measured by incidence and mortality when both sexes are included.14 It includes very common conditions such as gastro‐oesophageal reflux disease, non‐ulcer dyspepsia, and functional bowel disease, which although a significant proportion of the population probably self treat at some stage in their life, have a huge impact on primary and secondary care. Other common conditions include inflammatory bowel disease, coeliac and diverticular disease. Alcoholic liver disease remains a significant problem but with increasing obesity and lifestyle trends chronic liver disease due to non‐alcoholic fatty liver disease and hepatitis C is being increasingly seen. The wide spectrum of disorders requires a range of treatment involving self care, primary care through to secondary care, and highly specialised tertiary referral centres.
3.2 Incidence of gastrointestinal diseases
Gastrointestinal symptoms and complaints are common among the general population. About one in six admissions to hospital are for a primary diagnosis of gastrointestinal disease, and about one in six of the main surgical procedures in general hospitals are performed on the digestive tract. The following sections outline patterns of incidence and prevalence for some of the main gastrointestinal disorders in anatomical sequence: diseases of the oesophagus, followed by diseases of the stomach and duodenum, the small bowel and colon, the liver, pancreas and gastrointestinal cancers.
Incidence of diseases of the oesophagus
Gastro‐oesophageal reflux disease
Gastro‐oesophageal reflux disease (GORD or GERD when oesophagus is spelt as esophagus) occurs when reflux of stomach acid into the oesophagus is severe or frequent enough to impact the patient's life or damage the oesophagus, or both. It is the most common disorder of the gastrointestinal tract, resulting from failure of the gastro‐oesophageal sphincter. GORD is a chronic condition that, in most cases, returns shortly after discontinuing treatment.
Risk factors for GORD include hiatus hernia, certain foods, heavy alcohol use, smoking, and pregnancy. There is also a strong genetic component in the incidence of GORD: a first degree relative of a patient is four times more likely to be afflicted, while a recent study estimated that 50% of the risk of GORD is genetic.15 Other possible risk factors include concomitant drugs for treatment of hypertension, angina, and arthritis,16 and obesity.17
The risk of GORD increases with age, rising sharply above the age of 40. More than 50% of those afflicted are between the ages of 45 and 64. Incidence varies geographically, it is slightly higher in women than in men, and it is higher among white people than among Asian and Afro‐Caribbean ethnic groups.18,19
In Western countries, 10–40% of the adult population experience heartburn, which is the main symptom of GORD, although estimates vary according to the diagnostic criteria used.18,20,21 In the UK, a recent community based study reported a prevalence of 28.7% for GORD symptoms.22 Subjects with chronic GORD are at risk of developing Barrett's oesophagus (see below). About 10–15% of subjects who undergo endoscopy for GORD evaluation are found to have Barrett's oesophagus,16,23 while other complications of GORD include erosive oesophagitis, ulceration, strictures, and gastrointestinal bleeding.24
Barrett's oesophagus
Severe, longstanding gastro‐oesophageal reflux disease can damage the oesophagus and lead to a condition known as Barrett's oesophagus. This refers to an abnormal change or metaplasia in the cells of the lower end of the oesophagus. Barrett's oesophagus, or columnar‐lined oesophagus (CLO), occurs in about one in 400 of the general population, or about 15% of patients with reflux oesophagitis. It is a rare diagnosis in people aged under 40 years, but its prevalence increases sharply with age and with obesity. It is much more common in white people than in Asian and Afro‐Caribbean ethnic groups,18 among men than women, and among people in higher socioeconomic groups.25
Barrett's oesophagus is a major risk factor,16,23,24 and the only known precursor,26,27,28 for oesophageal adenocarcinoma, although the degree of risk is not very clearly defined as many people with Barrett's oesophagus remain undiagnosed. The diagnosed incidence of Barrett's oesophagus has been increasing sharply over time in the UK,29,30 indicating real increases in its prevalence.
Oesophagitis
Oesophagitis refers to the inflammation of the lower end of the oesophageal lining, arising mainly through the chronic reflux of stomach acid and digestive enzymes into the oesophagus. When the inflammation is severe, oesophageal ulcers may develop. Around 50% of people with GORD also have oesophagitis.31 Other, less common causes of oesophagitis include hiatus hernia, certain fungal infections such as monila and candida, viruses, irradiation, and caustic substances such as lye. The prevalence of oesophagitis increases with age and obesity, and it is also more common in men than in women, and among white people than in Asian and black ethnic groups.32,33
Oesophagitis is present in about 20% of patients at endoscopy,34 although case series from endoscopy units suggest that the diagnosis of oesophagitis is increasing over time. For example, one recent British study reported a diagnostic rate of 32%.35 It is likely that this reflects a true increase in the prevalence of oesophagitis, but the magnitude of the increase may not be entirely accurate owing to effective treatments for the condition, such as the advent of proton pump inhibitors.21
Dyspepsia
Functional gastrointestinal disorders are defined by symptoms in the absence of any structural abnormalities, and affect all areas of the GI tract, ranging from globus (feeling of a lump in the throat), non‐cardiac chest pain, functional dyspepsia in the upper GI tract, and irritable bowel syndrome (IBS) in the lower GI tract. Functional gastrointestinal disorders are characterised by poorly understood abnormalities of gut motility and sensory perception. These and rare motility disorders occur owing to dysfunctional interactions between the brain/central nervous system and the gut/enteric nervous system. Biological triggers underlying functional gastrointestinal disorders are being identified, leading to research aimed at providing effective treatments.
Dyspepsia describes pain or discomfort in the upper abdomen, rather than a defined condition, and it is a chronic, relapsing, and remitting symptom. Causes of dyspepsia include peptic ulcers, acid reflux disease, oesophagitis, anti‐inflammatory drugs, gastritis and duodenitis, hiatus hernia, gastric motility disorder, oesophageal or gastric cancers, although in many cases there is no underlying disease.
Dyspepsia has been defined in different ways by a number of expert groups. For example, the 1988 Working Party classification states that symptoms need to be referable to the upper GI tract, and need to be present for the past four weeks. The less inclusive Rome II criteria later stated that patients need to have predominant pain or discomfort centred in the upper abdomen for at least 12 weeks of the past year, and excluded patients with heartburn or acid reflux as their only symptoms. More recently, the BSG have defined dyspepsia as any group of symptoms that alert doctors to consider diseases of the upper GI tract.
Dyspepsia symptoms typically affect between 20 and 40% of the UK population, depending on the diagnostic criteria used.21 Most recent British studies have used the BSG definition and have typically reported dyspepsia prevalence rates of about 40% (table 3.2.1),36,37,38,39 although lower rates of 26%,40 29%,41 and 12%,42 have also been reported. Prevalence rates in the UK have often been higher than those reported for populations in other Western countries (table 3.2.1).
Table 3.2.1 Prevalence rates (% of population) of dyspepsia, as reported from various regional studies in the UK and in other Western countries.
| Country | Region | Year of study* | Study size | Prevalence (% of population)‡ | Authors and reference |
|---|---|---|---|---|---|
| UK studies: | |||||
| UK | Scotland | 1967 | 1 487 men | 29.0 | Weir RD and Backett BM, 196841 |
| UK | Hampshire | 1988 | 2 066 | 38.0 | Jones RH and Lydeard SE, 198939 |
| UK | England and Scotland ‐ 5 centres | 1989 | 7 428 | 41.0 | Jones RH et al, 199038 |
| UK | 150 Centres | 1994 | 2 112 | 40.3 | Penston JG and Pounder RE, 199636 |
| UK | north of England | 1997 | 3 179 | 25.7 | Kennedy TM et al, 199840 |
| UK | Glasgow | 1998 | 1 611 | 12.0 | Woodward M et al, 199942 |
| UK | Leeds | 1999 | 8 407 | 37.8 | Moayyedi P et al, 200037 |
| Foreign studies: | |||||
| Norway | 1979–1980 | 14 390 | 20 | Johnsen R et al, 198851 | |
| Norway | Sørreisa, | 1987 | 1 802 | 27.5 | Bernersen B et al, 199652 |
| USA | Olmsted County | 1988–1991 | 835 | 25.8 | Talley NJ et al, 199253 |
| Denmark | 1993 | 3 619 | 14–51 | Kay L and Jorgensen T, 199454 | |
| Germany | Essen | 1993 | 180 | 24.4 | Holtmann G et al, 199455 |
| Netherlands | 1994 | 500 | 17 | Schlemper RJ et al, 199556 | |
| Japan | 1994 | 231 | 32 | Schlemper RJ et al, 199556 | |
| USA | Olmsted County | 1996 | 2 200 | 19.8 | Locke GR et al, 199757 |
| Australia | Sydney | 1997 | 592 | 13.2 | Nandurkar et al, 199858 |
| Germany | Ludwigshafen | 1997 | 4 054 | 20.4 | Zober A et al, 199859 |
| Spain | 1998 | 264 | 23.9 | Caballero‐Plasencia AM et al, 199960 | |
| New Zealand | Wellington | 1999 | 817 | 34.2 | Haque M et al, 200061 |
| Sweden | Uppsala | 1999 | 1 422 | 14.5 | Agreus L et al, 200062 |
| Netherlands | Utrecht | 2000 | 500 | 13.8 | Boekema PJ et al, 200163 |
| Iceland | 2000 | 2 000 | 17.8 | Olafsdottir LB et al, 200564 | |
| Australia | New South Wales | 2001 | 2 300 | 11.4–36 | Westbrook JJ and Talley NJ, 200265 |
*The year before the year of publication is given, where the study period was not specified; ‡ranges of prevalence refer to prevalence rates obtained using different criteria for diagnosing dyspepsia.
Dyspepsia also accounts for between 1.2 and 4% of all consultations in primary care in the UK.34,43 Half of these consultations are for functional dyspepsia. Non‐cardiac chest pain may be of gastrointestinal origin but sufferers often persist in the belief that they have heart disease, resulting in severe morbidity. Fifty per cent of patients consulting their GP for chest pain,44 and a similar proportion seen in rapid access chest pain clinics,45 have no cardiac cause of their symptoms. Although mortality in people with functional gastrointestinal disorders is not raised compared with the general population, these disorders have a significant impact on quality of life. For example, two studies reported that 75% of people with non‐cardiac chest pain suffered persistent symptoms and impaired quality of life over periods of 10 years or more; 30–50% never returned to work and were unable to carry out household tasks.44,46
Peptic ulcers have been thought to account for a quarter of all cases of dyspepsia.47 Several British studies from the 1940s to the 1980s reported that 18%,48 26%,41 and 31% 39 of people referred with dyspepsia were found to have peptic ulcers, although more recently this percentage has fallen to around 10–15%.34,39,49,50
Incidence of diseases of the stomach and duodenum
Peptic ulcer
Peptic ulcer is the collective term that includes ulcers of the stomach and the duodenum. About 90–95% of duodenal ulcers and 70–80% of gastric ulcers are caused by the Helicobacter pylori infection. Other risk factors include non‐steroidal anti‐inflammatory drugs (NSAIDs) and corticosteroids, increased gastric acid secretion, blood group “O”, smoking, and heavy alcohol use.
Duodenal and gastric ulcer differ in their incidence by age and sex. The incidence of duodenal ulcer peaks at age 45–64 years, and is twice as common in men than in women, whereas gastric ulcer is more common in the elderly and more equally found in men and women.
The incidence of peptic ulcer in the UK increased during the first half of the 20th century. Since the 1950s, however, hospital admission rates for peptic ulcer have fallen among most age groups.66,67,68,69,70 Since the early 1980s, this is largely because of a reduction in recurrent ulcer disease consequent upon the identification and eradication of Helicobacter pylori infection in patients presenting with peptic ulcer. For example, admission rates for duodenal ulcer in Scotland fell by 38% from 157 to 98 per 100 000 population between 1975 and 1990,69 and the prevalence of peptic ulcer in primary care in England and Wales fell by 50% from 1994 to 1998.71 Hospital admissions for perforated peptic ulcer have also fallen over time in the UK; for example, by 26% in Oxford between 1976 and 1982,72 and by 44% in Scotland for perforated duodenal ulcer between 1975 and 1990.69
However, in contrast with this downward trend, hospital admissions for perforated peptic ulcer increased among elderly women in the UK during the 1970s and 1980s,69,73,74 and perforated duodenal but not gastric ulcer, and haemorrhagic peptic ulcers, increased among elderly people in England during the 1990s.75 These increases have been linked to the use of NSAIDs, which have been shown to cause both gastric and duodenal ulceration, including ulcer perforation and haemorrhage.76,77 Patients taking NSAIDs have been reported to be at 4.7 times greater risk of haemorrhagic peptic ulcer, with an increasing risk with age up to 13.2 in people aged over 60.21 Recent small reductions in the incidence of peptic ulcer among elderly women since the mid‐1980s, indicates increased awareness of the side effects of NSAIDs, and more selective prescribing of these drugs.12,70
Helicobacter pylori infection
Helicobacter pylori is a bacterial infection that was discovered in 1982 and is the causal agent in 90–95% of duodenal ulcers and 70–80% of gastric ulcers. It is also linked to other gastrointestinal diseases such as gastritis and dyspepsia,34,49 and it is estimated to be the cause of 73% of all gastric cancers.78,79Helicobacter pylori has been listed as a grade I carcinogen because gastric cancer can occur after Helicobacter pylori gastritis leads to atrophy and metaplasia.80
Risk of infection is strongly linked to social deprivation in childhood, and it is much higher in unsanitary or overcrowded living conditions with no fixed hot water supply.80 It is thought that the crowded living conditions of the expanding cities at the beginning of the industrial revolution led to a decline in hygiene and the spread of the infection early in life.12,81
The prevalence of the Helicobacter pylori infection in the UK has declined in recent decades, as the infection is progressively eradicated from patients presenting with peptic ulcer and also because of a declining incidence as conditions improved over time. Successive birth cohorts have had a lower risk of childhood infection: the prevalence of Helicobacter pylori in 20–30 year olds is 10–20%, rising with age to 50–60% in 70 year olds.
Up to half of the world's population is infected with Helicobacter pylori.80 Prevalence varies between about 80% for adults in developing countries, Japan, and South America, around 40% in the UK, and 20% in Scandinavia. Local differences in prevalence exist where there has been substantial immigration from countries with a higher prevalence of infection.
About 15% of people infected with Helicobacter pylori will develop peptic ulcer or gastric cancer as a long term consequence of the infection. Infection in infancy is thought to lead to pangastritis, which predisposes to gastric ulcer and gastric cancer, while infection in later childhood may lead to antral gastritis, which predisposes to duodenal ulcers and duodenitis.82 It has been estimated that one in 35 men and one in 60 women in England and Wales die from a Helicobacter pylori related disease.78 Eradication therapy for Helicobacter pylori infection has been shown to be effective for pylori peptic ulcer disease.49
Gastrointestinal haemorrhage
Gastrointestinal haemorrhage refers to bleeding from the bowel wall or mucosa anywhere along the GI tract. Presentation depends on the location and rate of haemorrhaging and includes melaena from rapid bleeding high in the gastrointestinal tract, iron deficiency anaemia from chronic slow blood loss, or red blood from the colon or ileum.
Acute upper gastrointestinal bleeding is the commonest emergency managed by gastroenterologists. About half of all upper gastrointestinal haemorrhages are caused by peptic ulcers and NSAIDs, while other causes include oesophageal or gastric varices, gastric erosions, Mallory‐Weiss tear in the lining of the oesophagus, angiodysplasia, and upper gastrointestinal malignancies. For example, a review of nine European studies from 1973 to 1995 reported that the main causes of haemorrhage were duodenal ulcer (24% of all cases), gastric ulcer (13%), varices (9%), gastritis/erosions (9%), oesophagitis (8%), malignancies (5%), and no diagnosis (14%).83
Lower gastrointestinal haemorrhage accounts for about 20% of all acute gastrointestinal haemorrhages. The most common causes are diverticular disease, inflammatory bowel disease, colonic polyps, ischaemic or infective colitis, gastroenteritis, haemorrhoids, angiodysplasia, and colorectal neoplasms. Most lower gastrointestinal haemorrhages occur in elderly people, and most of these bleeds settle spontaneously and do not require emergency surgery. It is estimated that 20–30% of all gastrointestinal haemorrhages are related to the use of non‐steroidal anti‐inflammatory drugs.
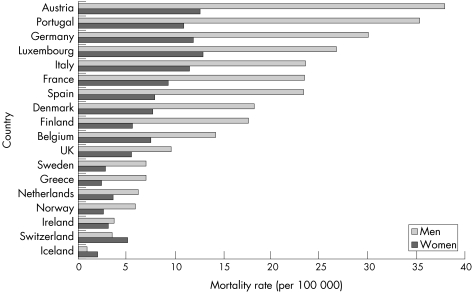
The incidence of upper gastrointestinal haemorrhage increases very sharply with age, it is higher in men than in women, and it tends to be highest in areas with high incidence of peptic ulcer—for example, in Scotland and the north of England rather than in southern regions. High hospital admissions rates of upper gastrointestinal haemorrhage have been reported in the west of Scotland (172 per 100 000 in 1992–93),92 Aberdeen (117 in 1991–93),90 and the north east of Scotland (116 in 1967–6886; table 3.2.2).
Table 3.2.2 Hospital admission rates (per 100 000 adult population) for upper gastrointestinal haemorrhage as reported from various regional studies in the UK and in other countries.
| Country | City/region | Study period | No of cases | Hospital admission rate per 100 000 adult population | Authors and reference |
|---|---|---|---|---|---|
| UK studies: | |||||
| UK | Oxford | 1953–1967 | 2149 | 47*† | Schiller KF et al, 197084 |
| UK | Oxford | 1981–1982 | 125 | 56*† | Berry AR et al, 198485 |
| UK | NE Scotland | 1967–1968 | 817 | 116 | Johnston SJ et al, 197386 |
| UK | Newport, Gwent | 1980–1981 | 330 | 52*† | Madden MV and Griffith GH, 198587 |
| UK | Nottingham | 1984–1986 | 1017 | 64*† | Katschinski BD et al, 198988 |
| UK | Bath | 1986–1988 | 430 | 70*† | Holman RA et al, 199089 |
| UK | NE Scotland | 1991–1993 | 1098 | 117 | Masson J et al, 199690 |
| UK | north west Thames | 1991–1993 | NA | 91 | Rockall TA et al, 199591 |
| UK | South west Thames | 1991–1993 | NA | 99 | Rockall TA et al, 199591 |
| UK | West Midlands | 1991–1993 | NA | 102 | Rockall TA et al, 199591 |
| UK | Trent | 1991–1993 | NA | 107 | Rockall TA et al, 199591 |
| UK | West of Scotland | 1992–1993 | 1882 | 172 | Blatchford O et al, 199792 |
| Foreign studies: | |||||
| Sweden | Varberg | 1957–1961 | 283 | 121* | Herner B and Lauritzen G, 196593 |
| Sweden | Sundsvall | 1980–1988 | 978 | 100* | Henriksson AE and Svensson JO, 199194 |
| Spain | Cordoba | 1983–1988 | 3270 | 160* | Mino Fugarolas G et al, 199295 |
| Denmark | Odense | 1990–1992 | 183 | 88 | Hallas J et al, 199596 |
| USA | San Diego | 1991–1994 | 258 | 102* | Longstreth GF, 199597 |
| Saudi Arabia | Abha | 1991–1993 | 240 | 31 | Ahmed ME et al, 199798 |
| Finland | Central province | 1992–1994 | 298 | 68 | Soplepmann J et al, 199799 |
| Estonia | Tartu county | 1992–1994 | 270 | 99 | Soplepmann J et al, 199799 |
| Netherlands | Amsterdam | 1993–1994 | 951 | 45 | Vreeburg EM et al, 199783 |
| Crete | Heraklion | 1998–1999 | 353 | 160 | Paspatis GA et al, 2000100 |
| Italy and Spain | Multicentre | 1998–2001 | 2813 | 40 | Laporte JR et al, 2004101 |
*Admission rates are expressed per 100 000 general population, instead of the usual 100 000 adult population for upper gastrointestinal haemorrhage, and therefore underreport incidence in comparison with the other studies; †admission rates are calculated from the cited number of cases and total populations served by the hospital(s).
A study of four health regions in the south of England and the Midlands reported an overall hospital admission rate of 103 per 100 000; which varied between 91 for north west Thames and 107 for Trent.91 However, lower hospitalised incidence rates of 45–70 per 100 000 were reported from earlier studies particularly in relatively affluent studies such as Bath and Oxford from the 1950s to the 1980s.84,85,88,89 With an ageing UK population, incidence is likely to continue to rise.91
Incidence rates of upper gastrointestinal haemorrhage in the UK are often higher than those reported in other recent studies in Europe and elsewhere. These include studies in Central Finland,99 the Netherlands,83 Saudi Arabia,98 Estonia,99 and a multicentre study in Spain and Italy. None the less, high incidence rates of 160 per 100 000 have been reported from studies in Crete in the late 1990s,100 and Spain in the 1980s.95
Incidence of diseases of the small bowel and colon
Inflammatory bowel disease
Ulcerative colitis and Crohn's disease are the two main idiopathic types of inflammatory bowel disease. Ulcerative colitis, otherwise known as idiopathic proctocolitis, causes inflammation and ulcers in the colon. Crohn's disease differs from ulcerative colitis because it can occur anywhere along the GI tract and causes inflammation deeper within the intestinal wall. Inflammatory bowel disease usually affects younger people and has a chronic relapsing course that impacts on educational, social, professional, and family life. Along with gastrointestinal cancers and liver disease, inflammatory bowel disease is one of the three most important areas for British gastroenterologists.
A total of about 150 000 people have inflammatory bowel disease in the UK, and a total of approximately 2.2 million across Europe.102 Although there is substantial regional variation (table 3.2.3), the prevalence of Crohn's disease in the UK is currently about 55–140 per 100 000 population, and that of ulcerative colitis is about 160–240 per 100 000, with a combined incidence of about 13 300 new cases diagnosed each year.103
Table 3.2.3 Incidence and prevalence rates (per 100 000 population) for Crohn's disease and for ulcerative colitis, as reported from various regional studies in the UK.
| City/region | Study period | Study sources* | No of cases | Incidence rate per 100 000 population | Prevalence per 100 000 population | Authors and reference |
|---|---|---|---|---|---|---|
| Crohn's disease: | ||||||
| Cardiff | 1931–90 | SP, HR, Lab | 86 | 2.3 in 1961–65 11.9 in 1981–85 8.6 in 1986–90 | – | Thomas GA et al, 1995121 |
| Cardiff | 1991–95 | SP, HR, Lab | 84 | 5.6 | – | Yapp TR et al, 2000122 |
| Oxford | 1951–60 | SP, HR | 24 | 0.8 | 9 in 1960 | Evans JG and Acheson ED, 1965131 |
| Derby | 1951–85 | HR, Lab | 225 | 0.7 in 1951–55 6.7 in 1981–85 | 85 in 1985 | Fellows IW et al, 1990124 |
| Nottingham | 1958–72 | SP, HR, Lab | 144 | 0.7 in 1958–60 3.6 in 1970–72 | – | Miller DS et al, 1974137 |
| Clydesdale | 1961–70 | HR | 357 | 1.2 in 1961–65 1.9 in 1966–70 | – | Smith IS et al, 1975138 |
| Gloucester | 1966–70 | HR, Lab | 19 | 1.5 | – | Tresadern JC et al, 1973139 |
| North Tees | 1971–77 | HR, Lab | 73 | 5.3 | 35 in 1977 | Devlin HB et al, 1980134 |
| NE Scotland and N Isles of Scotland | 1955–88 | SP, HR, Lab | 1008 | 1.3 in 1955–57 9.8 in 1985–87 | 147 in 1988 | Kyle J, 1992123 |
| Northern Ireland | 1966–81 | HR, Lab | 440 | 1.3 in 1966–73 2.3 in 1974–81 | – | Humphreys WG et al, 1990140 |
| Blackpool | 1968–80 | HR, Lab | 156 | 3.3 in 1971–75 6.1 in 1976–80 | 47 in 1980 | Lee FI and Costello FT, 1985125 |
| Leicestershire | 1972–89 | SP, HR, Lab | 582 | 3.2–4.7 (among Europeans) | – | Jayanthi V et al, 1992126 |
| North Tees | 1985–94 | SP | 200 | 8.3 | 145 in 1994 | Rubin GP et al, 2000136 |
| Trent | 2002 | SP, HR, Lab | 113 | – | 130 in 2002 | Stone MA et al, 2003141 |
| Ulcerative colitis: | ||||||
| Oxford | 1951–60 | SP, HR | 238 | 6.5 | 80 in 1960 | Evans JG and Acheson ED, 1965131 |
| NE Scotland | 1967–76 | SP, HR, Lab | 537 | 11.3 | – | Sinclair TS et al, 1983135 |
| Cardiff | 1968–87 | SP, HR, Lab | 6.4 in 1968–77 6.3 in 1978–87 | – | Srivastava ED et al, 1992133 | |
| North Tees | 1971–77 | HR, Lab | 146 | 15.1 | 99 in 1977 | Devlin HB et al, 1980134 |
| High Wycombe | 1975–84 | HR, Lab | 313 | 7.1 | 84 in 1984 | Jones HW et al, 1988132 |
| North Tees | 1985–94 | SP | 334 | 13.9 | 243 in 1994 | Rubin GP et al, 2000136 |
| Trent | 2002 | SP, HR, Lab | 211 | – | 243 in 2002 | Stone MA et al, 2003141 |
*Study sources: SP, survey of physicians; HR, review of hospital records or admission data; Lab, pathology data.
The causes of ulcerative colitis and Crohn's disease are not fully known. Although they are thought to be autoimmune diseases, it is not certain whether autoimmune abnormalities are a cause or result of the diseases. Suggested risk factors include appendectomy, diet, smoking, perinatal and childhood infections, and oral contraceptives,102 while a possible link with measles vaccination has been disputed.104,105 Inflammatory bowel disease predisposes strongly to cancer of the colon,106,107,108,109,110 to venous thromboembolism,111,112,113 and osteoporosis,114,115,116 and it is also associated with coeliac disease,117,118 and primary sclerosing cholangitis.119,120
There is a peak in incidence of inflammatory bowel disease between the ages of 10 and 19 years, and a smaller peak beyond 50 years of age. Women may be at a slightly increased risk of Crohn's disease than men, whereas the risk for ulcerative colitis is the same for men and women.
Studies of Crohn's disease in the UK, and in Europe, have typically reported large increases in incidence over the past 50 years, while others have reported incidence rates that have stabilised after earlier increases (table 3.2.3). There was a sharp increase in the incidence of Crohn's disease in Cardiff from the early 1960s to the early 1980s, before levelling off in the late 1980s,121 and subsequently declining during 1991–95.122 Other sharp increases in incidence of Crohn's disease up to the 1980s have been reported for the north east of Scotland,123 Derby,124 Blackpool,125 and among Europeans in Leicestershire.126
Incidence rates for ulcerative colitis have been more stable over time than those for Crohn's disease,127 although a few recent European studies have reported increasing,128,129 or decreasing rates.130 Several regional British studies have reported incidence rates of about six or 7 per 100 000 (table 3.2.3),131,132,133 although substantially higher rates of 11 to 15 have been reported for northern regions such as north Tees and the north east of Scotland.134,135,136
Although the incidence of inflammatory bowel disease may have shown a tendency to plateau in recent years, large increases in the incidence of paediatric Crohn's disease have continued to be reported in the UK. For example, in Scotland there was a threefold increase in paediatric incidence from 1968 to 1983,142 a further 50% increase from 1981–83 to 1990–92,143 and a 100% increase in north east Scotland from 1980–89 to 1990–99.144 In south Glamorgan there was a 140% increase in the incidence of paediatric disease from 1983–88 to 1989–93,145 although it is now thought to have reached a plateau.146 A recent comparison of two national British birth cohorts indicates that the prevalence of Crohn's disease has increased in younger people, although the prevalence of ulcerative colitis has remained stable.147
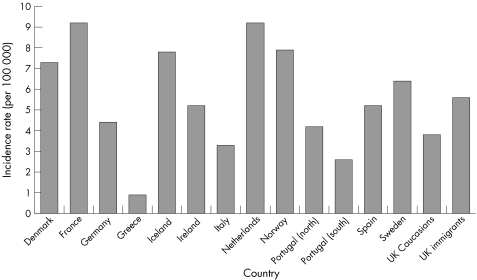
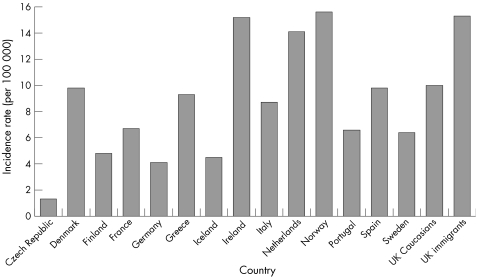
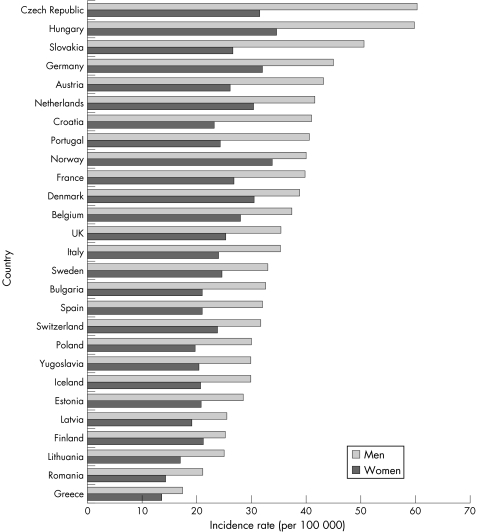
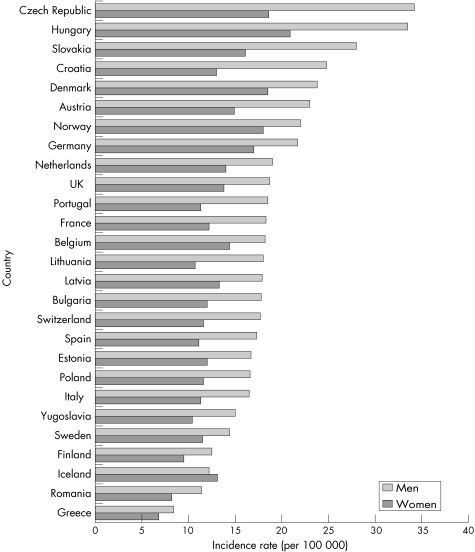
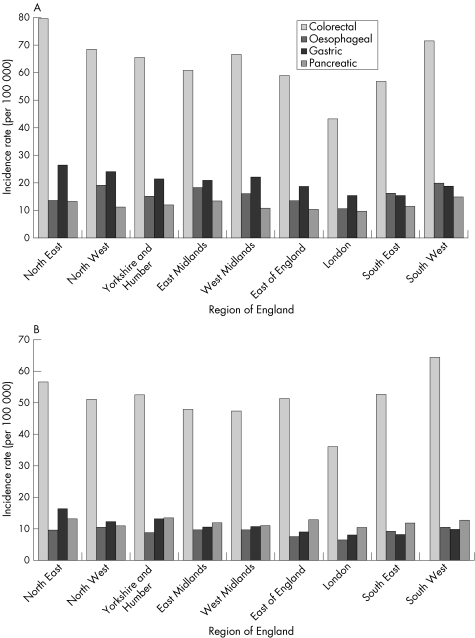
A comparison of incidence rates for Crohn's disease and ulcerative colitis in the UK, with those reported for various other European countries in 1991–93, is shown in figs 3.2.1 and 3.2.2.148 There is substantial international variation in the incidence of both types of inflammatory bowel disease. For Crohn's disease, incidence tends to be much higher in northern European countries, particularly in Scandinavia and the Netherlands.
Figure 3.2.1 Incidence rate (per 100 000 population) for Crohn's disease in the UK and in other European countries. Source: Shivananda et al, 1996.148
Figure 3.2.2 Incidence rate (per 100 000 population) for ulcerative colitis in the UK and in other European countries. Source: Shivananda et al, 1996.148
The incidence of ulcerative colitis among the UK white population (10.0 per 100 000) is similar to the average of all European countries reported here (9.4), but UK immigrants have a substantially higher rate (figs 3.2.1 and 3.2.2). The incidence of Crohn's disease in the UK white population (3.8) is lower than the European average (5.5), but UK immigrants have similar incidence (5.6).
Irritable bowel syndrome
Irritable bowel syndrome (IBS) refers to longstanding symptoms of abdominal pain, bloating, flatulence, diarrhoea, and/or constipation. It is the most common functional gastrointestinal disorder seen by GPs, and it is the most common disease diagnosed by gastroenterologists. Although not life threatening, IBS may severely impair quality of life, and it usually persists for several years. Like dyspepsia, IBS has been defined in a number of different ways according to different diagnostic criteria, which affects prevalence estimates.
IBS typically affects 10 to 25% of the general UK population. About half of people with IBS consult their GP, and of these about 20% are referred to a consultant.149 Consultation behaviour is often influenced by life events or psychological factors, as well as severity of symptoms. IBS constitutes about 20 to 50% of the outpatient gastroenterology workload.150,151,152
IBS can occur at any age, although it most commonly starts in late teenage years or early adulthood, and it is up to three times more common in women than in men. Although there is no consistent effect of age and ethnicity on symptoms,149 they vary according to which parts of the gut are affected.
Recent community based studies in the UK have reported an IBS prevalence of 10.5% in Birmingham,158 16.7% in Teeside,154 9.5% and 2.5% in Bristol,155,156 and 22% in Hampshire.43 In each of these studies, the prevalence in women was two to four times higher than in men. Prevalence also appears to be increasing in the UK. For example, a comparison of two British national birth cohorts revealed a prevalence rate that had risen from 2.9% in 1988 to 8.3% in 2000 among people aged 30 years.147
The prevalence of IBS ranges in all countries of the world from about 3% to 25%. Although differing diagnostic criteria affect comparability across studies, reported prevalence rates in the UK appear to be comparable with, or perhaps slightly higher than those reported in most other Western countries (table 3.2.4).
Table 3.2.4 Prevalence rates (expressed as percentages) of irritable bowel syndrome (IBS) reported from various studies in the UK and in other Western countries.
| Country | City/region | Year of study* | Study size | Prevalence (% of population)† | Authors and reference |
|---|---|---|---|---|---|
| UK studies: | |||||
| UK | Avon | 1979 | 301 | 13.6 | Thompson WG and Heaton KW, 1980157 |
| UK | Hampshire | 1991 | 1 620 | 22.0 | Jones RH and Lydeard SE, 199243 |
| UK | Bristol | 1991 | 1 896 | 9.5 | Heaton KW et al, 1992155 |
| UK | Teeside | 1997 | 3 179 | 16.7 | Kennedy TM and Jones RH, 2000154 |
| UK | Bristol | 1995–7 | 3 111 | 2.5 | Thompson WG et al, 2000156 |
| UK | Birmingham | 2003 | 4 807 | 10.5 | Wilson S et al, 2004158 |
| Other Western countries: | |||||
| USA | 1981 | 789 | 17.1 | Drossman DA et al, 1982159 | |
| USA | 1983 | 566 | 15.0 | Sandler RS et al, 1984160 | |
| Italy | Umbria | 1988 | 533 | 8.5 | Gaburri M et al, 1989161 |
| Japan | 1988–89 | 231 | 25.0 | Schlemper RJ et al, 1993162 | |
| USA | 1990 | 5 430 | 9.4 | Drossman DA et al, 1993163 | |
| The Netherlands | 1991 | 500 | 9.0 | Schlemper RJ et al, 1993162 | |
| USA | Olmsted County | 1992 | 643 | 8.5–20.4 | Saito YA et al, 2000164 |
| Sweden | Osthammar | 1988 | 1 290 | 14.0 | Agreus L et al, 1995165 |
| Denmark | Glostrup | 1993 | 4 581 | 6.6 | Kay L et al, 1994166 |
| Australia | Penrith, Sydney | 1996 | 3 240 | 4.4–13.6 | Boyce PM et al, 2000167 |
| Spain | 2000 | 2 000 | 2.1–12.1 | Mearin F et al, 2001168 | |
| France | 2001 | 15 132 | 4.7 | Dapoigny M et al, 2004169 | |
| Canada | 2001 | 1 149 | 12.1–13.5 | Thompson WG et al, 2002170 | |
| New Zealand | Dunedin | 1998–99 | 980 | 3.3–18.8 | Barbezat G et al, 2002171 |
| Iceland | 2000 | 2 000 | 30.9 | Olafsdottir LB et al, 200564 | |
| USA | Olmsted County | 2002 | 643 | 5.1–27.6 | Saito YA et al, 2003172 |
*Year before the year of publication is given, where the year of study was not specified; †ranges of prevalence refer to prevalence rates obtained using different criteria for diagnosing IBS.
Coeliac disease
Coeliac disease is an inflammatory condition of the small intestine resulting from sensitivity to gluten, a protein in wheat flour, and similar proteins in barley and rye. It develops in genetically predisposed people but can be diagnosed at any age from early childhood to old age. It appears that a “trigger factor” may be required to initiate that response. The trigger might be a viral infection but is usually not known. Removal of wheat gluten (as well as barley and rye) from the diet permits the intestinal mucosa to recover.
Coeliac disease is highly prevalent throughout the world, particularly in countries where wheat forms part of the staple diet, and it is one of the most important conditions managed by gastroenterologists. It is more prevalent in the families of those who are affected: it is estimated that as many as 10% of first degree relatives of patients are also affected.173 Previous underdiagnosis of coeliac disease in primary care reflects an evolving awareness of the diversity in the presentation of coeliac disease.174 Coeliac disease is often associated with other diseases such as ulcerative colitis, biliary cirrhosis, primary sclerosing cholangitis, osteoporosis, malignant lymphomas, and thyroid disorders,175,176,177,178,179,180,181,182,183 as well as being linked to increased risks of gastrointestinal cancer.182,184,185
The prevalence of coeliac disease is thought to be about 1% in the UK,186 which appears to be comparable with other countries, globally (table 3.2.5). The prevalence of coeliac disease has increased sharply in the UK in the last couple of decades; largely because of improved diagnosis rates as a result of the introduction of screening tools which can be used in primary care. In the diagnosis of coeliac disease, IgA antibodies to tissue transglutaminase and endomysium show good sensitivity and specificity for coeliac disease; however, it is recommended that the diagnosis is confirmed by small bowel biopsy. Cases of coeliac disease have been described in patients with normal biopsy and positive serology and visa versa. In patients with coeliac disease and IgA deficiency the serology will be negative, in such patients IgG transglutaminase and endomysial antibody should be determined.
Table 3.2.5 Prevalence rates of coeliac disease as reported from various international studies.
| Country | Screening method | Study size | Prevalence rate† | Authors and reference |
|---|---|---|---|---|
| The Netherlands | EMA* | 1 440 | 1 in 288 (a) | Schweizer JJ et al, 2004187 |
| Australia | EMA | 3 011 | 1 in 251 (a) | Hovell CJ et al, 2001188 |
| Sweden | TGA EMA | 1 850 | 1 in 205 (a) | Lagerqvist C et al, 2001189 |
| The Netherlands | EMA | 6 127 | 1 in 198 (c) | Csizmadia CG et al, 1999190 |
| Brazil | EMA | 2 371 | 1 in 183 (a) | Pratesi R et al, 2003191 |
| Argentina | AGA EMA | 2 000 | 1 in 167 (a) | Gomez JC et al, 2001192 |
| USA | AGA EMA | 4 126 | 1 in 133 (a) | Fasano A et al, 2003193 |
| Finland | EMA | 1 070 | 1 in 130 (c) | Kolho KL et al, 1998194 |
| Northern Ireland | AGA EMA | 1 823 | 1 in 122 (a) | Johnston SD et al, 1997195 |
| Finland | EMA | 3 654 | 1 in 99 (c) | Maki M et al, 2003196 |
| England | EMA* | 7 550 | 1 in 87 (a) | West J et al, 2003186 |
| Europe (Finland, Germany, Italy, Northern Ireland) | TGA EMA | 29 268 | 1 in 50–1 in 220 (a) 1 in 88–1 in 123 (c) | Mustalahti K et al, 2004197 |
Determination in serum of IgA antibodies against gliadin (AGA), endomysium (EMA), and tissue transglutaminase (TGA).
*Diagnosis not confirmed by small bowel biopsy; †a, adults; c, children.
Diverticular disease
Diverticular of the intestine is a major cause of mortality and morbidity in the UK, mainly among elderly people. It refers to diverticula, or small sacs or pouches that form in the wall of the colon. The most common complication is acute diverticulitis, which occurs when the diverticular become infected, and is sometimes associated with perforation, intestinal obstruction, fistula or abscess formation. Diverticular disease is very common in elderly people, but it is rare in younger age groups and in developing countries. It is thought to be caused mainly by longstanding constipation.198
Risk factors for diverticular disease include low fibre diets and low levels of physical activity, while vegetarians have a lower incidence of diverticular disease.199,200 Increased risks of perforated diverticula have been identified for NSAIDs,201,202,203,204 corticosteroids,205 and opiate analgesics,206 whereas calcium antagonists are thought to have a protective effect.203
Diverticular disease is much more common in the west than in less developed countries.203 For example, a study from the 1960s reported a hospital admission rate of 12.9 per 100 000 in Scotland that was over 60 times higher than those in Fiji, Nigeria, and Singapore.207 Westerners residing in those countries were also substantially more affected than the native populations. In Singapore, for instance, the admission rate among Europeans (5.4 per 100 000) was over 40 times that in the indigenous population.207
In the UK, diverticular disease is much more common among white people than among Asian ethnic groups,208 while incidence increases sharply with age. About 5% of people are affected when in their 40s, and about 50% of people when aged over 80.209 Diverticular disease is more common in men than in women among younger age groups, but it is more common in women among older age groups.203
Because uncomplicated disease is not associated with any particular symptoms, it is often not discovered until postmortem examination, while few studies have examined the progression from uncomplicated to complicated diverticular disease. Lower gastrointestinal haemorrhaging, which occurs in about 15–20% of cases, and infection resulting in peritonitis or abscesses are the most common complications, and are the causes of most admissions to hospital.210 For details of mortality associated with complicated and uncomplicated diverticular disease, see section 3.3.
With an ageing UK population, the incidence of diverticular disease is increasing.211,212 For example, hospital admissions for diverticular disease increased by 16% in men from 20 to 23 per 100 000, and by 12% in women from 29 to 32 per 100 000 in England during the 1990s,212 while emergency surgical admissions for diverticular disease increased significantly in the south west of England from 1974 to 1998.213
Incidence of diseases of the liver
Chronic liver disease and cirrhosis
Chronic liver disease and cirrhosis, traditionally referred to as liver cirrhosis, encompasses a wide range of acute and chronic liver conditions that are caused by a number of different agents. These conditions may lead to cirrhosis, resulting in scarring, injury, and dysfunction of the liver. They include heavy alcohol consumption, hepatitis B or C viral infections, prolonged exposure to certain drugs and toxins, inherited diseases such as haemochromatosis and Wilson's disease, autoimmune liver disease, and chronic liver diseases such as alcoholic fatty liver disease, primary biliary cirrhosis and other chronic diseases of the bile ducts. Around 25% of liver disease is alcohol related, and a similar amount is caused by hepatitis C.214
Alcoholic liver disease
Alcoholic liver disease refers to a handful of liver diseases that are attributed to the effects of alcohol. These include alcoholic cirrhosis, alcoholic fatty liver disease, alcoholic hepatitis, and alcoholic hepatic failure. Because of diagnostic difficulties, there is negligible reporting of population based incidence rates for the different aetiologies; while in most cases routine hospital data fail to distinguish between them. For example, in Scotland in 1999–2000, 71% of hospital discharges for alcoholic liver disease were diagnosed as “unspecified alcoholic liver disease”.215 Since only 15–30% of heavy consumers of alcohol develop advanced alcoholic liver disease,216 genetic and other environmental factors also have an important role.
Earlier, regional British studies reported incidence rates for alcoholic liver disease of 6.5, 14.6, and 2.8 per 100 000 population in respectively, west Birmingham in 1971–76,217 Tayside in 1975–79, and the Scottish Islands of Lewis and Harris in 1977–82.218 The study of west Birmingham also reported an increase in alcoholic liver disease from 2.3 to 9.5 per 100 000 from 1959–61 to 1974–76.217
More recent figures show a 160% rise in hospital admissions for alcoholic liver disease in Scotland between 1996 and 2000,219 while an earlier Scottish study also reported a 160% increase in admissions for liver cirrhosis from 1983 to 1995.220 The large increase in alcoholic liver disease in the UK in recent years has become a major public health concern and has led to the publication of a national alcohol reduction strategy.221
Incidence rates for alcoholic liver disease in the UK are still relatively low compared with those in many other Western countries. For example, a rate of 32 per 100 000 was recently reported for Los Angeles, which varied between 8 per 100 000 for Asian ethnic groups and 61 for Hispanics,216 while the incidence rate in Stockholm County increased from 8 to 24 per 100 000 during the 1970s before falling to 12 per 100 000 by the late 1980s.222
Non‐alcoholic fatty liver disease
Non‐alcoholic fatty liver disease (NAFLD), largely unheard of before the 1980s, is another liver disease on the increase, coinciding with the epidemic of obesity in the UK and in other Western countries. NAFLD is the term used to describe a number of liver conditions, including simple steatosis (fat accumulation in liver cells), steatosis with non‐specific inflammation, steatohepatitis (fat accumulation and liver cell injury), and hepatocellular cancer.223 It has also been suggested that cryptogenic cirrhosis may often actually be “burned out” non‐alcoholic steatohepatitis.224
NAFLD is commonly seen in conjunction with type 2 diabetes, obesity, hypertension, and dyslipidaemia, and is regarded as the liver's response to the metabolic syndrome. Although not the only risk factor, obesity is the most prevalent risk factor for NAFLD and is present in 65–90% of cases. Additional risk factors include advanced age and type 2 diabetes, while men and women are equally affected. Although many people with NAFLD remain undiagnosed, it is thought to affect about 20% of the general population in the UK,225 while the obesity epidemic is expected to result in increases in the prevalence of NAFLD in the future.
Non‐alcoholic steatohepatitis
Non‐alcoholic steatohepatitis (NASH) is a major cause of non‐alcoholic liver disease which closely resembles alcoholic liver disease, but occurs in people who consume little or no alcohol. As with alcoholic liver disease, an excess of fat is deposited in the liver, which leads to NASH, inflammation, and scarring, and can progress to cirrhosis. NASH is thought to progress to advanced liver disease in about 15–20% of cases. Most cases are asymptomatic and are diagnosed when abnormal liver blood results are discovered during routine investigations.226
Until relatively recently NASH was thought to be confined largely to middle aged obese women with diabetes. However, it has become increasingly recognised that NASH also occurs in people who are neither obese nor diabetic, and that it may be one of the most common liver diseases in the Western world.226 Unfortunately, figures on the incidence or prevalence of NASH in the UK are conspicuous by their absence.
Hepatitis C
The hepatitis C infection is caused by a virus, which is mainly passed through blood and blood products. Most new cases in western Europe are related to intravenous drug abuse, through using infected needles, and to the increased prevalence of hepatitis C infection in Eastern European immigrants.227 Other less common routes of infection in the UK include unprotected sex, through contaminated skin piercing and tattooing equipment, or from mother to baby.228 As symptoms from acute hepatitis C infection are uncommon, infection is often discovered by chance on routine screening or on testing after the patient's liver function tests have been found to be abnormal.
An estimated 0.5% of the general UK population, or about 300 000 people, are infected with hepatitis C. Since about one fifth of those infected appear to get rid of the virus naturally without treatment,228 the estimated prevalence of hepatitis C infection is about 0.4% or 240 000 people, which is about four times higher than the total number of 60 294 reported hepatitis C diagnoses in the UK up to the end of 2003.229
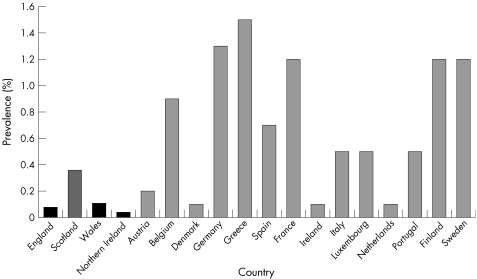
Prevalence rates, based on the total number of reported laboratory diagnoses in the UK, at the end of 2003 were 0.08% for the general population in England, 0.36% in Scotland, 0.11% in Wales, and 0.04% in Northern Ireland.229 These are typically lower than prevalence rates reported for other European countries (fig 3.2.3). There are an estimated five million hepatitis C carriers in western Europe,227 and 170 million in the world.230 Prevalence rates in the UK, and in Europe (1.0% of the population) are lower than in other parts of the world, such as Africa (5.3%), the Eastern Mediterranean (4.6%), and South East Asia (2.2%).230
Figure 3.2.3 Prevalence rates (% of population) for reported hepatitis C infections in England, Scotland, Wales, Northern Ireland, and other European countries. Notes: Rates in England, Scotland, Wales, and Northern Ireland are based on reported laboratory diagnoses.229 The data source for reported rates in the other European countries is Burroughs and McNamara.227
Greatly increased risks of hepatitis C infection are found among high risk subgroups of the UK population, such as injecting drug users. In Scotland, for example, reported prevalence rates for hepatitis C antibodies among injecting drug users varied between 23% in the Forth Valley and 62% in Greater Glasgow in 1999–2000,229 while a prevalence rate of 44% was reported for injecting drug users in London in 2001.231 Another document reported the highest prevalence in 2001–02 of about 45–50% in London and the north west of England, the lowest prevalence of about 15% in the north east, and a prevalence of 20–35% in other English regions.232
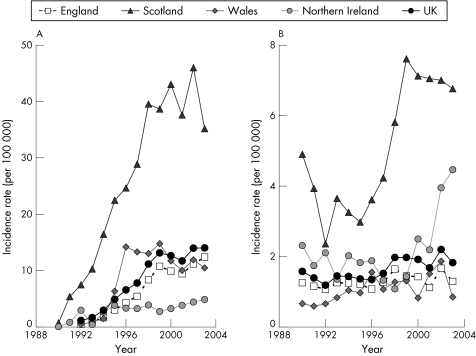
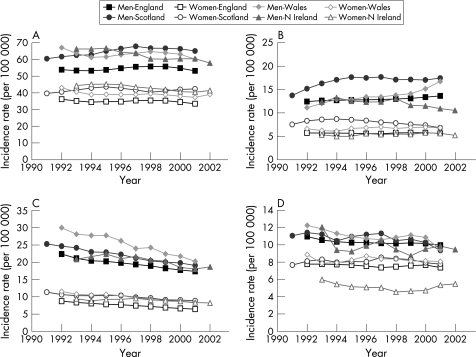
Reported incidence rates for hepatitis C increased alarmingly in the UK during the 1990s, particularly in Scotland (fig 3.2.4A). Based on reported diagnoses, the incidence is currently about 40 per 100 000 in Scotland, 10–15 per 100 000 in England, Wales, and in the UK overall, and around 5 per 100 000 in Northern Ireland.229 In Tayside, prevalence increased from 0.01% to 1.03% of the population from 1988 to 1998.233 The rise of hepatitis C infections has led to the recent publication of national English strategy and action plan documents.228,232
Figure 3.2.4 Trends in incidence rates (per 100 000 population) for reported hepatitis C and B infections in the UK, England, Scotland, Wales, and Northern Ireland, 1990–2003. (A) Hepatitis C infection; (B) hepatitis B infection. Notes: These incidence rates are based on reported laboratory diagnoses.229
About 40% of people with an acute hepatitis C infection have lifelong chronic infection, which often causes liver cirrhosis or cancer many years after the initial infection. Infected people who consume alcohol have accelerated liver damage, and increased incidence of liver cirrhosis and hepatocellular cancer.234,235 Hepatitis C infection invariably causes chronic illness, resulting in a major financial burden on healthcare resources. In western Europe, hepatitis C accounts for 70% of all cases of chronic hepatitis, 40% of all liver cirrhosis, and 60% of all hepatocellular cancer.227 Because of the increasing incidence of hepatitis C, it is estimated that the future burden of hepatitis C health care related to new incidence of cirrhosis will increase by 60% by 2008, and that there will be a fivefold increased need for liver transplantation.214
Hepatitis B
Hepatitis B is also caused by a virus; which, in Europe and North America, is mainly passed from person to person by unprotected sex. In the rest of the world it is mainly passed from infected mothers to their children or from child to child.226 Both hepatitis B and chronic hepatitis C are premalignant diseases leading to hepatocellular cancer. However, unlike hepatitis C, vaccination for hepatitis B has proved to be successful in reducing infection rates.227
The prevalence of hepatitis B in the UK is thought to be 0.1% of the general population or approximately 60 000 people,226 which compares with a total of about 13 000 reported diagnoses up to the end of 2003.229 In districts of the UK where there are high levels of immigration, prevalence can be much higher; as high as 2% of the population. In Europe, an estimated one million people are infected each year, although the infection is more common in South East Asia, the Middle and Far East, Africa, and southern Europe.
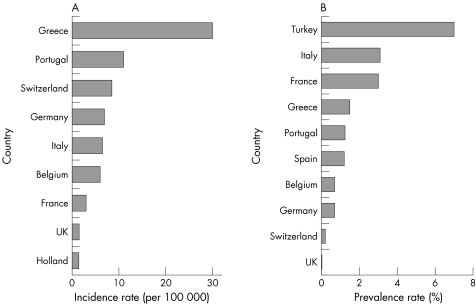
Compared with hepatitis C, there is a less discernible trend in the incidence of reported hepatitis B diagnoses in the UK in recent years (fig 3.2.4B), although there appears to have been quite sharp increases in Scotland during the late 1990s and in Northern Ireland during the past few years. Reported incidence rates for hepatitis B are about one fifth of those for hepatitis C. Recent World Health Organisation figures also indicate that the incidence and prevalence of hepatitis B in the UK is relatively low compared with many European countries; particularly south European countries such as Turkey and Greece (figs 3.2.5A and 3.2.5B).
Figure 3.2.5 Incidence rates (per 100 000 population) and prevalence rates (% of population) for hepatitis B infection in the UK and in other European countries. (A) Incidence rate; (B) prevalence rate. Source: World Health Organisation.230
Primary biliary cirrhosis
Primary biliary cirrhosis is a disease characterised by inflammatory destruction of the small bile ducts within the liver that eventually leads to cirrhosis of the liver. The cause of primary biliary cirrhosis is unknown, but because of the presence of autoantibodies, it is generally thought to be an autoimmune disease. However, other aetiologies such as infectious agents have not been completely excluded.
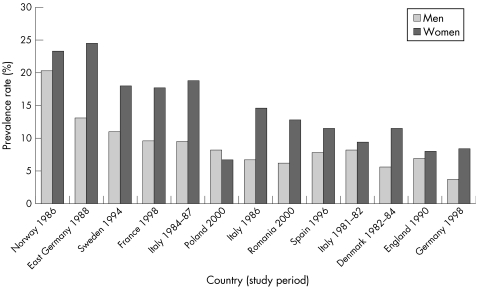
About 90% of primary biliary cirrhosis occurs in women, and most commonly between the ages of 40 and 60 years. Incidence appears to be increasing sharply in the UK (table 3.2.6).236 For example, the prevalence of primary biliary cirrhosis in northern England rose sevenfold between 1976 and 1987,237 and by 70% from 1987 to 1994.238
Table 3.2.6 Incidence and prevalence rates (per 100 000 population) for primary biliary cirrhosis as reported from various studies in the UK, and in other countries.
| Country | Region | Study period | Study sources* | No of cases | Incidence per 100 000 population | Prevalence per 100 000 population | Authors and reference |
|---|---|---|---|---|---|---|---|
| UK studies: | |||||||
| UK | Sheffield | 1976–79 | SP, Lab | 34 | 0.6 | 5.4 | Triger DR, 1980239 |
| UK | Northern England | 1976–87 | SP, Lab, HR | 347 | 1.9 | 1.8 in 1976 12.9 in 1987 | Myszor M and James OF, 1990237 |
| UK | Dundee | 1975–79 | LHD | 29 | 1.1 | 4.0 | Hislop WS et al, 1982240 |
| UK | NE England | 1972–79 | SP | 117 | 1.0 | 3.7 (rural) 14.4 (urban) | Hamlyn AN et al, 1983241 |
| UK | Glasgow | 1965–80 | Lab, LHD | 373 | 1.1–1.5 | 7.0–9.3 | Goudie BM et al, 1987242 |
| UK | Northern England | 1987–94 | SP, Lab, HR, LHD, ND | 770 | 2.3 in 1987 3.2 in 1994 | 20.2 in 1987 34.5 in 1994 | James OF et al, 1999238 |
| UK | Newcastle | 1987–94 | SP, Lab, HR, LHD, ND | 160 | 2.2 | 18.0 in 1987 | Metcalf JV et al, 1997243 |
| 24.0 in 1994 | |||||||
| UK | Swansea | 1995–96 | Lab, HR, LHD | 67 | – | 20.0 | Kingham JG and Parker DR, 1998244 |
| Foreign studies: | |||||||
| Sweden | Umea | 1972–83 | SP, Lab, HR | 86 | 1.3 | 15.1 | Danielsson A et al, 1990245 |
| Sweden | Malmo | 1973–82 | Lab, HR, ND | 33 | 1.4 | 9.2 in 1982 | Eriksson S and Lindgren S, 1984246 |
| Sweden | Orebro | 1976–83 | Lab | 36 | 1.4 | 12.8 in 1983 | Lofgren J et al, 1985247 |
| Europe | 10 centres | 1981 | SP | 569 | – | 2.3 (0.5–7.5) | Triger DR et al, 1984248 |
| Canada | Ontario | 1986 | SP | 206 | 0.3 | 2.2 | Witt‐Sullivan H et al, 1990249 |
| Spain | Granada | 1976–89 | SP, HR | 25 | 4.1 | 3.6 in 1976 | Caballero Plasencia AM et al, 1991250 |
| 6.2 in 1989 | |||||||
| Australia | Victoria | 1991 | SP, HR | 84 | – | 1.9 | Watson RG et al, 1995251 |
| Norway | Oslo | 1986–95 | HR | 25 | 1.6 | 14.6 in 1995 | Boberg KM et al, 1998252 |
| Estonia | 1973–92 | SP, Lab | 69 | 0.2 | 2.7 | Remmel T et al, 1995253 | |
| USA | Olmsted County | 1976–2000 | Lab, HR | 22 | 1.3 in men 0.5 in women | 6.3 in women | Bambha K et al, 2003254 |
| Alaska | 1984–2000 | Lab, HR | 18 | – | 16 (natives) | Hurlburt KJ et al, 2002255 | |
| Australia | Victoria | 1990–2002 | SP, Lab, HR | 249 | – | 5.1 | Sood S et al, 2004256 |
*Study sources: SP, survey of physicians; Lab, laboratory data on subjects with AMA; HR, review of hospital records or admission data; LHD, liver history data; ND, notification of deaths.
There are large geographical and secular variations in the prevalence of primary biliary cirrhosis world wide (table 3.2.6). The disease appears to be most common in north west Europe, particularly in northern Britain and Scandinavia: some of the highest reported prevalence rates are for northern England (34.5 per 100 000 population)243 and for northern Sweden (15.2).245 These compare with much lower prevalence rates of 1.9 in Victoria, Australia,251 2.2 in Ontario, Canada,249 and 2.7 in Estonia,253 while primary biliary cirrhosis is rarely found in Africa or Asia.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is a chronic inflammatory condition that occurs when the bile ducts inside and outside the liver become inflamed and scarred. As the scarring increases, blockage of the ducts leads to damage to the liver. Although the exact cause of primary sclerosing cholangitis is unknown, it is thought that the tissue damage is mediated by the immune system.257
Primary sclerosing cholangitis usually begins between the ages of 30 and 60 and is about twice as common in men as in women.120 Primary sclerosing cholangitis is closely associated with inflammatory bowel disease, particularly ulcerative colitis,257,258 and coeliac disease.177 Around 75–80% of northern European people with primary sclerosing cholangitis have underlying inflammatory bowel disease.120
Primary sclerosing cholangitis usually progresses to biliary cirrhosis, persistent jaundice, and liver failure. For patients with end stage primary sclerosing cholangitis, liver transplantation remains the only effective treatment. Primary sclerosing cholangitis also predisposes to cholangiocarcinoma in up to 30% of cases,120,259 and has been associated with increased risks of cancer of the colon, pancreas, gallbladder, and liver.260 It has also been shown to potentiate the risks of cancer of the colon in people with ulcerative colitis.261,262,263
Although the disease is becoming increasingly common, there is relatively little reported information on incidence or prevalence. Prevalence rates of 12.7 per 100 000 have been reported in south Wales in 2003,264 8.5,252 and 5.6265 per 100 000 population have been reported from Norwegian studies in the mid‐1990s, and 20.9 per 100 000 for Minnesota, USA in 2000.254
Gallstone disease
Gallstones or cholelithiasis occur when bile stored in the gallbladder hardens into pieces of stone‐like material. The two types of gallstones are cholesterol stones that are made primarily of hardened cholesterol, and account for about 80% of gallstones, and pigment stones that are darker and made of bilirubin. It is thought that cholesterol stones form when bile contains too much cholesterol, too much bilirubin, or not enough bile salts, or when the gallbladder does not empty for some other reason. However, the cause of pigment stones is uncertain, although they tend to occur in people who have cirrhosis, biliary tract infections, and hereditary blood disorders, such as sickle cell anaemia, in which too much bilirubin is formed.
Gallstone disease is the most common abdominal condition for which patients are admitted to hospital in developed countries.266 The incidence of gallstones increases with age and obesity, and it is higher in women than in men. Other risk factors include diabetes, Crohn's disease, cholesterol lowering drugs, gastric bypass surgery, hormone replacement therapy, fasting, and rapid weight loss. Gallstones are very common in the UK among older age groups, with reported prevalence rates of 12% among men, and 22% among women, who were aged over 60 years in an ultrasound survey in Bristol.267
Gallstones can block the normal flow of bile if they lodge in any of the ducts that carry bile from the liver to the small intestine. Complications of gallstones include chronic inflammation or infection of the gallbladder (cholecystitis), abscess formation, acute pancreatitis, and biliary obstruction.266 Gallstones have been shown to be the dominant aetiological agent in 30–60% of cases of acute pancreatitis in the UK, and in 25–75% of cases in other European or Western countries (table 3.2.7).
Table 3.2.7 Aetiology (expressed as percentages) of acute pancreatitis, as reported from various regional studies in the UK, and in other European or Western countries.
| Country | City/region | Study period | No of cases | Aetiology | Authors and reference | ||
|---|---|---|---|---|---|---|---|
| Gallstones (%) | Alcoholic (%) | Other and unknown (%) | |||||
| UK studies: | |||||||
| UK | Bristol | 1950–69 | 590 | 58 | 5 | 37 | Trapnell JE and Duncan EH, 1975284 |
| UK | Bristol | 1968–79 | 737 | 50 | 8 | 42 | Corfield AP et al, 1985285 |
| UK | Nottingham | 1969–76 | 214 | 46 | 8 | 45 | Bourke JB et al, 1979286 |
| UK | NE Scotland | 1983–85 | 378 | 41 | 15 | 44 | Thomson SR et al, 1987287 |
| UK | Wessex region | 1994–95 | 186 | 33 | 20 | 47 | Toh SK et al, 2000288 |
| UK | Glasgow | 1991–93 | 279 | 42 | 35 | 24 | De Beaux AC et al, 1995289 |
| UK | Somerset | 1991–95 | 263 | 56 | 12 | 32 | Norton SA et al, 2001290 |
| Other European or Western countries: | |||||||
| Finland | Tampere | 1967–68 | 97 | 53 | 16 | 31 | Mero M, 1982291 |
| Sweden | Gothenburg | 1974–75 | 204 | 26 | 66 | 8 | Svensson JO et al, 1979292 |
| Finland | Tampere | 1977–78 | 163 | 23 | 58 | 19 | Mero M, 1982291 |
| Norway | Buskerud | 1992 | 93 | 51 | 15 | 34 | Halvorsen FA and Ritland S, 1996293 |
| France | Nice | 1986–94 | 57 | 51 | 25 | 24 | Benchimol D et al, 1996294 |
| Spain | Alicante | 1991 | 473 | 52 | 20 | 28 | Minguez M et al, 1995295 |
| Italy | Bologna | 1990–94 | 204 | 60 | 13 | 27 | Gullo L et al, 2002296 |
| Greece | Thessaloniki | 1990–94 | 84 | 71 | 6 | 23 | Gullo L et al, 2002296 |
| Hungary | Gyor, Szeged | 1990–94 | 483 | 24 | 61 | 13 | Gullo L et al, 2002296 |
| France | Paris | 1990–94 | 65 | 35 | 39 | 26 | Gullo L et al, 2002296 |
| Germany | Ulm, Luneberg | 1990–94 | 232 | 35 | 38 | 27 | Gullo L et al, 2002296 |
| Portugal | Coimbra | 1994 | 91 | 59 | 24 | 17 | Milheiro A et al, 1995297 |
| Norway | Bergen | 1986–95 | 978 | 49 | 25 | 26 | Gislason H et al, 2004298 |
| Sweden | Malmo | 1985–99 | 929 | 42 | 25 | 33 | Lindqvist B et al, 2004299 |
| France | Nice | 1994–95 | 121 | 43 | 31 | 26 | Maes B et al, 1999300 |
| Iceland | Reykavic | 1998–99 | 50 | 42 | 32 | 26 | Birgisson H et al, 2002301 |
| New Zealand | Auckland | 1998–2001 | 112 | 42 | 29 | 29 | Flint R et al, 2004302 |
Hospital admission rates and operations for gallstones have mainly increased in the UK in recent decades,213,268,269,270,271 although admissions reflect the availability of hospital facilities and the prevalent medical practice, as well as the level of incidence.271 In England, for example, admissions increased by 30% in men, and by 64% in women, in England from 1989–90 to 1999–2000.271
Figure 3.2.6 shows prevalence rates for gallstones, as measured through cross‐sectional ultrasound surveys, in regional studies in England and in other European countries. The rates varied between 5 and 24%, they were typically 1.5 to two times higher in women than in men, and the highest rates were reported for Norway and for the former East Germany. The rates reported from the English study of Bristol are lower than those in most of the other European countries.
Figure 3.2.6 Prevalence rates (expressed as percentages) of gallstones among men and women in regional ultrasound surveys in the UK and in other European countries. Source: Aerts and Penninckx, 2003.266 Notes: The year before publication is stated where the study period was not specified. Study regions, overall study sizes and references for the different studies from left to right are: Norway—Schwedt, n = 1371272; East Germany—Neuruppin, 3226273; Sweden—Stockholm, 556274; France—Viduaban, 831275; Italy—multicentre study, 29 379276; Poland—national study, 10 133277; Italy—Sirmione, 1911278; Romania—Timisoara, 1323277; Spain—Guadalajara, 536279; Italy—Rome, 2320280; Denmark— Copenhagen, 3608281; England—Bristol, 1896267; Germany—Romerstein, 2498.282
Haemochromatosis
Haemochromatosis is an inherited condition that is characterised by the deposition of excessive iron in tissue and organs throughout the body, resulting in progressive damage and organ failure. Apart from liver disease, other conditions associated with iron overload include diabetes, joint damage, heart disease, and impotence. Excessive iron overload is associated with increased risks of mortality; mainly from liver cirrhosis, liver failure, liver cancer, and diabetes. Many patients with haemochromatosis remain undiagnosed for several years during the early stages of this condition.227 Although reliable prevalence data for haemochromatosis are not available for the UK, the disease is common in northern Europe. Prevalence rates of 1% and 0.93% have been reported for Germany and Ireland, with lower rates reported for France (0.5%), Sweden (0.5%), Denmark (0.38%), Iceland (0.37%), and Norway (0.34%).227
Incidence of diseases of the pancreas
Acute pancreatitis
Like liver disease, acute pancreatitis is also becoming increasingly common in the general population of the UK. It refers to a sudden inflammation of the pancreas that is activated by destructive pancreatic enzymes. Acute pancreatitis often lasts for a short period of time and, in many cases, it resolves. Severe cases of pancreatitis, however, particularly when necrotising pancreatitis occurs, usually lead to prolonged stays in hospital of three to six months, often with many weeks spent in intensive care and with a high mortality rate.
As there is no specific treatment for acute pancreatitis, surgery and manipulative endoscopy may be required for common duct stones or pancreatic necrosis, and especially for infected necrosis, which occurs in about 5–10% of cases of acute pancreatitis. However, surgery can carry a high mortality, particularly in the short term. Traditional open surgery for infected pancreatic necrosis carries a mortality rate of up to 50%, although a number of less invasive techniques, such as radiological drainage and a minimal access retroperitoneal approach, have been developed.283
The two main causes of acute pancreatitis are blockage of the pancreatic duct by gallstones and heavy alcohol consumption, although other causes can include abdominal trauma, surgery, hyperlipidaemia (types IV, V or VI), hyperparathyroidism, infections such as mumps, and some drugs such as corticosteroids, oral contraceptives, and thiazide diuretics. Because almost all people with an attack of acute pancreatitis are admitted to hospital, acute pancreatitis is one of few gastrointestinal diseases for which hospitalised incidence provides a good measure of true incidence.
Several British studies have shown sharp increases over time in the incidence of acute pancreatitis in recent decades (tables 3.2.7 and 3.2.8), although variation in the definition of incidence to some extent affects comparability across studies. One study of four counties in south east England reported a twofold increase in the incidence of acute pancreatitis from 4.9 to 9.8 per 100 000 population from 1963–74 to 1987–98.303 A recent national English study reported a 43% increase in incidence from 1989–90 to 2000–01,304 and an earlier study of Bristol reported a 35% increase in incidence from 1968 to 1979.285
Table 3.2.8 Incidence rates (per 100 000 population) for acute pancreatitis, as reported from various national and regional studies in the UK.
| Region | Study period | Study sources* | Incidence rate per 100 000 population | Authors and reference |
|---|---|---|---|---|
| Bristol | 1950–69 | HR, Lab, DR | 5.4 in 1961–67 | Trapnell JE and Duncan EH, 1975284 |
| Bristol | 1968–79 | HR, Lab, DR | 5.4–7.3 from 1968 to 79 | Corfield AP et al, 1985285 |
| Nottingham | 1969–76 | HR, Lab, DR | 5.7 | Bourke JB et al, 1979286 |
| Four counties of SE England | 1963–98 | HR | 4.9 in 1963–74 9.8 in 1987–98 | Goldacre MJ and Roberts SE, 2004303 |
| Wessex region, south of England | 1994–95 | HR, Lab | 15.2 | Toh SK et al, 2000288 |
| England | 1989/90–1999/2000 | HR | 14.5 in 1989–90 20.7 in 1999–2000 | Tinto A et al, 2002304 |
| Scotland | 1961–85 | HR, Lab | 6.9 (men) in 1961 75.0 (men) in 1985 11.2 (women) in 1961 48.4 (women) in 1985 | Wilson C and Imrie CW, 1990305 |
| NE Scotland | 1983–85 | HR, Lab | 24.2 | Thomson SR et al, 1987287 |
| Scotland | 1984–95 | HR | 25.8 in 1985 | McKay CJ et al, 1999306 |
| 41.9 in 1995 |
*Study sources: HR, review of hospital records or admission data; Lab, pathology records; DR, deaths records.
A study of Scotland reported an even greater, 10‐fold increase in incidence of acute pancreatitis among men, and a fourfold increase among women, from 1961 to 1985 (table 3.2.8),305 although a more recent Scottish study reported a more modest (62%) increase from 1985 to 1995.306 Increases in the incidence of acute pancreatitis have been attributed to a rise in alcoholic pancreatitis, linked to the increased use of alcohol in the community in the UK,284,303 and in Finland,291 although elsewhere in western Europe increases in incidence have been linked to gallstones.299 Table 3.2.7 shows trends in aetiology across studies, most notably a rise in alcoholic pancreatitis, and a fall in gallstones pancreatitis, across most British studies since the 1950s.
Despite differences in the measurement of incidence across studies, recently reported rates for acute pancreatitis are substantially higher in Scotland (about 25–65 per 100 000),287,305,306 than in England (about 8–25 per 100 000).285,288,303,304 Incidence rates in Scotland are typically comparable with the high rates reported in the Scandinavian countries,293,298,307,308 Iceland,301 and Germany,309 while rates in England are comparable with those in the Netherlands.310,311
Chronic pancreatitis
Chronic pancreatitis occurs when digestive enzymes attack and destroy the pancreas and nearby tissues, causing scarring and pain. It is not usually the result of recurrent attacks of acute pancreatitis but seems to develop separately. The pancreatic gland becomes fibrosed and possibly calcified. Chronic pancreatitis is a disease that is characterised by horrific pain, it severely impairs quality of life and shortens life expectancy, although the exact prognosis is difficult to quantify and it is poorly documented.
The most common cause of chronic pancreatitis is long term, heavy alcohol use. Alcohol has been shown to be the dominant aetiological agent in about 70–80% cases of chronic pancreatitis in recent European studies.312,313,314 However, chronic pancreatitis may be caused by blockage or narrowing of the pancreatic duct by gallstones. In other cases it is genetically linked, or it may be triggered by only one acute attack, especially if the pancreatic ducts are damaged, or it can be caused by the effects of malnutrition when calcification is present, and in other cases the cause cannot be determined. Some patients with chronic pancreatitis develop pancreatic cancer.
Chronic pancreatitis is more common in men than in women, and it often develops between the ages of 30 and 50. The prevalence of chronic pancreatitis in the UK is currently about 40–75 per 100 000 population,315 with an incidence of about eight new cases per 100 000. The tropical form of pancreatitis is a major health problem in southern Africa and Asia, with, for example, high prevalence rates of 114–200 and 25–50 per 100 000 reported for southern India,316 and Japan,317,318 respectively.
Although less common than acute pancreatitis, the incidence of chronic pancreatitis is also increasing,319 particularly with large increases in alcohol use in the UK population over the past 30 years.320,321 Between 1989–90 and 1999–2000 the hospital admission rate for chronic pancreatitis doubled in England.304
There is a large geographical variation in the reported incidence of chronic pancreatitis in Europe, partly reflecting differences in alcohol consumption. High rates of 26, 23, and 14 per 100 000 have been reported for France,322 Finland,314 and Stockholm County, Sweden,222 moderate rates of 5–8 per 100 000 in Luneberg County, Germany,309 Warsaw, Poland,323 and the Czech Republic,314 and a low rate of 1.3 in Switzerland.314
Incidence of gastrointestinal cancer
Gastrointestinal cancer is the most common type of cancer in Europe. Out of 2.1 million new cancers in Europe in 2000, gastrointestinal cancers accounted for 579 542 or 28.3% of the total.324 In the UK, there are about 60 000 new cases of gastrointestinal cancer each year.
Of all cancers in men in England and Wales in 1997, colorectal cancer was the third most common (incidence of 14 900, 13.7% of all new cases), while cancers of the stomach (5800, 5.3%), oesophagus (3600, 3.3%), and pancreas (2700, 2.5%) were ranked 5th, 7th, and 10th, respectively.14
Among women, colorectal cancer was the second most incident cancer (14 000, 12.4% of all new cancers), and cancers of the stomach (3300, 2.9%), pancreas (3000, 2.7%), and oesophagus (2500, 2.2%) were ranked 8th, 9th, and 12th, respectively. Together, gastrointestinal cancers represent around a quarter of all cancers in men (the most common cancer grouping by some margin), and one fifth of cancers in women, behind only breast cancer.14,103
Colorectal cancer
Colorectal cancer is the most common type of gastrointestinal cancer in the UK, with about 30 000 new cases a year, and an incidence rate of about 50 per 100 000 population. It accounts for just over half of all gastrointestinal cancers in the UK, and mainly affects people aged between 50 and 80.324 Of all cancers, when both sexes are included, colorectal cancer is the second most common cancer in England and Wales.14
Risk factors for colorectal cancers include a family history of bowel cancer, long term inflammatory bowel disease, high fat diets with low consumption of fibre, smoking, and lack of exercise. Table 3.2.9 illustrates the strong familial association with colorectal cancer. The predisposition to colorectal cancer among people with ulcerative colitis is well established,106,107,325 with relative risks as high as 21 cited.108,109 The link between Crohn's disease and colorectal cancer is less well documented, although in recent studies it has been reported as comparable to that for ulcerative colitis.108,326,327
Table 3.2.9 Familial association in the lifetime risk of developing colorectal cancer.
| Familial association | Lifetime risk |
|---|---|
| More than two first degree relatives affected | 1:3 |
| Two first degree relatives affected | 1:6 |
| One first degree relative aged <45 years affected | 1:10 |
| One first degree and one second degree relative affected | 1:12 |
| One first degree relative aged >45 years affected | 1:17 |
| General population | 1:50 |
Source: Keighley, 2003.324
From 1971 to 1997 in England and Wales, age standardised incidence of colorectal cancer increased by about 10% in men from about 45 to 50 per 100 000 population, and also increased slightly in women from about 30 to 35 per 100 000.14
Gastric cancer
Gastric cancer is twice as common in men as in women, and mainly affects older people: 80% of cases are diagnosed in people aged between 60 and 80. There are currently about 10 000 new cases a year in the UK, representing about 15% of all gastrointestinal cancers, with an incidence rate of approximately 17 per 100 000 population. In the past 30 years there has been a change in the distribution of gastric cancers, with an increase in the incidence of proximal tumours near the gastro‐oesophageal junction, but a larger decline in the incidence of antral cancers that used to dominate.324
Risk factors include longstanding infection with Helicobacter pylori, family history of gastric cancer, a history of gastric polyps, and other disorders such as atrophic gastritis and pernicious anaemia, poor hygiene and socioeconomic conditions, malnutrition, heavy alcohol consumption, smoking, and certain food products and preservatives, including salt and pickled foods. Diets high in fresh fruit and vegetables seem to protect against gastric cancers as they contain high levels of antioxidant vitamins that are thought to protect the stomach lining.
From 1971 to 1997 in England and Wales, the age standardised incidence of gastric cancer fell by about 50% in men from about 30 to 20 per 100 000, and almost halved in women from about 15 to 8 per 100 000.14
Oesophageal cancer
There are about 7000 new cases of oesophageal cancer a year in the UK, with an incidence rate of about 11 per 100 000 population. This represents about 11% of all gastrointestinal cancers in the UK, which is higher than the 5.9% in Europe as a whole. Oesophageal cancers mainly occur in people between the ages of 60 and 80, and are three times more common in men than in women.324
The most important risk factor is smoking, although others include severe acid reflux from the stomach, heavy alcohol consumption, obesity, a rare muscular disorder known as achalasia, diet, and chewing of betel nuts. Although the incidence of adenocarcinoma of the oesophagus is increasing in several European countries, squamous cell carcinoma remains the predominant histological type. In Europe, it is estimated that 63% of all squamous cell carcinomas in men and 33% in women are attributable to smoking.328
It is unclear why oesophageal adenocarcinoma is on the increase, although it is thought to be linked to the rise of gastro‐oesophageal reflux disease.21,329 From 1971 to 1997 in England and Wales, age standardised incidence of oesophageal cancer increased by about 50%, from 8 to 12 per 100 000 population in men, and from 4 to 6 per 100 000 in women.14
Pancreatic cancer
In the UK, there are about 6000 new cases of pancreatic cancer a year (about 8% of all gastrointestinal cancers), with an overall incidence rate of about 11 per 100 000 population. Cancers of the pancreas are more common in men than in women, and are predominantly diagnosed in the 50–70 year age group.324
Risk factors include pre‐existing chronic pancreatitis, liver cirrhosis, diabetes and a history of surgery to the upper digestive tract, smoking, family history, and environmental exposure to certain insecticides or chemicals such as gasoline. Chronic pancreatitis is an especially important risk factor for pancreatic cancer, with relative risks as high as 27 having been reported.330
From 1971 to 1997 in England and Wales, age standardised incidence of pancreatic cancer fell by approximately one sixth in men from about 12 to 10 per 100 000 population, but remained stable at about 7 per 100 000 in women.14
Liver cancer
Most liver cancers (about 95%) are metastatic: primary cancer sites in order of frequency are colon and rectum, pancreas, oesophagus, stomach, breast, lung, and kidney.324 There are about 2300 new cases of primary liver cancer a year, with an overall incidence of approximately 4 per 100 000 population. Primary liver cancer accounts for about 3% of all gastrointestinal cancers in the UK, and is more common in men than in women.
Risk factors for primary liver cancer include liver cirrhosis, either of alcoholic aetiology, through hepatitis B or C infection, or through inherited conditions such as haemochromatosis and α1 antitrypsin deficiency, exposure to certain chemicals such as vinyl chloride, smoking, and long term use of anabolic steroids.
Incidence of other gastrointestinal diseases and related conditions
Appendicitis
Appendicitis refers to the inflammation of the appendix when it becomes blocked. The blockage is thought to be caused by a build up of thick mucus within the appendix, or by a stool that enters the appendix from the caecum, or by swollen lymphatic tissue within the appendix. The most common complication of appendicitis is perforation, which is usually caused by a delay in treatment, and which can lead to a periappendiceal abscess or diffuse peritonitis.
Appendicitis can occur at any age, although it is rare in children under 2 years of age. Incidence peaks in late teens and early twenties, it declines with increasing age, and it is higher in men than in women. Appendicitis has also been linked to low fibre and refined carbohydrate diets, amoebiasis, bacterial gastroenteritis, and mumps.
About 10% of the UK population will develop acute appendicitis at some stage, and about 70 000 appendicectomies are performed each year. The incidence of acute appendicitis declined in the UK and in most other Western countries between the 1930s and the early 1990s, and there was a further reduction in hospital admissions for acute appendicitis, of 13% among men and 19% among women in England during the 1990s.331
Obesity
Obesity is not a gastrointestinal disorder but plays a significant part in many diseases of the liver and gut. A recent report by the Royal College of Physicians dealt with the growing epidemic of obesity in the UK and outlined its impact on a number of diseases, including heart disease, diabetes, and gastrointestinal disorders such as gallstones, liver disease, and gastrointestinal cancers.6 Other studies have also documented obesity as a risk factor for a wide range of gastrointestinal diseases, such as colorectal cancer,332,333,334 oesophageal cancer,334,335,336 gastric cancer,337,338 hepatocellular cancer,339,340 gallstone disease,328,333 alcoholic liver disease,341,342,343 non‐alcoholic fatty liver disease,333,339,342,344,345,346 gastro‐oesophageal reflux disease,17 Barrett's oesophagus,347,348 hiatus hernia,349 surgical complications,350,351 and prognosis for acute pancreatitis.352,353
The Royal College of Physicians recommend prevention strategies targeted towards improvements in nutritional labelling of foods, public education, and social marketing and retailing, promotion of leisure‐time sports and activities, NHS priorities and planning, promoting healthy schools, “active transport”, further research and development, and promotion of local level programmes.6
Alcohol related morbidity
Several of the gastrointestinal disorders covered in the previous sections of this report, such as alcoholic liver diseases, upper gastrointestinal haemorrhage from oesophageal varices, acute and chronic pancreatitis, gastric, oesophageal and liver cancer, and gastro‐oesophageal reflux disease are linked to alcohol consumption in varying proportions of cases. Alcohol has often been associated with a wide range of other non‐gastrointestinal diseases and conditions such as injury from traffic accidents, other trauma, violence, suicide, breast cancer, and haemorrhagic stroke, as well as being the direct cause of other disorders such as alcoholic psychoses and alcoholic dependence syndrome.354 In the UK in recent years, there have been reports of increasing numbers of people admitted to general hospitals with alcohol related illnesses, particularly in Scotland.220,355,356 For example, a recent study in Glasgow reported that during one month, 51% of all gastroenterology inpatients had been admitted owing to alcohol related conditions,357 and 65% of these were caused by alcoholic liver disease.
Infectious intestinal diseases and food poisoning
Food poisoning and infectious intestinal disease (IID) are important diseases in the UK. Food poisoning notifications and laboratory reports of pathogens responsible for IID have been falling in the past four years. However, in 2001, there were over 85 000 food poisoning notifications; and 1 in 60 people consulted a GP for IID in England and Wales.358
Defaecation problems
Faecal incontinence, the involuntary loss of rectal contents at a socially inappropriate time or place, is an underappreciated condition, which affects at least 2% of adults in the community. The prevalence in elderly people is up to 15%, and higher still among those living in residential or nursing homes. However, compared with urinary incontinence, the condition is neglected.359 Neurological related bowel problems present a heavy burden on nursing resources,360,361 with diseases or conditions such as multiple sclerosis,362,363,364 Parkinson's disease,365 spina bifida,366 stroke,367 and spinal cord injuries,368,369,370 associated with faecal incontinence or constipation, or both in 50% or more cases.371 The management of constipation alone can account for up to 10% of district nursing time.372
Biliary atresia
Biliary atresia is a disease of unknown cause in which all, or part of, the extrahepatic bile ducts are obliterated, leading to complete biliary obstruction. Biliary atresia is, however, a rare condition with fewer than 50 cases annually in the UK and Ireland.373
Short bowel syndrome (HPN)
Patients with a short small intestine as a result of disease or surgery may need additional feeding. Home parenteral nutrition (HPN) is a complex technology involving the intravenous infusion of all nutrients required for life directly into a central vein. These nutrients include carbohydrates, fat, amino acids, electrolytes, trace elements, and water. The patient, or carer, is taught to manage the complicated routine, enabling transfer of care to the home. Patient referral patterns for HPN treatment are inconsistent, with some regions in the UK having very few patients receiving HPN. However, there are several large centres in the UK where HPN is considered as an essential, life‐saving treatment. The point prevalence of patients receiving HPN in the UK in 2003 was 8.8 per million. Prevalence was higher in Scotland (12.9 per million) than in England (8.6), Wales (4.5), and Northern Ireland (9.6). There is considerable regional variation in period prevalence of patients receiving HPN in the UK: across strategic health authorities in the UK, prevalence varied between 1 and 21 per million population, with higher prevalence reflecting that HPN is more common in areas that are close to major referral centres.374
Iron deficiency anaemia
Iron deficiency anaemia (IDA) resulting from gastrointestinal bleeding is a common feature of many gastrointestinal disorders, including colorectal and gastric cancers. Patients investigated for IDA have been found to have gastrointestinal cancers in about 5–20% of cases,375,376,377 while IDA is also one of the most common presenting symptoms of coeliac disease.378
International comparisons of the incidence of gastrointestinal cancers
Figure 3.2.7 shows estimates of population based incidence rates for the main types of gastrointestinal cancer in the UK and in other regions of Europe in 1995.328 Among men, the UK had the third highest, age standardised incidence of oesophageal cancer in Europe (12.9 per 100 000), after France (17.0) and Hungary (14.9), with an overall rate of 9.9 for the whole of Europe. Among women, the UK had the second highest incidence of oesophageal cancer (5.9 per 100 000) after Ireland (6.6), with an overall rate of 1.9 for Europe.328
Figure 3.2.7. Estimates of age standardised population based incidence rates (per 100 000 population) for the main types of gastrointestinal cancer among men and women in the UK, eastern Europe, northern Europe, southern Europe, western Europe, and in Europe in 1995. (A) For colorectal cancer; (B) for oesophageal cancer; (C) for gastric cancer; (D) for pancreatic cancer. Notes: Western Europe includes Austria, Belgium, France, Germany, Luxembourg, Switzerland, and The Netherlands. Eastern Europe includes Belarus, Bulgaria, Czech Republic, Hungary, Poland, Republic of Moldova, Russia, Slovakia, and the Ukraine. Northern Europe includes Denmark, Estonia, Finland, Iceland, Ireland, Latvia, Lithuania, Norway, Sweden, and the UK. Southern Europe includes Albania, Croatia, Greece, Italy, Macedonia, Malta, Portugal, and Spain. Europe refers to all countries listed above for these four regions. Source: Bray et al, 2002.328
Incidence rates for gastric cancer among men and women in the UK were similar to those in western Europe and in northern Europe, but lower than in eastern Europe, southern Europe and Europe overall. Incidence of gastric cancer was highest in eastern Europe, and probably reflects the relatively low levels of affluence in these countries, and the resulting poor diet of their inhabitants. For both colorectal and pancreatic cancers, incidence in the UK among both men and women was very similar to those in Europe overall.328
Figure 3.2.8 shows incidence rates of colorectal cancers in the UK and in 26 other European countries in 2000. Incidence rates vary greatly across countries among men, although the highest rates were in eastern European states such as the Czech Republic (60.3 per 100 000), Hungary (59.8), and Slovakia (50.6). The rate for the UK (35.4) is similar to the average of these 27 countries (35.9). Among women, there is considerably less variation in national rates, with the UK incidence rate (25.3) similar to the European average of 24.2. The UK ranked as 13th of 27 for highest incidence of colorectal cancer in men, and 11th for women.
Figure 3.2.8 Standardised population based incidence rates (per 100 000 population) for colorectal cancer in 27 different European countries, 2000. Source: Keighley, 2003.324
3.3 Mortality from gastrointestinal diseases
Of 533 329 deaths in England and Wales in 2000, 59 959 (11.2%) had a gastrointestinal disease as the certified underlying cause of death. These include diseases of the digestive system (37% of all deaths from gastrointestinal disease), malignant neoplasms of the digestive system (62%), benign and other neoplasms of the digestive system (0.1%), intestinal infectious diseases (0.9%), and viral hepatitis (0.3%; tables 3.3.1 and 3.3.2).
Table 3.3.1 Population based mortality rates for the different ICD‐9 chapters in England and Wales, 1990 and 2000.
| Underlying cause of death | ICD‐9 chapter | ICD‐9 code | 2000 | 1990 | ||
|---|---|---|---|---|---|---|
| No of deaths | Mortality rate per 100 000 population | No of deaths | Mortality rate per 100 000 population | |||
| Infectious and parasitic diseases: | I | 001–139 | 3 767 | 7.1 | 3 046 | 6.0 |
| Intestinal infectious diseases | 001–009 | 547 | 1.0 | 187 | 0.4 | |
| Viral hepatitis | 070 | 200 | 0.4 | 112 | 0.2 | |
| All other infectious diseases | 010–069, 071–139 | 3 020 | 5.7 | 2 749 | 5.4 | |
| Neoplasms: | II | 140–239 | 134 793 | 254.6 | 144 577 | 285.1 |
| Malignant neoplasms of the digestive system | 150–159 | 37 004 | 69.9 | 40 965 | 80.8 | |
| Benign and other neoplasms of the digestive system | 210, 211, 230, 235.2–235.5 | 74 | 0.1 | 66 | 0.1 | |
| All other neoplasms | 140–149 etc, | 97 715 | 184.6 | 103 646 | 204.2 | |
| Endocrine, nutritional and metabolic disorders, and immunity disorders | III | 240–279 | 7 247 | 13.7 | 10 249 | 20.2 |
| Diseases of blood and blood‐forming organs | IV | 280–289 | 1 791 | 3.4 | 2 427 | 4.8 |
| Mental disorders | V | 290–319 | 10 866 | 20.5 | 13 395 | 26.4 |
| Diseases of the nervous system and sense organs | VI | 320–289 | 9 632 | 18.2 | 11 644 | 23.0 |
| Diseases of the circulatory system | VII | 390–459 | 207 228 | 391.4 | 259 247 | 511.1 |
| Diseases of the respiratory system | VIII | 460–519 | 92 461 | 174.6 | 61 018 | 120.3 |
| Diseases of the digestive system | IX | 520–579 | 22 134 | 41.8 | 18 429 | 36.3 |
| Diseases of the genitourinary system | X | 580–629 | 7 270 | 13.7 | 7 317 | 14.4 |
| Complications of pregnancy, childbirth and the puerperium | XI | 630–676 | 38 | 0.1 | 57 | 0.1 |
| Diseases of the skin and subcutaneous system | XII | 680–709 | 1 266 | 2.4 | 823 | 1.6 |
| Diseases of the musculoskeletal system and connective tissue | XIII | 710–739 | 3 407 | 6.4 | 5 286 | 10.4 |
| Congenital abnormalities | XIV | 740–759 | 1 165 | 2.2 | 1 621 | 3.2 |
| Certain conditions originating to the perinatal period | XV | 760–779 | 83 | 0.2 | 249 | 0.5 |
| Symptoms, signs and ill‐defined conditions | XVI | 790–799 | 13 656 | 25.8 | 4 897 | 9.7 |
| Injury and poisoning | XVII | 800–999 | 16 525 | 31.2 | 17 943 | 35.4 |
| Total gastrointestinal diseases | 001–009,070, 150–159,210, 211,230, 235.2–235.5, 520–579 | 59 959 | 113.3 | 59 759 | 117.8 | |
| All causes of death | I–XVII | 1–999 | 533 329 | 1007.4 | 562 225 | 1108.5 |
Table 3.3.2 Population based mortality rates for different gastrointestinal diseases in England and Wales, 1990 and 2000.
| Underlying cause of death | ICD‐9 code | 2000 | 1990 | ||
|---|---|---|---|---|---|
| No of deaths | Mortality rate per 100 000 population | No of deaths | Mortality rate per 100 000 population | ||
| Diseases of the digestive system: | |||||
| Diseases of oral cavity, salivary glands and jaws | 520–529 | 33 | 0.1 | 18 | 0.0 |
| Oesophagitis | 530.1 | 143 | 0.3 | 110 | 0.2 |
| Other diseases of oesophagus | 530.2–530.9 | 446 | 0.8 | 456 | 0.9 |
| Peptic ulcer | 531–534 | 4 022 | 7.6 | 4 381 | 8.6 |
| Gastritis and duodenitis | 535 | 168 | 0.3 | 96 | 0.2 |
| Other disorders of stomach and duodenum | 536–537 | 103 | 0.2 | 104 | 0.2 |
| Appendicitis | 540–543 | 139 | 0.3 | 148 | 0.3 |
| Hernia of abdominal cavity | 550–553 | 721 | 1.4 | 771 | 1.5 |
| Crohn's disease | 555 | 166 | 0.3 | 190 | 0.4 |
| Ulcerative colitis | 556 | 184 | 0.3 | 190 | 0.4 |
| Vascular insufficiency of intestine | 557 | 1 883 | 3.6 | 1 483 | 2.9 |
| Other non‐infective gastroenteritis and colitis | 558 | 501 | 0.9 | 341 | 0.7 |
| Intestinal obstruction without mention of hernia | 560 | 1 396 | 2.6 | 1 217 | 2.4 |
| Diverticular of intestine | 562 | 1 826 | 3.4 | 1 466 | 2.9 |
| Peritonitis | 567 | 562 | 1.1 | 313 | 0.6 |
| Other diseases of intestines and peritoneum | 564–566,568,569 | 1 134 | 2.1 | 698 | 1.4 |
| Chronic liver disease and cirrhosis | 571 | 4 770 | 9.0 | 3 063 | 6.0 |
| Other disorders of liver | 570,572,573 | 412 | 0.8 | 320 | 0.6 |
| Cholelithiasis, cholecystitis and other disorders of the gallbladder | 574–575 | 784 | 1.5 | 673 | 1.3 |
| Other disorders of biliary tract | 576 | 324 | 0.6 | 261 | 0.5 |
| Acute pancreatitis | 577.0 | 848 | 1.6 | 793 | 1.6 |
| Chronic pancreatitis | 577.1 | 77 | 0.1 | 88 | 0.2 |
| Other diseases of the pancreas | 577.2–577.9 | 37 | 0.1 | 30 | 0.1 |
| Gastrointestinal haemorrhage | 578 | 1 429 | 2.7 | 1 167 | 2.3 |
| Intestinal malabsorption | 579 | 26 | 0.0 | 52 | 0.1 |
| Total diseases of the digestive system | 520–579 | 22 134 | 41.8 | 18 429 | 36.3 |
| Benign and other neoplasms of the digestive system | 210, 211, 230, 235.2–235.5 | 74 | 0.1 | 66 | 0.1 |
| Malignant neoplasms, digestive system: | |||||
| Oesophagus | 150 | 6 061 | 11.4 | 5 259 | 10.4 |
| Stomach | 151 | 5 779 | 10.9 | 8 712 | 17.2 |
| Small intestine | 152 | 269 | 0.5 | 210 | 0.4 |
| Colon | 153 | 9 554 | 18.0 | 11 527 | 22.7 |
| Rectum, rectosigmoid junction and anus | 154 | 4 682 | 8.8 | 5 696 | 11.2 |
| Liver and intrahepatic ducts | 155 | 2 091 | 3.9 | 1 388 | 2.7 |
| Gallbladder and extrahepatic ducts | 156 | 527 | 1.0 | 813 | 1.6 |
| Pancreas | 157 | 6 105 | 11.5 | 6 145 | 12.1 |
| Retroperitoneum and peritoneum | 158 | 186 | 0.4 | 192 | 0.4 |
| Other and ill‐defined sites, digestive system | 159 | 1 750 | 3.3 | 1 023 | 2.0 |
| Total malignant neoplasms, digestive system | 150–159 | 37 004 | 69.9 | 40 965 | 80.8 |
| Intestinal infectious diseases: | |||||
| Cholera | 001 | 0 | 0.0 | 0 | 0.0 |
| Typhoid and paratyphoid fevers | 002 | 0 | 0.0 | 2 | 0.0 |
| Other salmonella infections | 003 | 13 | 0.0 | 68 | 0.1 |
| Shigellosis | 004 | 0 | 0.0 | 0 | 0.0 |
| Other food poisoning (bacterial) | 005 | 1 | 0.0 | 1 | 0.0 |
| Amoebiasis | 006 | 1 | 0.0 | 0 | 0.0 |
| Other protozoal intestinal diseases | 007 | 1 | 0.0 | 4 | 0.0 |
| Intestinal infections due to other organisms | 008 | 452 | 0.9 | 43 | 0.1 |
| Ill‐defined intestinal infections | 009 | 79 | 0.1 | 69 | 0.1 |
| Total intestinal infectious diseases | 001–009 | 547 | 1.0 | 187 | 0.4 |
| Viral hepatitis | 070 | 200 | 0.4 | 112 | 0.2 |
| Total gastrointestinal diseases | 001–009,070, 150–159,210, 211,230,235.2–235.5,,520–579 | 59 959 | 113.3 | 59 759 | 117.8 |
Diseases of the digestive system, which exclude gastrointestinal neoplasms and infectious diseases, ranked as the fourth ICD chapter that accounted for most deaths in England and Wales in 2000, after diseases of the circulatory system (207 228 deaths), neoplasms (134 793), and diseases of the respiratory system (92 461).
Figure 3.3.1 shows population based mortality rates for each major body system when deaths from cancer were allocated to their respective body systems; for example, when gastrointestinal cancers were included with diseases of the digestive system, when respiratory cancers were included with diseases of the respiratory system, etc. Then, gastrointestinal disease was the third body system that accounted for the most deaths (59 000), after circulatory diseases (207 000) and respiratory diseases (123 000). Among people aged 15–64 years, however, the mortality rate for gastrointestinal diseases was roughly equal to that from respiratory diseases, as the leading major cause of death after circulatory diseases among working aged people (fig 3.3.2).
Figure 3.3.1 Population based mortality rates for major disease groupings, in England and Wales, 2000: people of all ages. Source: ONS, 2001.379
Figure 3.3.2 Population based mortality rates for major disease groupings, in England and Wales, 2000: people aged 15–64 years. Source: ONS, 2001.379
The number of deaths from diseases of the digestive system in 2000 increased by 20% from 18 429 in 1990. Deaths from malignant neoplasms of the digestive system fell by 10% from 40 965 in 1990, and the small numbers of deaths from intestinal infectious diseases and from viral hepatitis respectively, almost trebled and increased by 80% from 1990 to 2000 (table 3.3.2).
The major causes of death from diseases of the digestive system, excluding gastrointestinal cancers, in 2000 were liver cirrhosis (22%), peptic ulcer (18%), vascular insufficiency of the intestine (9%), diverticular disease of the intestine (8%), and gastrointestinal haemorrhage (6%; fig 3.3.3).
Figure 3.3.3 Percentage causes of all deaths from diseases of the digestive system (excluding cancers) in England and Wales, 2000. Source: ONS, 2001.379
Gastrointestinal cancer is the most common cause of cancer death of all major cancer groupings. In England and Wales in 2000, gastrointestinal cancers caused 27% of all cancer deaths, followed by respiratory cancers (23%) and cancers of the genitourinary system (17%; fig 3.3.4). The gastrointestinal tract was also the most common site for all cancer deaths (fig 3.3.5).
Figure 3.3.4 Percentage causes of all deaths from cancer, according to major groupings of cancer, in England and Wales, 2000. Source: ONS, 2001.379
Figure 3.3.5 Percentage causes of all cancer deaths, according to the site of the cancer, in England and Wales, 2000. Source: ONS, 2001.379
Figure 3.3.6 shows the most common sites for all gastrointestinal cancer deaths. These were the colon and rectum (39% of all gastrointestinal cancer deaths), the pancreas, the oesophagus, and the stomach (16% each).
Figure 3.3.6 Percentage causes of all gastrointestinal cancer deaths, according to the site of the cancer, in England and Wales, 2000. Source: ONS, 2001.379
Table 3.3.3 shows the number of deaths and corresponding population based mortality rates in England and Wales in 2000 among working aged people (aged 15–64 years) for some of the most common diseases and causes of death in the general population. The mortality rate for diseases of the digestive system (16.3 per 100 000 population) was lower than that from all cancers (97.6) and from ischaemic heart disease (43.4), but was about twice as high as for stroke and for pneumonia, six times higher than for diabetes mellitus, and 12 times higher than for asthma. If gastrointestinal cancers are included with diseases of the digestive system as gastrointestinal diseases, the corresponding mortality rate (39.9) was only slightly lower than for ischaemic heart disease, and much higher than for stroke, pneumonia, diabetes mellitus, chronic airways obstruction, and asthma (fig 3.3.7).
Table 3.3.3 Population based mortality rates for selected causes of death among people aged 15–64 years in England and Wales, 2000.
| Cause of death | ICD‐9 code | No of deaths | Mortality rate per 100 000 population |
|---|---|---|---|
| Diseases of the digestive system | 520–579 | 5 488 | 16.3 |
| Diabetes mellitus | 250 | 864 | 2.6 |
| Ischaemic heart disease | 410–444 | 14 567 | 43.4 |
| Stroke | 431–434, 436 | 2 375 | 7.1 |
| Pneumonia | 480–486 | 2 879 | 8.6 |
| Asthma | 493 | 437 | 1.3 |
| Chronic airways obstruction | 496 | 1 700 | 5.1 |
| Neoplasms | 140–239 | 32 795 | 97.6 |
Source: ONS, 2001.379
Figure 3.3.7 Population based mortality rates (per 100 000 population) for selected causes of death among people aged 15–64 years in England and Wales, 2000. *Gastrointestinal diseases include diseases of the digestive system, malignant neoplasms of the digestive system, benign and other neoplasms of the digestive system, intestinal infectious diseases, and viral hepatitis. Source: ONS, 2001.379
Mortality statistics, based on underlying cause of death, to some extent underreport true mortality from gastrointestinal diseases and, importantly, this underreporting is greater for gastrointestinal diseases than for the two other major causes of death, circulatory and respiratory diseases. The following sections describe mortality rates and patterns in the UK for some of the main gastrointestinal disorders in anatomical sequence.
Mortality from diseases of the stomach and duodenum
Peptic ulcer
Although the incidence of peptic ulcer has fallen sharply in the UK in recent years, it was still the second largest cause of gastrointestinal death, after liver cirrhosis, in England and Wales in 2000, with over 4000 deaths and a mortality rate of 7.6 per 100 000 population (table 3.3.2).
Patient deaths after hospital admission for peptic ulcer in the UK between 1991 and 1994 was reported as 4.4%,381 with increased risks of mortality for patients who had no previous history of peptic ulcer (relative risk = 3), or who were undergoing surgery, were elderly or were current users of non‐steroidal anti‐inflammatory drugs. Much higher case fatality rates of 34% for perforated peptic ulcer,382 and 43% for perforated duodenal ulcer,383 have been reported in regional British studies.
Gastrointestinal haemorrhage
Gastrointestinal haemorrhage was the cause of almost 1500 deaths in England and Wales in the year 2000, with a population based mortality rate that had risen by over 17% since 1990 (table 3.3.2).
Patient deaths for upper gastrointestinal haemorrhage vary in the UK from about 5% to 15% (table 3.3.4), although lower rates of less than 4% have been reported.89,90 Case fatality varies strongly according to case mix, which would explain some of the geographical variation; while, as ever, case fatality is affected by factors such as the length of follow‐up and the inclusion of deaths after discharge with in‐hospital deaths.
Table 3.3.4 Case fatality rates (% of cases) after hospital admission for upper gastrointestinal haemorrhage, as reported from regional studies in the UK.
| City/region | Study period | No of cases | Case fatality rate (%) | Authors and reference |
|---|---|---|---|---|
| NW London | 1940–1947 | 687 | 9.9 | Jones AF, 1947384 |
| Aberdeen | 1941–1948 | 476 | 13.9 | Needham CD and McConachie JA, 1950385 |
| London | 1947–1958 | 325 | 13.0 | Coghill NF and Willcox RG, 1960386 |
| Oxford | 1953–1967 | 2149 | 8.9 | Schiller KF et al, 197084 |
| Birmingham | 1963–1974 | 158 | 12.0 | Hoare AM, 1975387 |
| NE Scotland | 1967–1968 | 817 | 13.7 | Johnston SJ et al, 197386 |
| Birmingham | 1971–1973 | 300 | 9.7 | Allan R and Dykes P, 1976388 |
| Cardiff | 1972–1978 | 583 | 10.3 | Mayberry JF et al, 1981389 |
| Bristol | 1974–1976 | 267 | 4.4 | Brown SG et al, 1981390 |
| West Lothian | 1980–1983 | 326 | 11.7 | Clason AE et al, 1986391 |
| Newport, Gwent | 1980–1981 | 330 | 15.2 | Madden MV and Griffith GH, 198587 |
| Oxford | 1981–1982 | 125 | 4.8 | Berry AR et al, 198485 |
| Bath | 1981–1985 | NA | 10–12 | Holman RA et al, 199089 |
| North London | 1986 | 292 | 4.8 | Sanderson JD et al, 1990392 |
| Bath | 1986–1988 | 430 | 3.7 | Holman RA et al, 199089 |
| Nottingham | 1986–1989 | 1147 | 6.1 | Daneshmend TK et al, 1992393 |
| Bridgend | 1990 | 109 | 4.6 | Clements D et al, 1991394 |
| NE Scotland | 1991–1993 | 1098 | 3.9 | Masson J et al, 199690 |
| 4 Health Regions in SE England and Midlands | 1991–1993 | 4185 | 14 | Rockall TA et al, 199591 |
| West of Scotland | 1992–1993 | 1882 | 8.1 | Blatchford O et al, 199792 |
| Newport, Gwent | 1993–1995 | 524 | 9.4 | Kapur KC et al, 1998395 |
| Sheffield | 1995–1998 | 900 | 8.1 | Sanders DS et al, 2004396 |
| Sutton Coldfield | 2002–2003 | 716 | 14.6 | Lim CH et al, 2006397 |
Case fatality for upper gastrointestinal haemorrhage is increased in surgical cases, and for cases of haemorrhages in inpatients. For example, surgical mortality rates of 13–41% have been reported from studies since the 1980s,84,85,89 while case fatality for haemorrhages in inpatients of 18–45% have also been reported.73,74,86,87,90,91,334,335,387 Other important risk factors include gastrointestinal malignancies or other pre‐existing comorbidity, shock, and advanced age.92,391,398 Despite improvements in treatment and management over time, a lack of impact on patient deaths is probably linked to older ages at presentation, increases in comorbidities,399 and less selective reporting over time.400
Mortality from diseases of the small bowel and colon
Inflammatory bowel disease
Inflammatory bowel disease is a major cause of debilitating morbidity, particularly among young adults, rather than a major cause of mortality. In the year 2000 in England and Wales, there were only 166 and 184 deaths, respectively, which were certified with Crohn's disease and ulcerative colitis as the underlying causes of death.
Most British population based studies have found no increased mortality among people with inflammatory bowel disease. For example, a study in Leicestershire reported standardised mortality ratios (SMRs) of 0.72 (compared to a mortality of 1.00 in the general population) for Crohn's disease,401 and 0.93 for ulcerative colitis,402 among European subjects; another study of three district hospital general centres reported SMRs of 0.94 for Crohn's disease and 0.93 for ulcerative colitis.403
However, some population based studies have reported increased mortality. For example, a study of Crohn's disease in Cardiff from 1934 to 1976 reported a significantly increased SMR of 2.2, that was particularly high in people aged under 20 (SMR of 11.0).404 Another study, a national UK primary care based study during the 1990s, reported significantly increased hazard ratios of 1.7 for Crohn's disease and 1.4 for ulcerative colitis. The hazard ratios were more highly increased among younger age groups: 3.8 among people aged 20–39 years with Crohn's disease, and 1.8 among those aged 40–59 with ulcerative colitis.405
Coeliac disease
Although coeliac disease is not usually recorded as an underlying cause of death, people with coeliac disease have been shown to be at moderately increased risks of mortality. For example, cohort studies in Scotland, Italy, and Sweden have reported increased mortality of 1.9‐ to 3.8‐fold respectively,406,407,408,409 with excess mortality often caused by malignant lymphomas or malignancies of the gastrointestinal tract.406,407,408,409,410
Diverticular disease
Diverticular of the intestine is quite a common cause of death in the UK, accounting for 1826 deaths in England and Wales in 2000. Population based mortality rates for diverticular disease increased greatly over the course of the 20th century, although this probably reflects an increase in the use of barium enema diagnostic testing and changing fashions of death certification, as well as a true increase in the prevalence of diverticular disease. From 1979 to 1999, age standardised population based mortality rates remained fairly constant in England at about 1.5 per 100 000 population in men and 2.25 per 100 000 in women.212
Mortality rates after hospital admission for diverticular disease are fairly low. From 1989–90 to 1990–2000 in England, age standardised in‐hospital case fatality rates were about 2.5% and 3.5% among men and women respectively,212 while a recent study in London reported case fatality of 9.5% at one year after admission.411
Higher mortality is associated with the severe complications such as perforated diverticular.203 For example, a recent study in Exeter reported a case fatality rate of 5.7% for acute complications of diverticular disease, which rose to 18% for those undergoing surgery412; a study in Birmingham reported case fatality of 11% for acute complications of diverticular disease from 1985–88413; and a study in Glasgow reported surgical mortality of 26% for perforated diverticular disease from 1976 to 1983.414
Mortality from diseases of the liver
Chronic liver disease and cirrhosis
Chronic liver disease and cirrhosis is one of the major causes of death from gastrointestinal disease in the UK. Cirrhosis mortality increased by 50% in England and Wales from 6 to 9 per 100 000 population during the 10 year period from 1990 to 2000 (table 3.3.2). From 1957–61 to 1997–2001 it increased by over threefold among men in England and Wales and in Scotland, by 250% among women in England and Wales, and by 160% among women in Scotland.415 Other studies have reported increases of 350% in England from 1970 to 1998,416 and 112% in the West Midlands from 1993 to 2000.417
This contrasts with a fall of almost 30% in the EU average cirrhosis mortality rate of 14 to 10 per 100 000 from 1970 to 1998. Together with a rise in national alcohol consumption,320,321 and in hospital admissions for alcoholic liver disease,220 the increase in cirrhosis mortality in the UK has led to the recent publication of a national alcohol harm reduction strategy.221
In recent years, the large increases in the number of people infected with the hepatitis C virus, who have a rapid progression of liver cirrhosis,234 and a poor outcome,418 have also contributed towards the increase in cirrhosis mortality.234 Hepatitis C infection has also been the subject of national strategy and action plan documents in England.228,232
Mortality after hospital admission with chronic liver disease and cirrhosis is extremely high, and does not appear to have improved in the past 40 years.217,419 Mortality varies greatly according to aetiology: case fatality rates of 40% for alcoholic cirrhosis and 17% chronic hepatitis were reported from an earlier study of west Birmingham in 1959–76.217 Mortality from chronic liver disease and cirrhosis is still relatively low compared with that in many other European countries (fig 3.3.8). However, while cirrhosis mortality rates have been falling in most European countries in recent years, there has been a sharp increase in mortality in the UK (fig 3.3.9).
Figure 3.3.8 Standardised mortality rates (per 100 000 population) for chronic liver disease and cirrhosis, in men and women in the UK and in 17 other European countries, 2000. Source: Burroughs and McNamara, 2003.227
Figure 3.3.9 Trends in standardised mortality rates (per 100 000 population) for chronic liver disease in the UK and in Europe, 1970–1998. Source: Department of Health.420
Gallstone disease
Cholelithiasis, cholecystitis, and other diseases of the gallbladder were the cause of almost 800 deaths in England and Wales in 2000. Age standardised mortality for cholelithiasis fell from about 8.5 to 5.5 per 100 000 population in England from 1979 to 1989, but has not fallen since.271
Case fatality after hospital admission for gallstones fell by one third in men (from 0.6% to 0.4%) and by 42% in women (from 0.5% to 0.3%) in England from 1989–90 to 1999–2000.271 Although death rates after admission for gallstones are low, reported risk factors include acute pancreatitis, liver cirrhosis, age, acute cholecystitis, and diabetes.421
Mortality from diseases of the pancreas
Acute pancreatitis
Acute pancreatitis was the underlying cause of about 850 deaths in England and Wales in 2000. Population based mortality for acute pancreatitis in England and Wales increased slightly from 1.56 per 100 000 population in 1990 to 1.60 in 2000 (table 3.3.2), which had increased from 1.37 per 100 000 in 1980. A slightly lower mortality rate of 1.23 per 100 000 population was reported for Northern Ireland in 1974–83,422 while mortality increased from 2.7 to 4.0 per 100 000 in Nottingham from 1969 to 1983.423
Almost all people with acute pancreatitis are admitted to hospital. Case fatality has fallen over time from about 30% to roughly 10%, although as there seems to have been little further improvement in recent years, it remains a lethal disease. Case fatality at one year after admission for acute pancreatitis fell only slightly in four counties of southern England from 13.5% in 1963–74 to 11.8% in 1987–98.303 However, it appears to have fallen more sharply in Scotland; with reported reductions from 17.6% in 1961–65 to 5.6% in 1981–85,305 and from 9.1% to 6.6% from 1984 to 1994.306
Other British studies have reported case fatality of 9.1% in the Wessex region in 1994–95,288 9.0% in the North West Thames region in 1988–92,424 6.3% in Somerset in 1991–95,290 5.4% in Nottingham in the late 1990s,425 and 17% in Cottingham in 1998.426 Reported mortality rates for acute pancreatitis in England have been comparable or slightly higher than those in Europe. For example, case fatality was 6.1% in Luneberg, Germany from 1980 to 1994,427 5% in an Italian multicentre study in 1996–2000,428 7.5% in north Jutland from 1981 to 2000,308 and 10.7% in the Netherlands in 1995.310
Prognosis depends strongly on disease severity, with much higher case fatality in severe cases; which can be as high as 50% for surgery or for infected pancreatic necrosis.283,429 For example, two Scottish studies reported case fatality of 38% and 43% for pancreatic necrosectomy,430,431 and a study in London reported mortality of 39% for severe cases.432 The Italian multicentre study reported case fatality that varied between 1.7% for mild acute pancreatitis and 17% for severe cases,428 a Swedish study reported mortality of 27% in severe cases,433 while a German study reported mortality of 17% for necrotising pancreatitis, compared with 5% overall.434
Chronic pancreatitis
Although chronic pancreatitis is rarely recorded as an underlying cause of death—in only 77 cases in England and Wales in 2000—it often leads to substantially increased risks of mortality. For example, an American study identified an SMR of 3.6 for people who underwent treatment for chronic pancreatitis, with prognosis influenced strongly by age at diagnosis, alcohol consumption, and smoking.435 In particular, chronic pancreatitis often leads to increased risks of pancreatic cancer,330,436,437,438,439,440 which carries a very poor prognosis.
Mortality from gastrointestinal cancers
Colorectal cancer
Colorectal cancer is the most common cause of death from gastrointestinal cancer, causing 39% of all gastrointestinal cancer deaths, and 11% of all cancer deaths, in England and Wales in 2000. There were over 14 000 deaths from colorectal cancers in England and Wales in 2000, with a population based mortality rate of 27 per 100 000, which has fallen in recent decades.14
Prognosis for colorectal cancer is substantially better than for most other gastrointestinal cancers. Five year survival rates after diagnosis with colorectal cancer were 35% for men, and 39% for women in England and Wales between 1996 and 1999.441 These had increased from 31% and 35% respectively, in 1991–95. Over 80% of people with colorectal cancers in Europe undergo surgical treatment, and five year survival after surgical resection ranges from 40% to 60% depending on the stage of the tumour.324
Survival rates for colorectal cancer in the UK have been rising steadily over the past three decades, but substantial international differences suggest that there is considerable scope for further improvement: five year survival in the UK is lower than in Europe as a whole (table 3.3.5). The contrast in survival for the UK and western Europe is particularly marked for colon cancer, which often presents in an advanced state as an emergency, with a relatively poor prognosis. This further indicates that the poor survival in the UK has been mainly due to late diagnosis. Some studies have linked late diagnoses in the UK to patients' GP consultation behaviour: for example, while rectal bleeding is a common symptom that affects up to 15% of adults,43 and is often an important symptom of colon cancer,442,443 many patients don't seek medical advice.444
Table 3.3.5 Percentage five year survival after diagnosis for the main types of gastrointestinal cancers in various European countries.
| Country | Gastrointestinal cancer | ||||
|---|---|---|---|---|---|
| Oesophageal (%) | Gastric (%) | Pancreatic (%) | Colorectal (%) | Liver (all cases) (%) | |
| Austria | 14 | 28 | 9 | 49 | 11 |
| Denmark | 5 | 14 | 2 | 41 | 1 |
| Estonia | 3 | 19 | 1 | 39 | 2 |
| Finland | 8 | 21 | 3 | 49 | 4 |
| France | 9 | 25 | 8 | 50 | 8 |
| Germany | 8 | 27 | 4 | 48 | 6 |
| Iceland | 25 | 24 | 3 | 52 | 9 |
| Italy | 8 | 24 | 4 | 37 | 4 |
| Netherlands | 12 | 20 | 2 | 55 | 0 |
| Poland | 3 | 11 | 4 | 25 | 3 |
| Slovakia | 8 | 19 | 8 | 39 | 5 |
| Slovenia | 3 | 16 | 3 | 35 | 0 |
| Spain | 9 | 28 | 5 | 48 | 10 |
| Sweden | 14 | 17 | 3 | 51 | 4 |
| Switzerland | 15 | 24 | 2 | 52 | 3 |
| UK | 9 | 12 | 3 | 41 | 4 |
| Average of the 16 European countries | 9.6 | 20.6 | 4.0 | 44.4 | 4.6 |
Source: Keighley, 2003.324
Survival rates are also lower in Europe than in the USA.445 This has also been attributed to diagnoses at earlier stages in the USA, as well as a higher proportion of cancers in the USA that are coded as adenocarcinoma in polyp, and which have a better prognosis.445
Oesophageal cancer
Cancers of the oesophagus represented over 6000 deaths in England and Wales in 2000, or 4.5% of all cancer deaths, with a mortality rate that has increased in recent decades.14
Five year survival after diagnosis with oesophageal cancer in the UK (9%) is slightly lower than a European average of 9.6% (table 3.3.5). The poor prognosis is largely due to the spread of tumours from the wall of the gullet, by the time of diagnosis. In Europe, only about one quarter of all oesophageal cancers are operable and, of these, five year survival is only about 20–30%.324
Gastric cancer
Cancers of the stomach are also responsible for 4.5% of all cancer deaths in the UK. In England and Wales in 2000, there were a total of 5779 deaths from gastric cancers, with a corresponding mortality rate of 10.9 per 100 000 population, which has fallen over time.14
The five year survival of about 12% in the UK is much lower than a European average of 21% (table 3.3.5). In Europe, only about 60% of gastric cancers are resectable when first diagnosed and surgical resection for cure is only achieved in about 40% of cases. Five year survival after surgical resection is closely related to the spread of the tumour, and varies from 95% for early cancers to only 20% for extensive lesions.324
Pancreatic cancer
Cancer of the pancreas also caused 4.5% of all cancer deaths in England and Wales in 2000 (6105 deaths), with a population based mortality rate of 11.5 per 100 000 that has remained fairly stable since the 1970s.14
Prognosis after diagnosis remains extremely poor. Survival is about 2% at five years among both men and women in England and Wales (table 3.3.5).441 A small minority (about 7%) of pancreatic cancers occur around the distal end of the bile and pancreatic ducts, present early and have relatively good prognosis. The rest, however, are located in the main body of the pancreas, present late and have dismal prognosis. In Europe, only 10% of pancreatic cancers are resectable, and the overall postoperative five year survival rate is only 10–15%.324 Prognosis in the UK is slightly worse than in the rest of Europe (table 3.3.5).
Liver cancer
Liver cancer caused 2091 deaths in England and Wales in 2000 and mortality has been increasing since the 1960s. Age standardised mortality rates per 100 000 population increased from 1.29 to 1.93 in women, and from 2.56 to 3.70 in men, between 1968 and 1996.446
Prognosis for liver cancer is also extremely poor (table 3.3.5). Five year survival in the UK was recently reported as 4%.324 This is largely because 95% of liver cancers are secondary deposits from tumours located elsewhere. Prognosis is slightly worse than the European average of 4.6% (table 3.3.5).
International comparisons of gastrointestinal cancer
Figure 3.3.10 shows population based mortality rates for each of the four main types of gastrointestinal cancer in the UK and, for comparison, with corresponding age standardised rates in other regions of Europe. Mortality from cancer of the oesophagus is particularly high in the UK (fig 3.3.10B), among both men and women, and it is higher than that in all other European countries presented here, except France (for men) and Ireland (for women).
Figure 3.3.10 Estimates of age standardised population based mortality rates (per 100 000 population) for different gastrointestinal cancers among men and women in the UK, eastern Europe, northern Europe, southern Europe, western Europe, and in Europe, 1995. (A) For colorectal cancer; (B) for oesophageal cancer; (C) for gastric cancer; (D) for pancreatic cancer. Notes: Western Europe includes Austria, Belgium, France, Germany, Luxembourg, Switzerland, and the Netherlands. Eastern Europe includes Belarus, Bulgaria, Czech Republic, Hungary, Poland, Republic of Moldova, Russia, Slovakia, and the Ukraine. Northern Europe includes Denmark, Estonia, Finland, Iceland, Ireland, Latvia, Lithuania, Norway, Sweden, and the UK. Southern Europe includes Albania, Croatia, Greece, Italy, Macedonia, Malta, Portugal, and Spain. Europe refers to all countries listed above for these four regions. Source: Bray et al, 1997.328
Mortality from gastric cancer, which is particularly high in eastern Europe (fig 3.3.9C), is substantially lower in the UK than in the rest of Europe. Death rates from colorectal cancer in the UK are similar to the European average, while mortality from pancreatic cancers in the UK is about average in women, but slightly lower in men.
Figure 3.3.11 shows large variation in mortality rates for colorectal cancer among men across 27 different European countries in 2000. Highest colorectal cancer mortality is found in eastern European countries such as the Czech Republic, Hungary, and Slovakia, with the UK mortality rate of 18.7 per 100 000 population similar to the European average of 19.1. Among women, there is much less variation, with the UK again similar to the European average.
Figure 3.3.11 Population based mortality rates (per 100 000 population) for colorectal cancer in the UK and in 26 other European countries, 2000. Source: Keighley, 2003.324
Figure 3.3.12 shows incidence to mortality ratios for colorectal cancers in the 27 European countries in 2000. The highest mortality ratios among men were in Lithuania (0.72), Latvia (0.70), and Denmark (0.61), and among women in Latvia (0.70), Iceland, and Lithuania (both 0.63). Mortality ratios in the UK, 0.55 and 0.53 for men and women, respectively, were similar to the corresponding European averages.
Figure 3.3.12 Mortality:incidence ratios for colorectal cancer in the UK and in 26 other European countries, 2000. Source: Keighley, 2003.324
3.4 Morbidity, quality of life
Although the data for mortality and activity in hospital and primary care are relatively reliable, they do not describe the burden of chronic GI diseases on the lives of sufferers. Several common chronic conditions—gastro‐oesophageal reflux disease (GORD), non‐ulcer dyspepsia, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD)—have mortality rates that are similar to those of the general population.447 Consulting rates vary, with some people more likely to opt for self care or alternative complementary therapies.53 Activity data reflect the burden on the health service, therefore, more than on the population.
Objective evidence or clinical assessment and self reported symptoms do not match well.20 Because of this there has been an increasing focus in health care generally, and in gastroenterology, in particular, on assessing patients' health related quality of life (HRQoL). Measurements of HRQoL can be used to identify problems of individual patients or populations, to enhance understanding of diseases, and to assess health technologies, treatments, and service delivery.448
Using self reported HRQoL, the prevalence of functional GI disorders in a population in Australia was found to be 34.6%.449 Sufferers were found to be more likely to have impaired mental health and physical functioning, measured by the SF12, an effect which was intensified amongst those who sought treatment. Halder et al emphasised the confounding effect of the psychological state, and suggested that some of the association between IBS/dyspepsia and HRQoL can be explained by psychological factors.450 Gastrointestinal symptoms in the elderly were found to be common in a study in Minnesota, with chronic constipation and chronic diarrhoea having prevalences of 24% and 14%, respectively. Faecal incontinence more than once a week was reported in 3.7%. IBS was estimated from reported symptoms to be a condition for 10.9%. Only 23% had seen a physician during the previous year, and attendance did not correlate well with symptom reporting.53
Borgaonkar and Irvine's review of HRQoL measures for GI diseases summarised research into the impact of chronic GI disorders on the quality of life of patients.447 HRQoL measures can be global, generic or disease‐specific. Disease‐specific tools have been developed to measure HRQoL for patients in each of the disease groupings below.
Symptoms of GORD occur in about 40% of adults each month, and in 7% daily. Symptoms such as heartburn, regurgitation, and chest pain substantially impair HRQoL and over half of patients require treatment. Patients with GORD were reported to feel as seriously affected as patients with cardiovascular disease, with SF36 physical functioning scores worse than for patients with acute myocardial infarction, and social function scores lower than for patients with congestive heart failure.
Dyspepsia occurs in 25% of the general population, with patients reporting considerable anxiety, abdominal pain, interruption of daily activities, and decreased sexual drive.
Irritable bowel syndrome is a commonly experienced disorder, with a prevalence of up to 22%. Sufferers report abdominal pain, altered bowel habit, and disturbed sensory and motor function, as well as symptoms elsewhere in the body—back pain, headache, dyspareunia, urinary symptoms, and sleep disturbances. People with IBS have significantly poorer SF36 scores than healthy controls, and patients have difficulty travelling, playing sports, and attending social events. Sufferers take time off work and finish their working lives at a young age.
Patients with IBD have been shown to have impaired HRQoL compared with healthy controls in physical, emotional, and social function. Family members and clinicians tend to underestimate the effects on patients compared with self reported health status. The most common problems reported are loose or frequent stools, abdominal pain, worries about disease flares, cancer or the need for surgery, and social restrictions. Eighty per cent of sufferers can maintain employment.
Anorectal disorders affect 4% of the population. Patients with anal fissure, constipation, or incontinence have all been reported to record depressed HRQoL life scores.
GI cancers account for 20% of all newly diagnosed cancers. Many do not respond to treatment and require palliative care. Patients experience side effects of treatment such as nausea, vomiting, pain, and fatigue, in addition to the symptoms directly caused by the cancer.
Patients with hepatitis C were found to record lower SF36 scores than those with hepatitis B across the dimensions of social functioning, physical role limitation, and energy and fatigue, although both groups displayed lower scores than healthy controls.
Overall the burden of GI disease on HRQoL in the general population is not well described, although there are efforts to assess impact in some conditions in studies carried out in various locations. Standardised measures for specific diseases are being developed and validated, which will help to understand and describe the burden and assess treatments and models of care.
3.5 Geographical variation
Peptic ulcer
The incidence of peptic ulcer has been higher in Scotland and in the north of England than further south,66,92 to some extent because of a higher prevalence of the Helicobacter pylori infection in the north.451 In primary care, the prevalence of peptic ulcer has also been reported as two to three times higher in the north of England than in the south.71
Gastrointestinal haemorrhage
The incidence of upper gastrointestinal haemorrhage is higher in Scotland and in the north of England than further south. High incidence rates of upper gastrointestinal haemorrhage have been reported in the west of Scotland (172 per 100 000 in 1992/93),92 and Aberdeen (117 per 100 000),90 compared with the lower rates of 107 for Trent, 102 for the West Midlands, 99 for South West Thames and 91 for North West Thames.91
Inflammatory bowel disease
Regional studies of the incidence of Crohn's disease in the UK show little systematic geographical variation (table 3.2.2). However, the highest incidence rates for ulcerative colitis have been reported in northern regions such as north east Scotland,134 and north Tees.135,136 The incidence of juvenile onset Crohn's disease has been reported as 50% higher (p<0.001) in northern Scotland than in southern Scotland during 1981–95, although no significant difference was found for ulcerative colitis.452
Alcoholic liver disease
There seems to be a substantially higher incidence of alcoholic liver disease in Scotland than in England. For example, in 1999–2000 the hospital admission rate for alcoholic liver disease in Scotland, 75.2 per 100 000 population,215 was about 2.5 times higher than the corresponding rate in England during the four year period, 1999–2000 to 2001–02, 31.4 per 100 000.453
Hepatitis B and C infection
Reported diagnoses of hepatitis B and C infections have been shown to vary geographically throughout the UK (fig 3.2.4). In particular, the incidence of both infections since the early 1990s has been highest in Scotland, with rates about four times higher than in the rest of the UK. The lowest rates of reported hepatitis B infections were in Wales, and the lowest rates for hepatitis C were in Northern Ireland.229
Primary biliary cirrhosis
Some of the highest prevalence rates for primary biliary cirrhosis in the world have been reported for northern England: 34.5 per 100 000 population,238 and 24 per 100 000.243 Relatively high rates of 20 and 9 per 100 000 have been reported for south Wales,244 and the west of Scotland.242
Acute pancreatitis
Reported incidence rates for acute pancreatitis, which is sometimes associated with heavy alcohol consumption, are normally substantially higher in Scotland,287,305,306 than in England.285,288,303,304
Gastrointestinal cancers
Figure 3.5.1 shows incidence rates among men and women, respectively, for the main types of gastrointestinal cancer in England, Scotland, Wales, and Northern Ireland during the period 1991–2002. Among both men and women, the incidence of colorectal cancer is lowest in England, oesophageal cancer is most common in Scotland, gastric cancer is most common in Wales among men, and pancreatic cancer shows little cross‐national variation in incidence among men, but a substantially reduced incidence rate in Northern Ireland among women.
Figure 3.5.1 Standardised incidence rates (per 100 000 population) for colorectal, gastric, oesophageal, and pancreatic cancers in England, Scotland, Wales, and Northern Ireland, for the period 1993–2001. (A) For men; (B) for women. Notes: Incidence rates are directly standardised to the standard European population. Sources: England: National Cancer Intelligence Centre, Office for National Statistics; Scotland: Information and Statistics Division, NHS in Scotland; Wales: Welsh Cancer Surveillance and Intelligence unit; Northern Ireland: Northern Ireland Cancer Registry.215,454,455,456
For colorectal cancer, there is evidence of a north‐south gradient in incidence among men in Scotland, with the highest incidence rates in the Shetlands, Highlands, the Grampian region, and the north of Scotland, but less of a geographical trend for women (fig 3.5.2). In Wales there is little geographical pattern in the incidence of colorectal cancer in either men or women (fig 3.5.3). Importantly, the incidence rates in Scotland and Wales are standardised using different standard populations, so no direct comparison of rates can be made across countries.
Figure 3.5.2 Incidence rates for colorectal cancer among men and women in different regions of Scotland, 1992–2001. Incidence rates are directly standardised to the standard European population. Source: Information and Statistics Division, NHS in Scotland.215
Figure 3.5.3 Incidence rates for colorectal cancer among men and women in different Welsh unitary authorities, 1992–2001. Incidence rates are directly standardised using the Welsh population. Source: Welsh Cancer Surveillance and Intelligence unit.455
Figure 3.5.4 shows incidence rates for the main types of gastrointestinal cancer in different regions of England during the calendar year 2001. For gastric cancer, there is a clear north‐south gradient, with incidence highest in the north and lowest in the south. For colorectal cancer, incidence among men is lowest in London and the south east and highest in the north and the south west, while for women there appears to be no clear pattern. Similarly for oesophageal and pancreatic cancers, no clear pattern is evident. Mortality to incidence ratios for each of the main types of gastrointestinal cancer also show little geographical pattern in England (fig 3.5.5).
Figure 3.5.4 Incidence rates (per 100 000 population) for colorectal, oesophageal, gastric, and pancreatic cancers in different regions of England, 2001. (A) For men; (B) for women. Source: England: National Cancer Intelligence Centre.454
Figure 3.5.5 Mortality: Incidence ratios for colorectal, oesophageal, gastric, and pancreatic cancers in different regions of England, 2001. (A) For men; (B) for women. Source: England: National Cancer Intelligence Centre, Office for National Statistics.
For each of the main types of gastrointestinal cancer, table 3.5.1 shows which Welsh unitary authorities have significantly increased or reduced incidence rates relative to the rest of Wales. Unlike England, there is no systematic geographical pattern in the incidence of any of the main gastrointestinal cancers.
Table 3.5.1 Significantly increased and reduced risks of gastrointestinal cancers among men and women resident in Welsh unitary authorities, 1992–2001.
| Unitary authority | Sex | Gastrointestinal cancer | ||||
|---|---|---|---|---|---|---|
| Colon | Rectum | Oesophagus | Stomach | Pancreas | ||
| Anglesey | Men | ↑ | ||||
| Women | ↑ | ↑ | ||||
| Blaenau Gwent | Men | ↑ | ↓ | ↓ | ||
| Women | ||||||
| Bridgend | Men | ↓ | ||||
| Women | ↓ | ↓ | ↓ | |||
| Caerphilly | Men | |||||
| Women | ↑ | |||||
| Cardiff | Men | ↓ | ↑ | |||
| Women | ||||||
| Carmarthenshire | Men | ↑ | ↓ | ↑ | ||
| Women | ↓ | |||||
| Ceredigion | Men | ↓ | ↓ | |||
| Women | ||||||
| Conwy | Men | |||||
| Women | ||||||
| Denbighshire | Men | |||||
| Women | ↓ | |||||
| Flintshire | Men | |||||
| Women | ||||||
| Gwynedd | Men | ↑ | ↑ | ↑ | ||
| Women | ||||||
| Merthyr Tydfil | Men | ↑ | ↓ | |||
| Women | ||||||
| Monmouthshire | Men | ↓ | ||||
| Women | ↓ | ↓ | ||||
| Neath and Port Talbot | Men | |||||
| Women | ↑ | |||||
| Newport | Men | |||||
| Women | ↓ | ↑ | ||||
| Pembrokeshire | Men | |||||
| Women | ||||||
| Powys | Men | ↓ | ↓ | |||
| Women | ↓ | |||||
| Rhondda Cynon Taff | Men | |||||
| Women | ↓ | ↓ | ||||
| Swansea | Men | ↑ | ||||
| Women | ↑ | ↑ | ||||
| Torfaen | Men | ↓ | ||||
| Women | ||||||
| Vale of Glamorgan | Men | ↓ | ||||
| Women | ||||||
| Wrexham | Men | ↓ | ||||
| Women | ↑ | |||||
↓ denotes significantly (p<0.05) reduced risk, relative to the rest of Wales; ↑ denotes significantly (p<0.05) increased risk, relative to the rest of Wales.
Source: Welsh Cancer Surveillance and Intelligence Unit.455
3.6 Socioeconomic factors
Dyspepsia
There is little evidence of an association between dyspepsia and social class.457 A historical study found a similar incidence of dyspepsia in private practice and in a dispensary in London around 1800,458 and a recent study in England and Scotland reported that symptom prevalence was unrelated to social class, but that social class affected consultation behaviour, rising from 17% in social class I to 29% in social class IV.38
Helicobacter pylori infection and peptic ulcer
There is a well recognised association between Helicobacter pylori infection, socioeconomic group,459 and childhood living conditions,460 which has persisted over time. For example, a recent study of [13C]urea breath testing for Helicobacter pylori infection among children in Glasgow, reported a significantly higher prevalence of 34% among children classified with the least affluent Carstairs' deprivation categories, compared with 16% among the most affluent categories, and 22% among intermediate groups.461
The incidence of peptic ulcer is strongly associated with lower social class or socioeconomic conditions,147,462,463 largely because of the higher prevalence of the Helicobacter pylori infection among people from lower socioeconomic backgrounds. However, gastric ulcers have been associated with manual social classes, and duodenal ulcers with non‐manual classes.464
Gastrointestinal haemorrhage
Gastrointestinal haemorrhage is also strongly related to social class, especially as the most common underlying cause of upper gastrointestinal haemorrhage is peptic ulcer. A recent study of the west of Scotland reported that the incidence of upper gastrointestinal haemorrhage was higher in areas of greater social deprivation: it was 2.2 times higher in the least affluent quarter than in the most affluent quarter.92
Inflammatory bowel disease
Inflammatory bowel disease is not thought to be related to social class or poverty. Studies of British national birth cohorts have found no association with social class for either Crohn's disease or ulcerative colitis.147,465 In Scotland, though, the incidence of juvenile onset Crohn's disease has been reported as significantly higher in areas of most affluence from 1981 to 1995, although no association was found for ulcerative colitis.452
Irritable bowel syndrome (IBS)
Some studies have reported of an increased prevalence of IBS in higher social classes, which has been considered as consistent with an allergic aetiology for IBS. These include studies in England466 and Australia.467 However, other British studies have reported of no significant association between IBS and social class,147,468 and a Danish study also reported no association for incidence or prevalence of IBS.166 Some studies,166,469,470 although not others,157,471,472 have reported that psychiatric illness or psychological factors may be of greater importance for IBS than socioeconomic and lifestyle factors.
Coeliac disease
There is not thought to be a strong association between coeliac disease and social class or poverty. One British study reported of a non‐significant tendency towards a higher prevalence among higher socioeconomic groups.186
Diverticular disease
Socioeconomic factors are thought to influence the incidence of diverticular disease of the intestine. A Scottish study reported that diverticular disease was more common in lower than in higher income groups. It is likely that higher income groups are more aware of the importance of dietary fibre and more able to afford protective foods such as fresh fruit and vegetables.473
Liver cirrhosis
A recent study reported that social class is a risk factor for alcohol related mortality, including liver cirrhosis, with men in manual occupations significantly more likely than professional men to die of alcohol related causes. Alcohol seems to be similar to other psychoactive substances in that problem use is linked to social structural factors such as poverty, disadvantage, and social class.474 Another recent study reported that social class differentials in mortality from liver cirrhosis increased from 1961 to 1981 in England and Wales and in Scotland.475
Hepatitis B and C infections
Both hepatitis B and C are linked to deprivation and poverty. For example, a study of routine neonatal screening in Scotland found the highest prevalence of hepatitis C infections in high deprivation areas, particularly the most deprived areas in Greater Glasgow476; and a USA study reported of a strong association between both hepatitis B and C with deprivation, that was largely related to the impact of poverty on the spread of the two viruses.477
Acute pancreatitis
The incidence of acute pancreatitis is often much higher in areas of higher alcohol consumption and lower affluence—for example, in Scotland compared with the south of England. However, one prominent British study found no association between social class and the incidence of acute pancreatitis in the Nottingham region, but instead found a large excess for people resident in areas with “particularly hard drinking water”.286
Gastrointestinal cancers
The incidence of gastric cancer, in particular, and cancer of the oesophagus is highest in deprived or poor areas. In Scotland from 1991 to 1995, for example, there was a strong social gradient for gastric and oesophageal cancers, with the highest incidence in areas having the highest Carstairs' deprivation scores, and the lowest incidence found in areas that were the most affluent (fig 3.6.1). Socioeconomic variation in gastric cancer incidence occurs to some extent because of the association between Helicobacter pylori infection and poverty. However, there were no significant associations between deprivation and incidence of colorectal and pancreatic cancers; although colorectal cancer incidence appears to be highest in the most affluent areas.
Figure 3.6.1 Standardised incidence and mortality rates (per 100 000 population), and five year survival, for colorectal, oesophageal, gastric, and pancreatic cancer shown for Carstairs deprivation categories in Scotland: cases diagnosed between 1991 and 1995. (A) Incidence; (B) mortality; (C) five year survival. Notes: Incidence and mortality rates are standardised using the European population. Incidence and mortality rates for oesophageal and gastric cancers are strongly associated with deprivation (both p<0.001), but those for colorectal or pancreatic cancers are not. Five year survival for colorectal (p<0.01), oesophageal (p = 0.01), and gastric (p = 0.04) cancers are all associated with deprivation, but that for pancreatic cancers is not. Carstairs deprivation categories are measured in quintiles. Source: Information and Statistics Division, NHS in Scotland.215
In England and Wales, use of the ONS longitudinal Study from 1976 to 1990 showed significantly higher incidence of gastric cancers among lower social groups, no social inequalities in incidence of pancreatic cancer, and a significantly higher incidence of colorectal cancer among women but not among men in advantaged social groups.478 Colorectal cancer has similarly been associated with professional or managerial occupations in another British study,464 and colon cancer with sedentary occupations in Sweden.479
Population based mortality in Scotland shows similar patterns to those for incidence, although mortality from colorectal cancer in the most affluent areas is comparable to that in the rest of the population (fig 3.6.1). Five year survival rates in Scotland are positively and significantly correlated with affluence for colorectal cancer, in particular, and also for gastric and oesophageal cancer. However, for pancreatic cancers, which have the poorest prognosis, there is much less scope for socioeconomic variation in survival.
In England, inequalities in survival for colorectal cancer have been attributed to earlier surgical resection among people from more affluent backgrounds, reflecting inequalities in access to treatment,480 while others have reported lower uptake of screening among people from more deprived areas.481,482 For details of the impact of socioeconomic and demographic factors on consultations in primary care for diseases of the digestive system, see section 4.3.2.
3.7 Costs to society
Costs to the NHS of GI disease are reported in section 4.5 below. In addition to health services costs, however, GI disease imposes a considerable burden on the other parts of the UK economy, as well as to patients and their families.
It was not possible to undertake a study of the full burden of illness within the time and financial constraints of this review. However, in 1996 the British Society for Gastroenterology commissioned the Unit for Policy Research in Science and Medicine (PRISM) of the Wellcome Trust to undertake a study to estimate the burden of GI disease in the UK.483 The following estimates are based on that report.
One major element of burden is the years of working life lost by those who die of GI diseases before reaching retirement age. The PRISM study estimated that in 1997 approximately 147 400 person years were lost (from age of death (if 20+) to 65) from GI diseases in both men and women. They reported that “… the burden of gastrointestinal disease in terms of premature death has been approximately constant in recent years”. On the assumption that this burden has remained constant since 1997 and applying their valuation method updated with current average earnings, the estimated cost of early death by GI disease in 2004 is £3230m.
A second major element of the burden of GI disease is the lost productivity due to long term sickness absences from work. The PRISM study estimated that GI disease causes 46 680 person years of lost productivity or roughly 1.7% of long term sickness absence in the UK. Applying their valuation method updated with current average earnings produces an estimated value of lost productivity in 2004 of £1050m.
With respect to short term sickness absence, the PRISM study crudely estimated that one fifth of all short term sickness absences were due to GI diseases. Updating their estimate with current earnings produces a value of lost productivity estimate of £2900m.
On this basis, the total estimated cost to the British economy in 2004 is thus £7180m. Although this figure may be crude, it identifies an order of magnitude which clearly indicates that GI morbidity and mortality impose major costs on the British economy.
In addition to costs for the economy, GI diseases impose considerable burden on individual patients and their families. This includes travel and other costs incurred in receiving treatment and the cost of over the counter drugs, which are not included in the NHS costs reported in section 4.5.
4 Current service provision in the UK
4.0 Methods and data limitations
The main methods used for the activity analysis in this chapter involved using routine data sources in the UK to provide information on hospital activity and costs. The main source used for hospital inpatient activity was hospital episode statistics (HES) in England, produced by the Department of Health. Record linkage allows hospital activity to be determined for the numbers of people receiving inpatient care, as well as the numbers of episodes of care. Linked hospital episode data were provided by the Unit of Health‐Care Epidemiology, University of Oxford.484
Hospital activity for surgical procedures was also obtained from hospital episode statistics. However, because outpatient activity data are not yet available for the UK, data were obtained for outpatients from the USA.
For activity in primary care, the most recent comprehensive study of consultation patterns in primary care is the fourth national morbidity study in England and Wales in 1991–92.485 This comprised a representative national sample of 60 general practices, covering just over half a million registered patients or 1% of the population of England and Wales. This study followed the third national morbidity survey in 1981–82.486
Some of the main data limitations for investigating activity include concerns about the accuracy of routine hospital episode statistics,8,9,10,11 as well as increasing doubts that the finished consultant episode is still a valid measure in a health service where changing roles and teamwork are increasingly becoming the norm.11,487,488
A limitation of the investigation of primary care activity is that the latest comprehensive and freely available study of consultation patterns in primary care in England and Wales is the fourth national morbidity study which covers the period 1991–92.
The literature review described in section 5.0.1 has also contributed to some sections in this chapter. Workforce data have been collected by an annual census of consultant gastroenterologists taken on 30 September each year. These data are cross checked with the Royal College of Physicians annual census data (coordinated to 30 September each year). Advertisements in the BMJ and consultant gastroenterology advisory appointment committees are constantly monitored. Specialist registrars also complete an annual census, with data being cross checked against information from the consultant census, Joint Committee for Higher Medical Training, and by monitoring movements of the specialist registrar workforce as they occur. Data on nurses and non‐consultant career grade (NCCG) doctors are collected from the consultant census, from the RCN directory, and from an unpublished survey of nurses.
4.1 Organisation
The current provision of services for patients with gastrointestinal disorders has been summarised in a joint report from the British Society of Gastroenterology and Royal College of Physicians in 2003.489 Common problems include indigestion, reflux, irritable bowel syndrome, and constipation. Many of these problems can be diagnosed and treated by the patient's family practitioner. Those with worrying or persistent symptoms will usually be referred to a consultant gastroenterologist in outpatients, to identify or exclude organic disease and receive advice on treatment. Investigations will often include blood tests, endoscopy, and imaging. If problems arise suddenly or appear very serious, urgent inpatient assessment and treatment may be required. Such problems include bleeding from peptic ulcer, jaundice, acute liver disease, and severe exacerbations of colitis and Crohn's disease. In hospitals many GI disorders require a team approach involving physicians, surgeons, radiologists, pathologists, specialist and non‐specialist nurses, dieticians, nutritionists, physiotherapists, clinical scientists, physiologists, speech and language therapists, hypnotherapists, and psychologists. Some problems will require referral to a tertiary centre where a specific concentration of expertise is required to manage serious or rare disorders, both medically and surgically.
Conventional services for patients with gastrointestinal disorders reflect the traditional division between the primary and secondary care sectors. General practitioners usually have direct access to laboratory, radiological, and endoscopic investigations, but are required to refer patients to consultant colleagues in hospitals when a specialist opinion or care is needed. Not all hospitals provide a full range of diagnostic and treatment facilities and tertiary referral to a subregional or regional hospital is often necessary for complex problems.
The management of many gastroenterological conditions has been reviewed in evidence based guidelines produced by the British Society of Gastroenterology (table 4.1.1), National Institute for Health and Clinical Excellence (NICE; table 4.1.2), and Scottish Intercollegiate Guidelines Network (SIGN; table 4.1.3). This document will not examine the clinical management of individual disorders except in the context of the location and nature of the services required.
Table 4.1.1 BSG published guidelines, including work‐in‐progress.
| The following guidelines have been published: |
| Oesophageal manometry and pH monitoring (revised 2006) |
| Antibiotic prophylaxis in gastrointestinal endoscopy (revised 2001) |
| Management of patients with short bowel (2006) |
| Complications of gastrointestinal endoscopy (2006) |
| Management of inflammatory bowel disease in adults (2004) |
| Dyspepsia management guidelines (revised 2002). Now NICE |
| Management of patients with coeliac disease (revised 2002) |
| Initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease (1997) |
| A structured approach to colorectal biopsy assessment (1997) |
| Management of acute pancreatitis (revised 2005) |
| Informed consent for endoscopic procedures (1999 and 2006) |
| Use of liver biopsy in clinical practice (2004) |
| Indications for referral and assessment in adult liver transplantation (2000) |
| Osteoporosis in coeliac disease and IBD (2000) |
| Management of iron deficiency anaemia (revised 2005) |
| UK guidelines on the management of variceal haemorrhage in cirrhotic patients (2000) |
| Management of irritable bowel syndrome (2000) |
| Treatment of hepatitis C incorporating the use of PEG interferon (revised 2003) |
| Management of oesophageal and gastric cancer (2002) |
| Management of osteoporosis associated with chronic liver disease (2002) |
| Non‐variceal upper gastrointestinal haemorrhage (2002) |
| Colorectal cancer screening in high risk groups (2002) |
| Management of patients with coeliac disease (2002) |
| Diagnosis and treatment of cholangiocarcinoma (Nov 2002) |
| Diagnosis and treatment of hepatocellular carcinoma (HCC) in adults (2003) |
| Investigation of chronic diarrhoea (2003) |
| Resection of colorectal cancer liver metastases (2006) |
| Enteral feeding in adult hospital patients (Dec 2003) |
| Pancreatic cancer (2005) |
| Use of oesophageal dilatation in clinical practice (Feb 2004) |
| The following guidelines have been published in Gut: |
| Management of patients with pancreatic, peri‐ampullary and ampullary carcinomas (2005) |
| Management of acute pancreatitis (revised 2005) |
| Management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (2005) |
| Management of ascites in cirrhosis (2006) |
| Diagnosis and management of Barrett's oesophagus (2006) |
http://www.bsg.org.uk/bsgdisp1.php?id = 48c1b0bcae9daa89d36aandh = 1 (accessed 18 December 2006).
Table 4.1.2 NICE guidelines relating to GI disorders.
| The following guidelines have been published: |
| Eating disorders: core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders (Jan 2004) |
| Colorectal: service guidance for the NHS in England and Wales improving outcomes for colorectal cancer (Jun 2004) |
| Dyspepsia: managing dyspepsia in adults in primary care (Aug 2004) |
| Nutrition support in adults (Feb 2006) |
| The following guidelines are in development: |
| Obesity (Dec 2006) |
| Faecal incontinence (June 2007) |
| Irritable bowel syndrome (Feb 2008) |
http://www.nice.org.uk/page.aspx?o = cg (accessed 18 December 2006).
Table 4.1.3 SIGN guidelines relating to GI disorders.
| The following guidelines have been published: |
| Management of colorectal cancer (Mar 2003) |
| Dyspepsia (Mar 2003) |
| Management of obesity in children and young people (April 2003) |
| Management of harmful drinking and alcohol dependence in primary care (Sep 2003, updated Dec 2004) |
| Management of oesophageal and gastric cancer (June 2006) |
| The following guidelines are currently in development: |
| Management of continence within primary care |
http://www.sign.ac.uk/guidelines/ (accessed 18 December 2006).
4.2 Workforce
Most patients with persistent symptoms suggestive of gastrointestinal disease will be managed by a team led by a gastroenterologist. The Royal College of Physicians has set out a description of the specialty490 and defined the workload of a consultant‐led gastroenterology team. It is recommended that a consultant‐led team should look after no more than 20–25 inpatients at any one time, the majority being admitted on emergency take days. In outpatients a consultant physician in gastroenterology, working alone, in a new patient clinic, should see 6–8 patients; each allotted 20–30 minutes. When reviewed, 12–15 patients should be seen in a single session.
The Royal College of Physicians recommends that 65–74 consultant programmed activity sessions are required to serve a population of 250 000, which indicates a need for about six consultants for such a population. This takes into account the need to allow for education and training, audit, and service management. For diagnostic upper gastrointestinal endoscopy or flexible sigmoidoscopy, a maximum of 10–12 procedures should be carried out in a single session, allowing 15–20 minutes for each procedure. Therapeutic procedures will take at least twice as long, and diagnostic and therapeutic colonoscopy will usually take 30–40 minutes for each procedure.
The workforce in gastroenterology has expanded substantially over the past few years. The robust data on consultant gastroenterologist numbers show a rise from 335 to 725 in England and Wales (fig 4.2.1), with an average expansion of 6.3% a year over the whole of that period (fig 4.2.2). At present growth rates it will take 13 years to reach the recommended six consultants per 250 000 population. The total number of academic gastroenterologists is 118 (England 104, Scotland 10, Wales 3, and Northern Ireland 1). Currently, in excess of 400 stoma care nurses are listed in the RCN directory and, from a recent unpublished survey, there are about 100 IBD nurses in the UK. No data are available on workforce numbers in the allied professions that support the care of patients with GI disorders, but there are concerns that expansion has not matched that in medicine and nursing.
Figure 4.2.1 Numbers of consultant gastroenterologists in England and Wales against time.
Figure 4.2.2 Consultant gastroenterologists expansion rates (England and Wales).
Expansion in consultant numbers has been greater in the past five years, averaging 7% in England with similar expansion in Wales and Northern Ireland and slightly less in Scotland (table 4.2.1). Thus the numbers of gastroenterology consultants in the UK total 826 (as of 30 September 2004) (table 4.2.2).
Table 4.2.1 Annual expansion (%) of consultants in different parts of the UK by year.
| UK regions | 30 Sep 2000 | 30 Sep 2001 | 30 Sep 2002 | 30 Sep 2003 | 30 Sep 2004 |
|---|---|---|---|---|---|
| England | 7.6 | 5.7 | 8.0 | 7.2 | 6.5 |
| Wales | 0 | 20.0 | 6.7 | 6.3 | 8.1 |
| Scotland | 8.8 | 8.1 | 3.0 | 2.9 | 6.6 |
| Northern Ireland | 0 | 5.3 | 5.0 | 9.5 | 8.0 |
Table 4.2.2 Numbers of consultants in different parts of the UK by year.
| UK regions | 30 Sep 2000 | 30 Sep 2001 | 30 Sep 2002 | 30 Sep 2003 | 30 Sep 2004 |
|---|---|---|---|---|---|
| England | 523 | 552 | 600 | 643 | 688 |
| Wales | 25 | 30 | 32 | 34 | 37 |
| Scotland | 62 | 67 | 69 | 71 | 76 |
| Northern Ireland | 19 | 20 | 21 | 23 | 25 |
This consultant workforce is supported by at least 418 associate specialists (371 England, 18 Wales, 16 Scotland, 13 Northern Ireland) and 312 nurses undertaking duties that a few years ago would have been deemed the province of doctors—for example, endoscopy nurses (268 England, 13 Wales, 28 Scotland, 3 Northern Ireland). Neither of the groups is evenly distributed through regions or principalities, varying from 3 specialist nurses in Northern Ireland, 6 in Oxford to 32 in Trent and 35 in North Thames (East). For NCCG doctors this variation ranges from 14 in South Thames (West) to 39 in South Thames (East). The lack of correlation (direct or inverse) between consultant numbers, specialist nurse, and NCCG doctors suggests the distribution has developed in an ad hoc fashion rather than by formal planning based on population needs (see table 4.2.3).
Table 4.2.3 Numbers of nurses and associate specialists contributing to gastroenterology service provision in the UK.
| UK regions | Number of nurses | Non‐consultant career grades |
|---|---|---|
| England | 268 | 371 |
| Wales | 13 | 18 |
| Scotland | 28 | 16 |
| Northern Ireland | 3 | 13 |
| Total | 312 | 418 |
In addition the specialist registrar trainees provide substantial service work and any reduction in training numbers (as seems likely as the number of consultants plateau at a required level) would need to be replaced by consultants or other workers of similar skill. Five hundred and fifty specialist registrars or equivalent currently have posts in the UK (as of 30 September 2004), though 131 are out of programme or undertaking research so contribute only a proportion of their time to service delivery (table 4.2.4).
Table 4.2.4 SpR/NTN posts in the UK (as of 30 September 2004).
| SpR/NTN posts | England | Wales | Scotland | Northern Ireland |
|---|---|---|---|---|
| Specialist registrar (clinical) | 265 | 10 | 22 | 6 |
| Senior registrar | 1 | |||
| Research registrar | 99 | 4 | 9 | 3 |
| Out of programme | 11 | 1 | 1 | 3 |
| Visiting registrar, inc FTTA | 53 | 10 | ||
| LAT | 28 | 1 | 2 | |
| Locum/hon consultant | 21 | |||
| Total | 478 | 26 | 34 | 12 |
In detailed work reported in Consultant physicians working with patients490 the need for approximately 1950 consultant gastroenterology posts in the UK as a whole (assuming a population of 59.6 million), providing 1665 whole time equivalent posts, was demonstrated to deliver acceptable levels of care. This number allows for a proportion of part time consultants as estimates suggest such work is increasingly popular. Larger numbers may be required with the predicted expansion of the population to 65 million. Typically six to seven will serve a population of 250 000, with extra needed where other duties are fulfilled. These will include specialist training (for example, endoscopy courses), undergraduate teaching, academic and research roles over and above those expected in a district hospital. Each team will require additional support staff such as non‐consultant career grade doctors, specialist nurses (with roles in nutrition, endoscopy, inflammatory bowel disease and more technical functions—for example, pH and manometry, videocapsule endoscopy, etc) and the service time provided by specialist registrars. No clear information exists on likely need but one could imagine a team consisting of six whole time equivalent consultants, one to two whole time equivalent non‐consultant career grade doctors, two endoscopy nurses, and two to three specialist nurses (each providing additional help with IBD, pH, etc) and one to two specialist registrars being trained in gastroenterology and general medicine.
The MINuET study491 has concluded that more diagnostic endoscopies could be undertaken by nurses. The implications of this are that at least one whole time equivalent specialist nurse, trained in endoscopy, would be required in each medium sized district general hospital. In practice, it is unlikely that a nurse endoscopist would wish only to undertake endoscopies and it is more probable that other specialist nurse roles would be included in the job description. On this basis it is predicted that two whole time equivalent specialist nurses would be required for each hospital. A survey of 196 endoscopy units, registered with the Joint Advisory Group for Gastrointestinal Endoscopy in 2004, identified 149 nurse endoscopists in post in 96 units (64% of the 150 units that responded).492 On this basis it can be predicted that approximately 200 nurse endoscopists will need to be found and trained in the UK, if the majority of diagnostic procedures are to be undertaken by nurses.
4.3 Activity
4.3.1 Primary care
Routinely collected clinical data are coded and analysed using the International Classification of Diseases (ICD) for diagnosis and Office for Population Censuses and Surveys Classification (OPCS) for surgical operations and procedures.
During 1991–92, 78% of people consulted a general practice on at least one occasion. Table 4.3.1 shows the prevalence rate or percentage of people who consulted for the major disease groupings (ICD‐9 chapters). Of the different gastrointestinal diseases, 8.7% of people consulted for diseases of the digestive system, 4.1% consulted for intestinal infectious diseases, 0.1% consulted for malignant neoplasms of the digestive system, and 0.04% for viral hepatitis (table 4.3.1).
Table 4.3.1 Rates per 10 000 population for patients consulting general practice, and for the total consultation rate, for the different ICD‐9 chapters, England and Wales, 1991–1992.
| Diagnosis at consultation | ICD‐9 chapter | ICD‐9 code | Percentage of patients consulting general practice | Consultation rate per 10 000 population | ||
|---|---|---|---|---|---|---|
| All consultations | Serious | All consultations | Serious | |||
| Intestinal infectious diseases: | 001–009 | 4.09 | NA | 517 | NA | |
| Infectious and parasitic diseases | I | 001–139 | 13.99 | 0.09 | 2 006 | 12 |
| Viral hepatitis | 070 | 0.04 | NA | 8 | NA | |
| All other infectious diseases | 010–069, 071–139 | NA | NA | 1 489 | NA | |
| Neoplasms: | II | 140–239 | 2.39 | 0.90 | 492 | 287 |
| Malignant, digestive system | 150–159 | 0.13 | NA | 54 | NA | |
| Benign and other neoplasms, digestive | 210,211,230, 235.2–235.5 | NA | NA | 9 | NA | |
| All other neoplasms | 140–149, etc | NA | NA | 436 | NA | |
| Endocrine, nutritional, metabolic and immunity disorders | III | 240–279 | 3.77 | 1.85 | 710 | 419 |
| Diseases of blood and blood‐forming organs | IV | 280–289 | 0.97 | 0.08 | 151 | 12 |
| Mental disorders | V | 290–319 | 7.28 | 1.13 | 1 761 | 350 |
| Diseases of the nervous system and sense organs | VI | 320–389 | 17.32 | 1.99 | 2 848 | 378 |
| Diseases of the circulatory system | VII | 390–459 | 9.31 | 3.67 | 2 397 | 977 |
| Diseases of the respiratory system | VIII | 460–519 | 30.70 | 5.79 | 6 200 | 1314 |
| Diseases of the digestive system | IX | 520–579 | 8.66 | 2.29 | 1 495 | 414 |
| Diseases of the genitourinary system | X | 580–629 | 11.33 | 0.31 | 2 050 | 53 |
| Complications of pregnancy, childbirth and the puerperium | XI | 630–676 | 1.08 | 0.19 | 183 | 25 |
| Diseases of the skin and subcutaneous system | XII | 680–709 | 14.55 | 0.00 | 2 289 | 0 |
| Diseases of the musculoskeletal system and connective tissue | XIII | 710–739 | 15.21 | 5.34 | 3 070 | 1067 |
| Congenital abnormalities | XIV | 740–759 | 0.53 | 0.29 | 69 | 41 |
| Certain conditions originating from the perinatal period | XV | 0.13 | 0.03 | 16 | 4 | |
| Symptom, signs and ill‐defined conditions | XVI | 760–779 | 15.10 | 0.07 | 2 340 | 7 |
| Injury and poisoning | XVII | 800–999 | 13.90 | 0.61 | 1 946 | 90 |
| Total gastrointestinal diseases | 001–009, 070, 150–159, 210,211, 230, 235.2–235.5, 520–579 | NA | NA | 2 083 | NA | |
| All illnesses | I–XVII | 001–999 | 78.03 | 19.84 | 30 021 | 5450 |
Source: McCormick et al, 1995.485
Diseases of the digestive system formed one of the leading ICD chapters as the cause of people consulting their GP, following respiratory diseases (30.7% of all people), diseases of the nervous system (17.3%), musculoskeletal diseases (15.2%), diseases of the skin and subcutaneous tissue (14.6%), infectious diseases (14.0%), injury and poisoning (13.9%), genitourinary diseases (11.3%), and diseases of the circulatory system (9.3%).
The percentage of patients consulting general practice for diseases of the digestive system rose by one fifth from 7.2% of people in 1981–82 to 8.7% in 1991–92 (fig 4.3.1), which was closer to the 8.2% and 10.0% of people consulting for diseases for the digestive system in the historical national morbidity surveys in 1955–56 and 1971–72, respectively.12 The proportion of people consulting for most other ICD‐9 chapters also increased, although there were reductions for infectious diseases, mental disorders, and ill‐defined diseases.
Figure 4.3.1 Percentage of patients consulting general practice for the different ICD‐9 disease chapters in England and Wales, 1981–82 and 1991–92; a comparison over time between the last two national morbidity surveys. Source: McCormick et al, 1995.485
The total consultation rate for gastrointestinal diseases was 2083 per 10 000 population; or just over one consultation for every five people in the general population (table 4.3.2). These comprised 1495 consultations per 10 000 for diseases of the digestive system, 517 for intestinal infectious diseases, 54 for malignant neoplasms of the digestive system, nine for benign and other neoplasms of the digestive system, and eight per 10 000 for viral hepatitis.
Table 4.3.2 Consultation rates (per 10 000 population) for patients consulting general practice for gastrointestinal diseases in England and Wales, 1991–1992.
| Diagnosis at consultation | ICD‐9 code | Consultation rate per 10 000 population |
|---|---|---|
| Diseases of the digestive system: | ||
| Diseases of oral cavity, salivary glands, and jaws | 520–529 | 185 |
| Diseases of oesophagus | 530 | 169 |
| Peptic ulcer | 531–534 | 90 |
| Gastritis and duodenitis | 535 | 101 |
| Disorders of function of stomach | 536 | 224 |
| Other disorders of stomach and duodenum | 537 | 1 |
| Appendicitis | 540–543 | 13 |
| Hernia of abdominal cavity | 550–553 | 104 |
| Crohn's disease | 555 | 20 |
| Ulcerative colitis | 556 | 26 |
| Vascular insufficiency of intestine | 557 | 0 |
| Other non‐infective gastroenteritis and colitis | 558 | 21 |
| Intestinal obstruction without mention of hernia | 560 | 8 |
| Diverticular of intestine | 562 | 40 |
| Peritonitis | 567 | 1 |
| Other diseases of intestines and peritoneum | 564–566, 568, 569 | 413 |
| Chronic liver disease and cirrhosis | 571 | 10 |
| Other disorders of liver | 570, 572, 573 | 1 |
| Cholelithiasis, cholecystitis, and other disorders of gallbladder | 574–575 | 36 |
| Other disorders of biliary tract | 576 | 5 |
| Diseases of pancreas | 577 | 6 |
| Gastrointestinal haemorrhage | 578 | 16 |
| Intestinal malabsorption | 579 | 5 |
| Total diseases of the digestive system | 520–579 | 1495 |
| Malignant neoplasms of the digestive system: | ||
| Oesophagus | 150 | 10 |
| Stomach | 151 | 10 |
| Small intestine | 152 | 0 |
| Colon | 153 | 18 |
| Rectum, rectosigmoid junction, and anus | 154 | 11 |
| Liver and intrahepatic ducts | 155 | 1 |
| Gallbladder and extrahepatic ducts | 156 | 0 |
| Pancreas | 157 | 4 |
| Retroperitoneum and peritoneum | 158 | 0 |
| Other and ill‐defined sites of the digestive system | 159 | 0 |
| Total malignant neoplasms of the digestive system | 150–159 | 54 |
| Benign and other neoplasms of the digestive system | 210, 211, 230, 235.2–235.5 | 9 |
| Infectious intestinal diseases: | ||
| Cholera | 001 | 0 |
| Typhoid and paratyphoid fevers | 002 | 0 |
| Other salmonella infections | 003 | 6 |
| Shigellosis | 004 | 2 |
| Other food poisoning (bacterial) | 005 | 2 |
| Amoebiasis | 006 | 0 |
| Other protozoal intestinal diseases | 007 | 1 |
| Intestinal infections due to other organisms | 008 | 9 |
| Ill‐defined intestinal infections | 009 | 497 |
| Total infectious intestinal diseases | 001–009 | 517 |
| Viral hepatitis | 070 | 8 |
| Total gastrointestinal diseases | 001–009, 070, 150–159, 210 211, 230, 235.2–235.5, 520–579 | 2083 |
Source: McCormick et al, 1995.485
Consultation rates per 10 000 population for individual gastrointestinal diseases are also shown in table 4.3.2. The most common causes of consultation were ill‐defined intestinal infectious diseases (497 consultations per 10 000 population), disorders of the function of the stomach (224 per 10 000), diseases of the oral cavity, salivary glands and jaws (185), diseases of the oesophagus (169), hernia (104), gastritis and duodenitis (101), and peptic ulcer (90; table 4.3.2).
For people consulting GPs with gastrointestinal diseases, the most common causes of consultation were infectious intestinal diseases (4.0% of people), functional disorders not elsewhere classified (2.1%), disorders of function of stomach (1.5%), diseases of the oesophagus (1.0%), and gastritis and duodenitis (0.7%; fig 4.3.2).
Figure 4.3.2 Percentage of patients consulting general practice for the most common gastrointestinal diseases in primary care in England and Wales, 1991–1992 (at least 0.10% of the general population consulting). Source: McCormick et al, 1995.485
Socioeconomic and demographic influences on consultations for diseases of the digestive system in primary care
Consultations for diseases of the digestive system in primary care in 1991–92 show a strong social class gradient, with significantly increased rates of consultation for the manual social classes IV, V, and III manual, and reduced consultation levels for social classes I, II, and among men in the III non‐manual class (fig 4.3.3A).
Figure 4.3.3 Standardised consulting ratios (general population = 100) for diseases of the digestive system in general practice in England and Wales, 1991–92 according to (A) social class; (B) ethnic group; (C) employment status; (D) housing. Note: Standardised consulting ratio in the general population = 100. Vertical bars represent 95% confidence intervals. Source: McCormick et al, 1995.485
Consultation rates for digestive diseases were greatly increased for Pakistani and Bangladeshi ethnic groups, by 84% among men and 139% among women, but were not significantly increased or reduced for all other classified ethnic groups (fig 4.3.3B).
Consulting was reduced among people in full‐time employment, and among women in part‐time employment, but was increased among people who were unemployed or who were registered long term sick, and for women who were classified as looking after the home or family (fig 4.3.3C). People living in council housing and other rented accommodation also had increased rates of consultation, while people in owner occupied housing and women in communal accommodation had reduced consultation rates (fig 4.3.3D).
Increased rates of consultation were also reported for men in the Midlands and Wales, widowed or divorced people, and smokers, while people in southern England, people in rural areas of residence, single women, and non‐smokers had reduced rates of consultation (fig 4.3.4).
Figure 4.3.4 Standardised consulting ratios (general population = 100) for diseases of the digestive system in general practice in England and Wales, 1991–92 according to (A) region of England and Wales; (B) urban or rural residence; (C) marital status; (D) smoking habit in past week. Note: Standardised consulting ratio in the general population = 100. Vertical bars represent 95% confidence intervals. Source: McCormick et al, 1995.485
4.3.2 Inpatients
Out of 39 million finished consultant episodes (FCEs) in England during the four year period 1998–99 to 2001–02, 6.5 million (17%) had a gastrointestinal disease as the principal diagnosis (table 4.3.3); although about 45% of these admissions were day cases and mainly refer to endoscopic assessments. Of these, 5.2 million were for diseases of the digestive system, one million were for malignant neoplasms of the digestive system, 225 820 were for benign and other neoplasms of the digestive system, 160 160 were for intestinal infectious diseases, and 20 232 were for viral hepatitis.
Table 4.3.3 Annual population based hospital admission rates (per 10 000 population) for ICD‐10 chapters, based on numbers of finished consultant episodes (FCEs) and on numbers of people admitted in England, 1998–99 to 2001–02.
| ICD chapter | ICD‐10 code | No of FCEs | Admission rate per 10 000 population | No of people admitted | Admission rate per 10 000 population |
|---|---|---|---|---|---|
| Certain infectious diseases: | A00‐B99 | 596 468 | 30.5 | 511 032 | 26.1 |
| Intestinal infectious diseases | A00‐A09 | 160 160 | 8.2 | 143 536 | 7.3 |
| Viral hepatitis | B15‐B19 | 20 232 | 1.0 | 15 484 | 0.8 |
| All other infectious diseases | A10‐B14,B16‐B99 | 416 476 | 21.3 | 352 012 | 18.0 |
| Neoplasms: | C00‐D48 | 5 317 876 | 271.8 | 2 367 584 | 121.0 |
| Malignant neoplasms, digestive | C15‐C26 | 987 680 | 50.5 | 274 792 | 14.0 |
| Benign and other neoplasms, digestive | D00‐01,D12‐D13,D37 | 225 820 | 11.5 | 191 480 | 9.8 |
| All other neoplasms | C00‐C14, etc | 4 104 376 | 209.8 | 1 900 792 | 97.1 |
| Diseases of the blood and blood‐forming organs | D50‐D89 | 661 852 | 33.8 | 341 752 | 17.5 |
| Endocrine, nutritional and metabolic disorders | E00‐E90 | 590 408 | 30.2 | 379 100 | 19.4 |
| Mental and behavioural disorders | F00‐F99 | 326 748 | 16.7 | 246 500 | 12.6 |
| Diseases of the nervous system | G00‐G99 | 923 780 | 47.2 | 677 176 | 34.6 |
| Diseases of the eye and adnexa | H00‐H59 | 1 505 464 | 76.9 | 1 342 436 | 68.6 |
| Diseases of the ear and mastoid process | H60‐H95 | 371 340 | 19.0 | 351 500 | 18.0 |
| Diseases of the circulatory system | I00‐I99 | 4 273 688 | 218.4 | 3 083 240 | 157.6 |
| Diseases of the respiratory system | J00‐J99 | 2 945 864 | 150.6 | 2 266 772 | 115.8 |
| Diseases of the digestive system | K00‐K93 | 5 160 944 | 263.8 | 4 377 780 | 223.7 |
| Diseases of the skin and subcutaneous system | L00‐L99 | 1 050 452 | 53.7 | 905 000 | 46.3 |
| Diseases of the musculoskeletal system and connective tissue | M00‐M99 | 2 589 824 | 132.4 | 2 168 676 | 110.8 |
| Diseases of the genitourinary system | N00‐N99 | 3 087 232 | 157.8 | 2 576 980 | 131.7 |
| Pregnancy, childbirth and the puerperium | O00‐O99 | 908 180 | 46.4 | 812 756 | 41.5 |
| Certain conditions originating in the perinatal period | P00‐P96 | 96 748 | 4.9 | 82 824 | 4.2 |
| Congenital malformations | Q00‐Q99 | 321 192 | 16.4 | 24 9348 | 12.7 |
| Symptoms, signs and abnormal findings | R00‐R99 | 5 317 348 | 271.7 | 4 393 120 | 224.5 |
| External causes of morbidity and mortality | V01‐Y89 | 2 966 692 | 151.6 | 2 565 944 | 131.1 |
| Total gastrointestinal diseases | A00‐A09,B15‐B19,C15‐C26, D00,D01,D12,D13, D37,K00‐K93 | 6 554 836 | 335.0 | 5 003 072 | 255.7 |
| All diseases and conditions | A00‐Y89 | 39 012 100 | 1993.7 | 29 699 520 | 1517.8 |
Diseases of the digestive system was the second ICD chapter after neoplasms, and excluding “symptoms, signs, and abnormal findings”, that was the principal diagnosis for most FCEs (table 4.3.3). Using record linkage to identify person based admission rates, as well as episode based rates, diseases of the digestive system was the ICD chapter that was the principal cause for most people being admitted to hospital (4.4 million in England from 1998–99 to 2001–02).484
Figure 4.3.5 shows the percentage of inpatient admissions for each major body system, after deaths from cancer were allocated to their respective body systems—for example, when gastrointestinal cancers were included with diseases of the digestive system, when respiratory cancers were included with diseases of the respiratory system, etc. Then, gastrointestinal diseases were the leading major cause of hospital admission, either as FCEs (6.55 million; 17% of the total) or as people admitted (5.00 million; 17% of the total).
Figure 4.3.5 Percentage hospital admissions for major ICD‐10 disease groupings in England, 1998–99 to 2001–02 (A) based on the number of finished consultant episodes (FCEs). Source: Department of Health, 2004453; (B) based on the number of people admitted. Sources: Unit of Health‐Care Epidemiology, Oxford, 2004.484
In other words, 1 in 39 people were admitted each year with a principal diagnosis of gastrointestinal disease. This compares with 1 in 60 people admitted each year for genitourinary disease, 1 in 63 for circulatory disease, 1 in 76 for accidents and injury, 1 in 81 for respiratory disease, and 1 in 90 for musculoskeletal disorders.
Using the criterion of “any diagnosis” rather than “main diagnosis”, a total of 7.5 million people were admitted with “any diagnosis” of gastrointestinal disease during the four year period (one in 26 people per year), with a corresponding total of 10.0 million FCEs (26% of all FCEs).
Table 4.3.4 Annual population based hospital admission rates (per 10 000 population) for different gastrointestinal diseases, based on numbers of finished consultant episodes (FCEs) and on numbers of people admitted in England, 1998–99 to 2001–2002.
| Diagnosis at admission | ICD‐10 code | No of FCEs | Admission rate per 10 000 population | No of people admitted | Admission rate per 10 000 population |
|---|---|---|---|---|---|
| Diseases of the digestive system: | |||||
| Diseases of oral cavity, salivary glands, and jaws | K00–K14 | 700 664 | 35.8 | 669 884 | 34.2 |
| Oesophagitis | K20 | 150 944 | 7.7 | 135 476 | 6.9 |
| Gastro‐oesophageal reflux disease | K21 | 227 032 | 11.6 | 208 516 | 10.7 |
| Other diseases of oesophagus | K22, K23 | 211 860 | 10.8 | 161 348 | 8.2 |
| Peptic ulcer | K25–K28 | 217 280 | 11.1 | 157 828 | 8.1 |
| Gastritis and duodenitis | K29 | 339 248 | 17.3 | 308 424 | 15.8 |
| Dyspepsia | K30 | 148 200 | 7.6 | 144 816 | 7.4 |
| Other diseases of stomach and duodenum | K31 | 33 848 | 1.7 | 28 256 | 1.4 |
| Appendicitis | K35–K37 | 146 476 | 7.5 | 138 136 | 7.1 |
| Other diseases of appendix | K38 | 5 432 | 0.3 | 5 108 | 0.3 |
| Hernia of abdominal cavity | K40–K46 | 635 620 | 32.5 | 596 204 | 30.5 |
| Crohn's disease | K50 | 71 632 | 3.7 | 44 496 | 2.3 |
| Ulcerative colitis | K51 | 89 620 | 4.6 | 67 140 | 3.4 |
| Other non‐infective gastroenteritis and colitis | K52 | 303 500 | 15.5 | 260 488 | 13.3 |
| Diverticular of intestine | K57 | 211 688 | 10.8 | 182 284 | 9.3 |
| Other diseases of intestines | K55,K56,K58–K63 | 752 812 | 38.5 | 632 892 | 32.3 |
| Diseases of peritoneum | K65–K67 | 31 496 | 1.6 | 25 344 | 1.3 |
| Liver cirrhosis | K70, K73, K74 | 81 080 | 4.1 | 46 200 | 2.4 |
| Other diseases of liver | K71,K72,K75–K77 | 29 072 | 1.5 | 22 176 | 1.1 |
| Cholelithiasis, cholecystitis, and other diseases of the gallbladder | K80–K82 | 363 620 | 18.6 | 263 420 | 13.5 |
| Other diseases of biliary tract | K83, K87 | 37 820 | 1.9 | 25 800 | 1.3 |
| Acute pancreatitis | K85 | 64 560 | 3.3 | 43 044 | 2.2 |
| Other diseases of the pancreas | K86 | 33 644 | 1.7 | 19 212 | 1.0 |
| Other diseases of the digestive system | K90–K93 | 273 796 | 14.0 | 191 288 | 9.8 |
| Total gastrointestinal diseases | K00–K93 | 5 160 944 | 263.8 | 4 377 780 | 223.7 |
| Malignant neoplasms of the digestive system: | |||||
| Oesophagus | C15 | 123 848 | 6.3 | 38 240 | 2.0 |
| Stomach | C16 | 109 532 | 5.6 | 37 068 | 1.9 |
| Small intestine | C17 | 8 612 | 0.4 | 3 156 | 0.2 |
| Colon | C18 | 375 660 | 19.2 | 88 924 | 4.5 |
| Rectosigmoid junction | C19 | 56 328 | 2.9 | 13 976 | 0.7 |
| Rectum | C20 | 199 924 | 10.2 | 48 524 | 2.5 |
| Anus and anal canal | C21 | 10 484 | 0.5 | 4 124 | 0.2 |
| Liver and intrahepatic ducts | C22 | 20 660 | 1.1 | 9 200 | 0.5 |
| Gallbladder | C23 | 3 708 | 0.2 | 1 516 | 0.1 |
| Other and unspecified parts of biliary tract | C24 | 7 432 | 0.4 | 3 480 | 0.2 |
| Pancreas | C25 | 62 064 | 3.2 | 22 796 | 1.2 |
| Other and ill‐defined digestive organs | C26 | 9 428 | 0.5 | 3 788 | 0.2 |
| Total malignant neoplasms of the digestive system | C15–C26 | 987 680 | 50.5 | 274 792 | 14.0 |
| Benign and other neoplasms of the digestive system | D00–01,D12–13,D37 | 225 820 | 11.5 | 191 480 | 9.8 |
| Intestinal infectious diseases: | |||||
| Cholera | A00 | 40 | 0.0 | 40 | 0.0 |
| Typhoid and paratyphoid fevers | A01 | 1 020 | 0.1 | 856 | 0.0 |
| Other salmonella infections | A02 | 5 488 | 0.3 | 4 572 | 0.2 |
| Shigellosis | A03 | 412 | 0.0 | 372 | 0.0 |
| Other bacterial intestinal infections | A04 | 33 656 | 1.7 | 28 192 | 1.4 |
| Other bacterial food borne intoxications | A05 | 632 | 0.0 | 536 | 0.0 |
| Amoebiasis | A06 | 400 | 0.0 | 296 | 0.0 |
| Other protozoal intestinal diseases | A07 | 1 316 | 0.1 | 1 160 | 0.1 |
| Viral and other specified intestinal infections | A08 | 80 064 | 4.1 | 74 248 | 3.8 |
| Diarrhoea and gastroenteritis of presumed infectious origin | A09 | 37 132 | 1.9 | 33 264 | 1.7 |
| Total intestinal infectious diseases | A00–A09 | 160 160 | 8.2 | 143 536 | 7.3 |
| Viral hepatitis | B15–B19 | 20 232 | 1.0 | 15 484 | 0.8 |
| Total gastrointestinal diseases: | A00–A09, B15–B19, C15–C26, D00, D01, D12, D13, D37, K00–K93 | 6 554 836 | 335.0 | 5 003 072 | 255.7 |
Gastrointestinal cancer is the most common cause of hospital admission of all major cancer groupings. It was the principal diagnosis of 23% of all cancer FCEs, followed by lymphatic and haematological cancer (22%) and genitourinary cancer (18%; fig 4.3.6). FCEs for GI cancers mainly comprised colorectal cancer (60%), followed by cancers of the oesophagus (12%), stomach (11%), pancreas (7%), and the liver and intrahepatic ducts (2%).
Figure 4.3.6 Percentage hospital admissions, based on number of FCEs, for major groupings of cancer in England, 1998–99 to 2001–02. Sources: Department of Health, 2004.453
The main causes of hospital admission for (non‐cancer) diseases of the digestive system include hernia (12% of all FCEs), non‐infective gastroenteritis and colitis (9%), cholelithiasis, cholecystitis and other diseases of the gallbladder, and gastritis and duodenitis (7%; fig 4.3.7).
Figure 4.3.7 Percentage of hospital admissions, based on number of FCEs, for diseases of the digestive system in England, 1998–99 to 2001–02. Sources: Department of Health, 2004.453
Figure 4.3.8 shows hospital admission rates, based on number of FCEs and on number of people admitted, for some of the most common diseases and conditions in the general population. The number of people admitted for gastrointestinal diseases was more than double that for all types of accident, four times that for ischaemic heart disease, over 10 times that for stroke and pneumonia, and 20–40 times that for diabetes, asthma, and all traffic accidents.
Figure 4.3.8 Annual population based hospital admission rates (per 10 000 population) based on numbers of FCEs, and on numbers of people admitted, for gastrointestinal diseases and for other selected diseases and conditions in England, 1998–99 to 2001–02.*Gastrointestinal disease includes diseases of the digestive system, malignant neoplasms of the digestive system, benign and other neoplasms of the digestive system, intestinal infectious diseases, and viral hepatitis. Sources: Department of Health, 2004.453
4.3.3 Outpatients
Currently HES only includes data on admitted patients. In the near future, outpatient data will be available on HES online, but this will not include clinical data.
However, using figures for the USA in 2000, these show that out of an estimated total of 27.4 million outpatient visits for gastrointestinal symptoms, the leading gastrointestinal complaint was abdominal pain, cramps and spasms (12.3 million outpatient visits), followed by diarrhoea (4.06 million), nausea (3.32), vomiting (2.89), dyspepsia (1.82), constipation (1.33), anal or rectal bleeding (1.26), and melaena (1.18).493
The leading physician diagnoses for the same 27.4 million outpatient visits were abdominal pain (5.24 million), gastro‐oesophageal reflux disease (4.62 million), gastroenteritis (3.43), gastritis (2.4), haemorrhoids (1.57), irritable bowel syndrome (1.56), non‐inguinal hernia (1.54), benign neoplasms of the colon (1.52), malignant colorectal neoplasm (1.49), inguinal hernia (1.24), and diverticulosis of the colon (1.00).
4.3.4 Procedures
Table 4.3.5 shows the total number of surgical procedures undertaken in England in 2000–01 for the different OPCS‐4 chapters, as well as the total numbers of “main procedures” during a given episode, and the numbers of day case procedures.
Out of a total of 12.7 million procedures, 1.21 million (9.5%) were performed on the digestive tract and a further 128 886 (1.0%) on other abdominal (principally digestive) organs. Considering main procedures only, 1.03 million out of a total of 6.5 million procedures (16%) were performed on the digestive tract, and a further 97 102 (1.5%) on other abdominal organs (table 4.3.5).
A total of 5.8 million of the 12.7 million procedures (45%) were undertaken as day case admissions, including 59% of the procedures on the digestive tract and 11% of the procedures on the other abdominal organs (table 4.3.5).
Table 4.3.5 Numbers of surgical procedures: all, main, and day case procedures according to OPCS‐4 chapter in England 2000–2001.
| Surgical procedure | OPCS‐4 chapter | OPCS‐4 code | All procedures | Main procedures | Day case procedures | |||
|---|---|---|---|---|---|---|---|---|
| No | (%) | No | (%) | No | (%) | |||
| Nervous system | A | A01‐A84 | 238 932 | (1.9) | 202 455 | (3.1) | 127 048 | (2.2) |
| Endocrine system and breast | B | B01‐B37 | 99 845 | (0.8) | 90 301 | (1.4) | 25 592 | (0.4) |
| Eye | C | C01‐C86 | 705 740 | (5.6) | 410 667 | (6.3) | 547 550 | (9.5) |
| Ear | D | D01‐D28 | 125 523 | (1.0) | 94 788 | (1.5) | 73 919 | (1.3) |
| Respiratory tract | E | E01‐E63 | 318 165 | (2.5) | 205 984 | (3.2) | 99 373 | (1.7) |
| Mouth | F | F01‐F58 | 28 0249 | (2.2) | 235 335 | (3.6) | 173 268 | (3.0) |
| Upper digestive tract | G | G01‐G82 | 635 154 | (5.0) | 561 572 | (8.6) | 403 190 | (7.0) |
| Lower digestive tract | H | H01‐H62 | 571 089 | (4.5) | 474 073 | (7.3) | 328 653 | (5.7) |
| Other abdominal organs—principally digestive | J | J01–J72 | 128 886 | (1.0) | 97 102 | (1.5) | 13 678 | (0.2) |
| Heart | K | K01–K71 | 295 807 | (2.3) | 195 351 | (3.0) | 93 205 | (1.6) |
| Arteries and veins | L | L01–L97 | 293 139 | (2.3) | 182 761 | (2.8) | 86 426 | (1.5) |
| Urinary | M | M01–M83 | 672 821 | (5.3) | 525 198 | (8.1) | 323 036 | (5.6) |
| Male genital organs | N | N01–N34 | 112 939 | (0.9) | 97 304 | (1.5) | 74 337 | (1.3) |
| Lower female genital tract | P | P01–P31 | 105 450 | (0.8) | 73 654 | (1.1) | 56 414 | (1.0) |
| Upper female genital tract | Q | Q01–Q56 | 632 020 | (5.0) | 434 024 | (6.7) | 364 276 | (6.3) |
| Female genital tract associated with pregnancy, birth, and puerperium | R | R01–R34 | 947 964 | (7.5) | 526 861 | (8.1) | 2 159 | (0.0) |
| Skin | S | S01–S70 | 446 162 | (3.5) | 315 840 | (4.9) | 246 583 | (4.3) |
| Soft tissue | T | T01–T96 | 436 789 | (3.4) | 306 272 | (4.7) | 128 271 | (2.2) |
| Bones and joints of skull and spine | V | V01–V54 | 91 021 | (0.7) | 71 820 | (1.1) | 34 480 | (0.6) |
| Other bones and joints | W | W01–W92 | 653 005 | (5.1) | 536 079 | (8.2) | 177 102 | (3.1) |
| Miscellaneous and subsidiary operations | X | X01–X59 | 1 106 086 | (8.7) | 872 017 | (13.4) | 544 845 | (9.4) |
| Subsidiary classification of operations | Y–Z | Y01–Z92 | 3 806 538 | (30.0) | 0 | (0.0) | 1 869 195 | (32.3) |
| All operations | A–Z | A01–Z92 | 1 2703 240 | (100.0) | 6 509 426 | (100.0) | 5 792 598 | (100.0) |
Source: Department of Health, 2004.453
Figure 4.3.9 shows the percentage breakdowns of surgical procedures on the digestive tract and other abdominal organs in England in 2000–2001 (A) for all surgical procedures and (B) for the main surgical procedure during the episode. Most procedures were performed as day case admissions for endoscopy examination. The other most common gastrointestinal procedures were excisions of gallbladders (42 013, 3% of all gastrointestinal procedures), emergency excisions of appendices (36 657; 3%), and destruction of haemorrhoids (21 720; 2%; fig 4.3.9).
Figure 4.3.9 Percentage of different surgical procedures undertaken on the digestive tract, and on other abdominal organs in England, 2000–2001 for (A) all surgical procedures. Source: Department of Health, 2004453; (B) main surgical procedures. Source: Department of Health, 2004.453
A total of 17.4 million (17 364 212) bed days were associated with the total of 6.51 million main procedures. Of these, 2.6 million (2 629 352; 15.1%) were for procedures on the digestive tract, 1 389 613 on the upper digestive tract, and 1 239 739 on the lower digestive tract. An additional half a million (519 393) bed days were for procedures on the other abdominal organs.
Therefore, a total of 3.15 million bed days (18.1% of the total) were for gastrointestinal procedures, which was second only to procedures on “other bones and joints” (3.17 million) as the heaviest burden on hospital beds (fig 4.3.10).
Figure 4.3.10 Total number of bed days for main surgical procedures in England, 2000–2001. Source: Department of Health, 2004.453
Table 4.3.6 shows surgical procedures on the digestive tract with mean waiting times to admission in excess of 90 days in England in 2000–01. Eight of 69 procedures on the upper digestive tract, 10 of 52 procedures on the lower digestive tract, and 7 of 61 procedures on other abdominal organs had waiting times of 90 days or more.
Table 4.3.6 Surgical procedures of the digestive tract and other abdominal organs with waiting times in excess of 90 days in England, 2000–01.
| Surgical procedure | Mean waiting time (days) | No of admissions | Surgical procedure | Mean waiting time (days) | No. of admissions |
|---|---|---|---|---|---|
| Plastic operations on stomach | 319 | 214 | Other connection of ileum | 121 | 184 |
| Connection of stomach to transposed jejunum | 229 | 75 | Excision of pilonidal sinus | 121 | 5 684 |
| Repair of gall bladder | 217 | 11 | Repair of diaphragmatic hernia | 118 | 289 |
| Antireflux operations | 213 | 2 121 | Open introduction of prosthesis into bile duct | 118 | 34 |
| Repair of liver | 192 | 96 | Revision of antireflux operations | 116 | 107 |
| Fixation of rectum for prolapse | 172 | 357 | Other open operations on gall bladder | 115 | 29 |
| Excision of haemorrhoids | 167 | 9 177 | Extirpation of lesion of jejunum | 114 | 21 |
| Excision of gall bladder | 161 | 38 373 | Excision of lesion of anus | 111 | 7 792 |
| Incision of gall bladder | 157 | 179 | Other abdominal operations for prolapse of rectum | 110 | 660 |
| Repair of anus | 151 | 584 | Intra‐abdominal manipulation of ileum | 107 | 237 |
| Other open operations on bile duct | 133 | 66 | Other operations on pilonidal sinus | 98 | 5 850 |
| Open endoscopic operations on colon | 126 | 50 | Other operations on haemorrhoids | 93 | 907 |
| Perineal operations for prolapse of rectum | 126 | 1 028 |
Source: Department of Health, 2004.453
4.4 Voluntary sector patient support groups
Voluntary organisations provide support for patients with coeliac disease, inflammatory bowel disease, irritable bowel syndrome, liver disease, and after bowel surgery. These organisations are listed in Appendix 1. The voluntary sector plays a major part in the care and support of patients with chronic gastrointestinal disorders and has conducted many surveys which document the considerable impact of chronic GI disorders on the physical, mental, social, and financial health of those affected.494,495,496,497
4.5 Costs to the NHS
As with non‐NHS costs reported in section 3.7, a comprehensive costing of all NHS resources devoted to GI disease was not possible within the time and resource constraints of the present study. Several key cost areas such as GI cancers (recorded under cancer rather than GI disease) are not included. For others, cruder methods were used here than would have been the case in a more detailed costing exercise. Nevertheless the costs below give a general picture of costs in the relevant areas.
Hospital costs
Data on the number of FCEs in England for all HRGs for diseases of the digestive system were obtained from the Royal College of Physicians iLab using Hospital Episode Statistics. Activity data for each HRG were multiplied by the relevant NHS reference cost.498 Table 4.5.1 below shows the total number of emergency, overnight elective and day cases in 2001–02 and the associated costs. Total hospital costs (England only) were £1400m.
Table 4.5.1 Number of finished consultant episodes and costs (£000) digestive system HRGs: England 2001–02.
| Emergency | Elective admissions | Day cases | |
|---|---|---|---|
| Total FCEs | 648 000 | 250 000 | 839 000 |
| Total costs | 762 000 | 382 000 | 291 000 |
| Overall total cost | 1 435 000 | ||
Source: HES activity data from iLab, NHS reference costs.
Drugs
In 2002, 60 million prescriptions were issued for diseases of the gastrointestinal system (represented by therapeutic group 1 in the British National Formulary). The net ingredient cost of these drugs was £802m, of which £596m was for ulcer healing drugs and £51m for laxatives, which represents 7.9% of the total number of prescriptions issued in the UK and 9.5% of the total UK drug cost.499 These reported costs are solely for the “net ingredient cost” of the drugs and do not include other cost items such as containers or dispensing fees.
Primary care
The Office of Health Economics has estimated the cost of general practitioner consultations in the UK in 2000–01 for diseases of the gastrointestinal system to be £136m. This represents 7.8% of the cost of all GP visits.
These three elements of NHS costs due to GI diseases give a total of under £2400m but it must be emphasised that this understates total NHS costs for the reason given above.
4.6 Problems with existing service provision
4.6.1 Access
Statistical data on actual usage of resources are covered in section 4.3 and 4.5. Many of the underlying problems concerned with rising demand and limited access are examined, providing a more comprehensive reflection than the brief summary given here. Although many studies give a passing mention to problems of access, only three studies were found to cover this topic for GI services in any significant detail. Indeed, as one would expect, there are clear concerns for a range of services including endoscopy,500 outpatient management,501 and open access gastroscopy,502 with specific problems being excessive workload,502 ways to restrict access as a means to control costs,500,502 and the inappropriate use of services.501 It seems that an extensive and systematic study on the problem of access for the delivery of GI services has yet to be carried out.
4.6.2 Inequalities
No significant publications were found on the problems of inequalities in the delivery of GI services. Only one study—a brief, opinion based commentary on the topic of the inverse care law—was found to highlight general issues of inequalities in the health service.503 One likely reason for the lack of reliable evidence is that inequality is a much wider ranging concern, and is generally not confined to specific disciplines of medicine or health care. Readers concerned with this topic are therefore advised to consult other sources for an overview of this problem.
4.6.3 Waiting lists
Five studies were included in this section. Unsurprisingly, a literature review by Dunnill and Pounder504 found that waiting times (whether for an appointment or in the outpatient department) form the bulk of patients' concerns. Guidelines set out by the Association of Coloproctology of GB and Ireland recommend that surgeons should expect to achieve waiting times of four weeks or less between making a diagnosis of colorectal cancer and the start of treatment.505 But an audit by Duff et al506 carried out in the north west of England found that the median time between referral from the surgeon and the start of radiotherapy was 40 days, while only four patients (6% of the sample) received radiotherapy within 28 days of referral.
For bowel cancer, Flashman et al507 showed that most patients were not referred according to the two week standard set out by the Department of Health (that is, that all patients suspected by their GP of having bowel cancer should be seen by a specialist within two weeks). Clinics did not shorten the overall time to treatment or improve the stage of disease because the time lags before referral and after the outpatient appointment caused major delays. A brief report by Hellier508 outlined the problems with meeting the two week standard in endoscopy clinics, and emphasises that in order to make it a realistic possibility and to avoid distorted referral practices, funding needs to be targeted at GI outpatient and endoscopy facilities.
4.6.4 Patient safety
Several papers have dealt with patient safety for the treatment of GI disease. These can be generally divided into the safety of methods of treatment (such as the use of NSAIDs and complementary medicine) and procedures (for example, endoscopy and surgery). A wide breadth of studies cover various aspects of treatment,509,510,511,512,513,514,515,516,517,518 and are generally beyond the scope of this report. However, it is worth noting that the main concerns in this area are focused on ensuring that sufficient evidence and research is carried out to assess the safety of new drugs and treatments for GI disease,509,510,515 which include over‐the‐counter drugs,513 unlicensed and off‐label drugs,511 prescription of NSAIDs,512,514,516 complementary medicines used by children,518 and endoscopic therapy for acute non‐variceal upper GI haemorrhage.517
As far as the safety of procedures is concerned, most studies have generally found that upper GI endoscopies are safe regardless of age and where they are performed.519,520,521,522 However, some caveats remain in the area of how the service is delivered, including a restriction of upper GI endoscopy in elderly patients (85 years and over) to cases of bleeding (overt and suspected) and anaemia in order to reduce costs.522 There are also suggestions that simple diagnostic endoscopies can be performed safely in the primary care setting, leaving secondary care units to concentrate on those patients requiring sedation, who are acutely ill, and who require therapeutic procedures519—problems on location of care are covered in greater detail in section 5. Despite these positive findings, endoscopy does carry some risk. In a study by Quine et al,521 out of 13 036 patients undergoing endoscopic endoscopy without any therapeutic intervention, there were seven deaths, and this was expected to have been an underestimate owing to the reliance on self reporting by doctors. Another study520 reported significant complication and death from diagnostic oesophageal gastroduodenoscopy as 1 in 1000 and 1 in 10 000 procedures respectively, but that patients' sex, age, or preference for sedation or endoscopist did not affect the morbidity rate.
A report in 2004 from the National Confidential Enquiry into Patient Outcome and Death523 identified a low mortality from therapeutic endoscopy, with the exception of percutaneous endoscopic gastroscopy (PEG), which had a mortality of 6%. The report made many recommendations to improve the structure and process of therapeutic endoscopy, including the importance of careful selection for PEG insertion and ERCP, and the importance of endoscopy for gastrointestinal haemorrhage being undertaken only by experienced endoscopists.
More complications can be found for GI related surgery, especially for older patients.524,525 For malignant bowel obstruction, it is suggested that patients should only undergo surgery if their life expectancy is at least two months,526 but endoscopic enteral stents for patients with this disease is a safe and cost effective alternative.527
4.6.5 Information to patients and practitioners
There seems to be widespread encouragement for initiatives aimed at improving the information flow between patients and practitioners.511,513,528,529,530,531,532,533,534 Key issues include the need for nurses and doctors to give relevant and holistic information to patients undergoing gastroscopy at the right time532; information leaflets on drugs, illnesses, and diet511,513,529,530,531; and the need for practitioners to be more alert and vigilant in identifying the need to provide information.528,533,534
Studies also highlight specific problems due to poor communication. Sewitch et al found that a poor or ineffectual conversation between patients and practitioners increased the risk of intentional non‐adherence to IBD drugs by patients.535 It is also interesting to note that in a survey of around 800 patients undergoing colorectal cancer screening in the USA by Greiner et al,536 61% felt they had inadequate or no time to discuss colorectal cancer with their physician. It was suggested that new and creative methods are needed to satisfy patients' information needs and encourage discussion. In the UK, a study in 1997537 highlighted that the opportunity to educate and inform patients about IBD in outpatient clinics is often wasted, as practitioners neglect to mention key information sources such as the National Association for Colitis and Crohn's Disease, especially to patients with long term chronic diseases. There is evidence that patients are more satisfied with the information given before and after endoscopy, when it is given by nurses rather than doctors.523
4.6.6 Speed of diagnosis and complications of care
Not surprisingly, a considerable number of reports have been published on diagnosis for GI disease. Thirty five studies examining the topic in great detail were included in this report. For the most part, these highlight the need for a quick and accurate diagnosis for a spectrum of GI illnesses, including IBD,538,539 IBD in children,540,541 IBS,542,543 abdominal pain in the elderly,544 coeliac disease,174 gastro‐oesophageal reflux disease,545 dyspepsia,546,547,548 disorders of the large bowel,549 ultra‐short bowel disease,550 functional bowel disorders,551 Crohn's disease,552 and acute bowel ischemia.553 Complementing these are studies which deal specifically with the diagnosis for GI related cancers.554,555,556,557,558,559,560,561 Some research was also found on the use of diagnostic procedures (which were largely effective) such as colonoscopy and biopsy,562 oesophago‐gastroduodenoscopy,563 imaging techniques (for example, computed tomography, ultrasonography, and MR scan),553,558 push enteroscopy,564 and molecular‐pathological diagnosis.565
However, there is also evidence which points to the possible complications which may arise during the treatment of patients or due to the procedures mentioned above, or both. Lang et al suggest that resources and costs double for patients who develop complications after undergoing gastroenterological surgery.566 For upper GI endoscopy, Quine et al warn of the risk of perforation during diagnostic and therapeutic procedures,521 a problem which seems to be occurring at a significant rate owing to inexperienced practitioners. A similar concern is also voiced by a study by Schofield,567 where alleged negligence comes from the activity of GPs, gynaecologists, and colorectal surgeons, and patients receive laparoscopic injuries such as bowel perforation, bleeding, and major vascular damage. In a small study of coeliac disease by Hin et al,378 attention has also been given to problems of underdiagnosis and misdiagnosis.
Clearly, a strong body of evidence exists on providing adequate diagnostic services, which require appropriate training and stringent assessment to ensure patient safety. If this is achieved, the problem that remains is not one of effectiveness, but of ensuring that a sufficient level of service to support the inevitable rise in demand is available.
4.7 Drivers for change
4.7.1 New evidence and guidelines about best care
A plethora of literature can be found on a range of topics concerning guidelines for the care of GI diseases. A total of 45 studies were included for this section of the report, but only a brief summary will be given here owing to the sheer amount of information they cover; readers with a special interest in this area are advised to examine these and other related documents in greater detail. As with previous sections, evidence here can be broadly divided into two areas—treatment and procedures. Some examples include guidelines for the treatment of colorectal cancer,505,556,568,569 bowel cancer,507 other GI related cancers,570,571,572 Barrett's oesophagus,573 dyspepsia,546,574,575,576,577,578,579,580,581,582 IBD,583,584,585H pylori eradication,586,587,588, and other GI related diseases.589,590,591,592,593,594,595,596,597,598 For procedures: colonoscopy,599,600 endoscopy,600,601,602 and coloproctology.603 Despite the quantity of such studies, there remains a distinct lack of reference to service provision—in those where such topic was examined, only tentative suggestions are given, or where more substantial studies have been carried out, conclusions lack an evidence base.574,576,578,598,604,605,606 In light of these findings, and the general lack of an evidence based framework for GI service delivery, there is clearly a pressing need for more research and planning of how services should be delivered and the resources required to meet the demand.
4.7.2 Changing incidence of cancer
Colorectal cancer incidence increased by about 20% among men and by 5% among women from 1971 to 1997. However, reflecting large improvements in prognosis over time, mortality rates fell by 20% among men and by 34% among women (fig 4.7.1A).
Figure 4.7.1 Trends in standardised incidence and mortality rates (per 100 000 population) for gastrointestinal cancers, among men and women in England and Wales, 1971–97 (A) for colorectal cancer; (B) for oesophageal cancer; (C) for gastric cancer; (D) for pancreatic cancer. Source: Quinn et al, 2001.14
For cancers of the oesophagus, incidence and mortality both increased by about 60% in men 1971 and 1997, illustrating the poor prognosis associated with oesophageal cancers. Among women, incidence increased by about 40% and mortality increased by about a quarter (fig 4.7.1B).
The incidence of gastric cancer fell sharply by 40–50% in both men and women. Reflecting, improvements in diagnosis and treatment, mortality fell slightly more sharply than incidence; by about 60% in both men and women (fig 4.7.1C).
The incidence of pancreatic cancers fell by about one sixth in men from 1971 to 1997, but remained stable in women. With extremely poor prognosis for pancreatic cancers, annual mortality rates closely tracked incidence rates (fig 4.7.1D).
Figure 4.7.2 shows slightly updated trends up to 2002 for the incidence of each of the four main gastrointestinal cancers separately in England, Wales, Scotland, and Northern Ireland. They show little further trend for colorectal and pancreatic cancers, but further increases in the incidence of oesophageal cancers among men in Scotland and in Wales, and further reductions in gastric cancer among men and women in all four countries.
Figure 4.7.2 Trends in standardised incidence rates (per 100 000 population) for gastrointestinal cancers, among men and women in England, Wales, Scotland, and Northern Ireland, 1991–2002 for (A) colorectal cancer; (B) oesophageal cancer; (C) gastric cancer; (D) pancreatic cancer. Sources: England: National Cancer Intelligence Centre, Office for National Statistics; Scotland: Information and Statistics Division, NHS in Scotland. Wales: Welsh Cancer Surveillance and Intelligence Unit; Northern Ireland: Northern Ireland: Northern Ireland Cancer Registry.215,454,455,456
4.7.3 Changing incidence of other gastrointestinal and liver diseases
For a few gastrointestinal diseases, such as acute appendicitis and peptic ulcer in most age groups, there has been a fall in incidence in the UK in recent years. However, for most other gastrointestinal and liver diseases, there have been increases in incidence or prevalence over time (see earlier section 3.2).
These include, in particular, liver diseases such as liver cirrhosis, including alcoholic liver disease, non‐alcoholic fatty liver disease, primary biliary cirrhosis and hepatitis C infection, which will have a major impact on health care in this area.
There have also been increases in the incidence of acute and chronic pancreatitis, gallstones disease, upper gastrointestinal haemorrhage, diverticular disease of the intestine, coeliac disease, irritable bowel syndrome, and Barrett's oesophagus.
For some gastrointestinal diseases, such as inflammatory bowel disease, there is little evidence of a discernible upward or downward trend in incidence in recent years, even sometimes after earlier increases during previous decades. However, because of improvements in treatment, care, and prognosis, the overall prevalence of these diseases continues to rise.
In summary, the overall burden of gastrointestinal and liver diseases has increased greatly in the past few decades, and will continue to rise in the future.
4.7.4 Screening programmes
A significant amount of research has been carried out into screening and surveillance methods for GI diseases. This is reflected by a total of 32 studies included in this report. On the whole, there is strong support for the development and use of widespread screening programmes for a wide variety of GI diseases, where the poor prognosis of GI cancers is mainly attributed to delays in diagnosis.324 Most of the evidence relates to GI cancers607,608,609,610,611 but also covers diseases such as Barrett's oesophagus,573,612,613Helicobacter pylori,614 GERD,615 and diarrhoea.616 The main problems in this area are the economic costs associated with such programmes–that is, that they need to be adequately managed and feasible608,610,614,615,617,618,619,620,621,622,623; need to control and ensure high quality screening practices573,607,612,613,624,625,626,627,628; and need to provide a greater awareness of the effectiveness of existing and new methods for screening.611,629,630,631,632,633,634,635
Currently the British Society of Gastroenterology recommends colonoscopic surveillance of patients with inflammatory bowel disease636 and colonic polyps.636
A national screening programme for bowel cancer is to start in England in 2006.637 This will have significant implications for endoscopy services.
4.7.5 Genetics
Medical genetics, in the form of Cancer Genetics Services already impacts on the delivery of GI services, albeit to only a relatively small extent, for those patients, and their relatives, who are at increased risk of GI tumours owing to some form of genetic predisposition and hence require some form of GI surveillance, usually by colonoscopy.638,639,640 Cancer genetics is a rapidly developing field, becoming increasingly sophisticated, and in the future, clinical genetics input is likely to extend to other common GI conditions—for example, IBD and coeliac disease.641,642 Advances in genetics will improve not only the ability to predict who is, or is not, at risk of certain conditions, but also improve diagnosis, partly through molecular pathology.643,644
Medical genetics will also play a part in other areas of GI services—for example, predicting a person's responses to drugs, including adverse events (pharmacogenetics), and predicting response of tumours to treatment (somatic genetics, as opposed to germline genetics). It is likely that pharmacogenetics will impact first on avoiding adverse events, with individual tailoring of prescriptions following later.641,642,645,646 Although the science of predicting response to treatment from an analysis of a tumour's genetics is in its infancy, certainly as far as GI medicine is concerned, it promises to deliver truly individualised treatment. A considerable amount of work needs to be done, however, to translate this into practice.644,646,647,648,649,650 More widespread molecular genetic testing of tumours will also reveal more people who are genetically predisposed and thus warrant the attention of cancer genetics services.
4.7.6 Prevention
Studies on the prevention of GI diseases are not as prolific as might be expected. Only five studies were found to examine the subject in any significant detail,512,651,652,653,654 and even in these varied in the topics and diseases covered. Among those included in this review, are the prevention of H pylori,654 traveller's diarrhoea,653 and NSAID related morbidity and mortality.512 Muller and Sonnenberg emphasise the beneficial effects of endoscopy for reducing mortality due to colorectal cancer and cancers of the large bowel, and outline its crucial role as a preventative procedure.652 Hulscher et al also discuss the role of interventions to increase preventative activities in primary care, and the need for more research to determine their effectiveness.651
4.7.7 Development of managed clinical networks
The complexity of some disorders has been a driver for the development of clinical networks that cover many disciplines across different healthcare organisations. The Calman‐Hine report was the catalyst for clinical networks to support the care of patients with cancer and it has been proposed that similar networks be set up for liver disease and hepatopancreatobiliary surgery.214
4.7.8 Quality assessment of endoscopy
At present, there is no agreed national approach to quality assessment of endoscopy, but this is now being remedied, after the appointment of a national clinical lead for endoscopy by the Department of Health. The following activities are in progress:
Development of a global rating scale
This is a scale that provides an indication of how a patient will experience having an endoscopy in an endoscopy unit. There are 12 items on the scale that reflect two dimensions: quality and safety of care, and customer care. A recent census in England using this scale was completed by >90% of endoscopy units. Further measurements will be done twice yearly. The scale has been underpinned with objective measures, and a web reporting system for the scale has been completed (http:www.grs.nhs.uk, accessed 18 January 2007). The scale is designed to support quality improvement and help inform patient choice as well as quality assure endoscopy units.
Ensuring the appropriateness of endoscopy and referral pathways
This work aims at streamlining the patient pathway. It is likely that the “Map of Medicine” commissioned by the National Electronic Library for Health will provide an electronic framework for referral pathways linked to choose and book systems (http://www.mapofmedicine.com, accessed 21 December 2006).
Development of a competency framework
A competency framework for all health professionals working in endoscopy is currently being prepared. This will form the basis of certification of trainee endoscopists and endoscopy assistants.
Re‐validation of established endoscopists
A re‐validation methodology for established colonoscopists is currently being tested. Only those who have successfully completed this process will be allowed to perform colonoscopy on patients referred for colonoscopy through the bowel cancer screening programme which began in 2006.
Accreditation of endoscopy units
A process for accreditation of endoscopy units is currently being designed and tested. This peer review type process will replace the self completed questionnaire accreditation process required by the JAG (Joint Advisory Committee on Gastrointestinal Endoscopy). Formal accreditation of units which began in 2006.
Development of quality and safety markers
The BSG endoscopy committee is currently preparing quality and safety markers for endoscopy that will underpin the Global Rating Scale and the accreditation process.
5. Models of service delivery and their effectiveness: overview of the research literature
5.0 Methods
5.0.1 Systematic review of evidence
To promote a reliable, consistent, and unbiased reflection of existing research is the principal idea behind the use of systematic reviews. The establishment of numerous organisations such as the Cochrane and Campbell Collaborations which provide up‐to‐date reviews in the area of health, educational, and social research, and of course, a proliferation of such reviews and associated methodology in the traditional academic arena, underlines the sort of attention directed towards them over the past decade or so. In comparison with conventional literature reviews, systematic reviews are designed to answer a specific question based on research evidence rather than to provide a general overview of a topic. Thus, the practical advantages of systematic reviews and the associated analyses are generally transparent—to deliver a holistic summary and synthesis of individual pieces of research evidence which together constitute a stronger body of evidence. However, the immense resources needed for the retrieval, appraisal, and synthesis of the relevant literature are a prominent drawback. Indeed, the inspection of literally thousands of publications is not uncommon in systematic reviews, all of which require careful screening for inclusion or exclusion, from which only a small percentage can be deemed appropriate for the research question.655,656,657 Moreover, the processes undertaken during a review can be contentious, including claims that the review can be used as a means of exerting political control over new research; criticisms of the outcomes derived from it (with respect to relative importance and methods used); and the way in which users are to be involved throughout (see, for example, Davies658 and Gough and Elbourne659 for a more detailed discussion). Although these concerns are beyond the scope of this report, they represent real and substantial problems, which should be borne in mind throughout. What is clear, is that any critical evaluation of literature should take into account the techniques used in systematic reviews so as to promote consistency and reliability and obtain an accurate reflection of the work that is already out there. For these reasons, particular attention was placed on the design and development of suitable review methods with which to conduct a literature review and synthesis for this report.
At the heart of any systematic review is its review protocol. This consists of explicit criteria for the retrieval of relevant literature, and includes factors such as keywords, sources of information (such as databases, periodicals, and reports), and systematic methods for conducting and managing the search to enable repeatability—that is, a search that can be performed as many times as necessary by any researcher using the same criteria. The final list of factors deemed appropriate for the task is often referred to as the inclusion and exclusion criteria, which clearly specify the types of study to be included in the final analysis, as well as how, where, and with what the search is to be carried out. These factors ultimately determine the shape of the literature search, and hence the final outcomes of the study. The protocol thus represents the methodology which underpins the research, and forms the basis for the evaluation of the data obtained. A crucial element in this report was therefore the design and implementation of a protocol which adheres closely to the established conventions of systematic reviews and one which could be applied with a high degree of repeatability and consistency using the available resources so as to enhance the quality of information for the final analysis.
Design aspects of review protocols660 were taken into account to enable a reliable method of literature and data retrieval to be constructed, particularly for the central aspects of the investigation. It can also be seen later in this section that an extensive quality appraisal and grading of evidence was carried out to enhance the interpretation of the findings. The first subject to be examined was specification of the research question. This was deemed to be of two parts: (a) the current burden of GI disease and services (representing the general areas of the report), and (b) service provision and its effectiveness for GI disease in the UK (section 5). In order to tackle these areas, two sets of criteria for the protocol (such as those mentioned above) needed to be established. The first protocol would be used for sections dealing with general GI topics (the burden of GI disease), while the second would be developed to focus on service provision and its effectiveness for GI treatment. Figure 5.1.1 provides a map of the various stages of the protocol to be incorporated.
Figure 5.1.1 Conceptual map of the review protocol (adapted from Horvath and Pewsner661 and Khan et al662).
As can be seen in fig 5.1.1, the report follows the general structure of a systematic review, with the main difference being that a broad set search strategy is used for related topics of interest, while a separate criteria is used for the main area of study. This approach thus allows key points of interest to be reviewed systematically, and related areas to be incorporated into other sections of the review. The remainder of this chapter outlines the various components of the review protocol used in this study.
General search strategy
The design of this search is to examine the current burden of GI disease and services in the UK. It was expected that a wide a variety of literature would be encountered during the study, which includes paper based and electronic articles, research articles, general reports, and systematic reviews. To determine the basis for inclusion and exclusion, a consideration was made of the following topics:
Relevance of content
Searches in electronic databases were carried out using the keywords shown in table 5.1.1; only those which adhered to the aims of the report were included. Manifestly, it was not possible to incorporate every conceivable synonym under each subject heading as this would not only make the search process unmanageable but would probably also make the search too broad for the requirements of this report. Nevertheless, the words shown in table 5.1.1 were defined after thorough consultation with subject experts and librarians, and can be considered accurate for the purpose. Furthermore, terms used as part of medical subject headings (MeSH) produced by the National Library of Medicine were used where permissible (such as Cochrane) as a thesaurus to cover a broad range of keywords and synonyms. Although keywords, synonyms, and MeSH terms allow a considerable amount of literature to be retrieved, the specificity and appropriateness of content might require a more detailed examination because publications may or may not be relevant even with the presence of certain keywords. Consequently, an in‐depth assessment of abstracts and, where required, the entire article or report, was carried out by researchers and subject experts where the appropriateness of content was uncertain. A more detailed description of this and actual search techniques can be found below.
Table 5.1.1 Keywords.
| A. Gastroenterology | B. Burden | C. Services | D. Evaluation/study type | E. Setting/population |
|---|---|---|---|---|
| 1. Biliary | 1. Burden | 1. Colonoscopy | 1. Appraisal | 1. UK |
| 2. Bowel | 2. Delay | 2. Community care | 2. Assessment | 2. UK |
| 3. Digestive system | 3. Epidemiology | 3. Diagnostic | 3. Audit | 3. Britain |
| 4. Dyspepsia | 4. Load | 4. Emergency | 4. Benefit | 4. England |
| 5. Gastroenterology | 5. Morbidity | 5. Health maintenance organisation | 5. Best practice | 5. Ireland |
| 6. Gastrointestinal | 6. Mortality | 6. Management | 6. Cohort | 6. Scotland |
| 7. Hepatology | 7. Need | 7. Nurse practitioners | 7. Cost | 7. Wales |
| 8. Intestine | 8. Open access | 8. Economic | ||
| 9. Liver | 9. Organisation | 9. Effectiveness | ||
| 10. Pancreatic | 10. Pathway | 10. Estimate | ||
| 11. Stomach | 11. Planning | 11. Evaluation | ||
| 12. Postoperative | 12. Evidence | |||
| 13. Primary care | 13. Experiment | |||
| 14. Professional roles | 14. Health promotion | |||
| 15. Provision | 15. Meta‐analysis | |||
| 16. Rapid access | 16. Observation | |||
| 17. Resources | 17. Outcome | |||
| 18. Role substitution | 18. Qualitative | |||
| 19. Secondary care | 19. Review | |||
| 20. Self care/management | 20. Study | |||
| 21. Self referral | 21. Survey | |||
| 22. Service(s) | 22. Trial | |||
| 23. Surgery | 23. Volume | |||
| 24. Tertiary care | 24. Waiting (time, list) | |||
| 25. Economic evaluation | ||||
| 26. Cost effectiveness analysis | ||||
| 27. Cost utility analysis | ||||
| 28. Cost benefit analysis |
Setting and population
Although a comparison of results from other countries would have been useful, this was outside what could be realistically achieved in the given time frame. Publications were thus restricted primarily to those relevant to the UK, but no stipulations were made about the population studied (such as men and women).
Date of research
Despite the emphasis on current issues pertaining to the burden of GI disease, older articles and reports are also of interest because they allow for interesting comparisons, particularly for the rate of development. As a consequence, no restrictions were placed on date (in the majority of databases used in this study, this would include studies published between 1966 to present), but primary focus was placed on more up‐to‐date literature.
Research methods
No specific requirements were made of certain study types or experimental designs. The expectation was that a wide variety of publications would be obtained for general concerns of GI disease (owing to the breadth of the subject), including survey, evaluative, and experimental studies. All types of study design were thus included in the search criteria, and included those published in peer reviewed academic journals, relevant reports, and systematic reviews. Owing to the wide range of sources, an important concern was that of literature assessment with respect to the overall quality of the articles used (that is, the reliability of the results) and the grading of evidence (see below).
Language
An inherent problem for any extensive literature review is that highly relevant articles and reports may be written in a language other than English. Given the report's primary focus on GI disease in the UK, this particular problem was not expected to be too important. Nevertheless, to guard against possible exceptions, and in particular, the obvious pitfall of excluding potentially relevant studies, the decision was made not to exclude on the basis of language. As far as resources permitted, the aim was to obtain and translate relevant foreign publications for the report where English titles and abstracts indicated potential relevance.
Inclusion and exclusion procedure
After a systematic search of the relevant sources (a discussion of the criteria is given below), a detailed screening of the articles retrieved was required to determine final inclusion or exclusion. To maximise the consistency and accuracy of this process, a pilot test was undertaken in which two researchers (one of whom is a gastroenterologist) carried out inclusion and exclusion on the same set of articles. Although perfect agreement is difficult to achieve, discrepancies were examined, from which a standard protocol was developed. The final set of articles were then individually screened and categorised into one of two groups—include (including borderline cases with some degree of relevance) and exclude.
The next stage was thus the development of the actual search strategy to incorporate these various requirements. The crux of this process is defined by the terms set out in table 5.1.1. Although these help to increase the accuracy and specificity of the search, there are literally tens of thousands of combinations (that is, searches) possible by using a word from each of the five columns, making the workload virtually unmanageable. Despite this apparently colossal task, the use of Boolean operators (AND, OR, NOT) allowed the search to be conducted with greater efficiency. The fields in which individual terms were searched are article/report keywords, abstracts, title, and where possible, MeSH categories.
The configuration of Boolean operators and keywords is shown in fig 5.1.2. This procedure sets the specificity of the search, and can easily be broadened or narrowed, if necessary, depending on the quantity of articles retrieved. Should it be found, for instance, that a search combining all five columns in table 5.1.1 yields results which are too specific (exemplified by a low number of studies retrieved), the search can be broadened by combining terms from only four columns, and so on. Although great care must be exercised throughout (it might be the case that the paucity of studies is due to the fact that very little has been written about the subject in question, rather than an inherent problem with the search strategy), this iteration was employed during the search process until the team was satisfied that coverage and specificity were adequate.
Figure 5.1.2 Search strategy.
The primary sources of literature are shown in table 5.1.2. Most of these are electronic databases available via the internet where the search strategy described above is implemented. Although these provide comprehensive coverage of relevant sources of information, further searches were carried out of other sources (such as general internet searching, citations from relevant articles, and articles identified by existing GI projects within the department) using the same stipulations on content as described above.
Table 5.1.2 Primary literature sources.
| Academic journals: hand searching; journal databases; reference lists; existing projects |
| Centre for Reviews and Dissemination (CRD—includes DARE database) |
| Cochrane Collaboration |
| Embase |
| Health management and policy database (HMIC) |
| Medline |
| National Institute for Health and Clinical Excellence (NICE) |
| NHS Service Delivery and Organisation (SDO); Department of Health |
| Other internet based sources—for example, Institute for Food Research; Gut Week |
| SIGLE (grey literature) |
Search criteria for service provision
After the general search, a separate search strategy was developed for the key area of the report: the provision of services and their effectiveness for GI treatment. Among the criteria, attention was placed on specifying the literature with a more appropriate set of search terms. Inevitably, there would be a degree of overlap between this and the general search described in the previous section owing to certain similarities in the nature of content. But because search strategies are rarely foolproof and do not find all the desired material, this search helped to identify a greater number of relevant articles.
The definition of new keywords for the key area was established by using those shown in table 5.1.1 and after a further consultation with subject experts. The revised search terms shown in table 5.1.3 are similar to those for the general search, with the differences being four columns as opposed to five (the burden of disease is excluded, thus making this search broader than the previous one), and four additional keywords relating to the subject of effectiveness. This aside, all other stipulations are the same as those described in the previous section: characteristics of literature; use of keywords from each of the four columns with Boolean operators (as before, the number of columns can be reduced to allow coverage to be broadened as necessary); literature sources; and the process of inclusion and exclusion. The new search strategy was brought together using the technique shown in fig 5.1.2.
Table 5.1.3 Keywords for key areas of report.
| A. Gastroenterology | B. Services | C. Effectiveness/study type | D. Setting/population |
|---|---|---|---|
| 1. Biliary | 1. Colonoscopy | 1. Appraisal | 1. UK |
| 2. Bowel | 2. Community care | 2. Assessment | 2. UK |
| 3. Digestive system | 3. Diagnostic | 3. Audit | 3. Britain |
| 4. Dyspepsia | 4. Emergency | 4. Benefit | 4. England |
| 5. Gastroenterology | 5. Health maintenance organisation | 5. Best practice | 5. Ireland |
| 6. Gastrointestinal | 6. Management | 6. Cohort | 6. Scotland |
| 7. Hepatology | 7. Nurse practitioners | 7. Change | 7. Wales |
| 8. Intestine | 8. Open access | 8. Conventional | |
| 9. Liver | 9. Organisation | 9. Cost | |
| 10. Pancreatic | 10. Pathway | 10. Economic | |
| 11. Stomach | 11. Planning | 11. Effectiveness | |
| 12. Postoperative | 12. Estimate | ||
| 13. Primary care | 13. Evaluation | ||
| 14. Professional roles | 14. Evidence | ||
| 15. Provision | 15. Experiment | ||
| 16. Rapid access | 16. Future | ||
| 17. Resources | 17. Innovation | ||
| 18. Role substitution | 18. Health promotion | ||
| 19. Secondary care | 19. Meta analysis | ||
| 20. Self care/management | 20. Observation | ||
| 21. Self referral | 21. Outcome | ||
| 22. Service(s) | 22. Qualitative | ||
| 23. Surgery | 23. Review | ||
| 24. Tertiary care | 24. Study | ||
| 25. Survey | |||
| 26. Trial | |||
| 27. Volume | |||
| 28. Waiting (time, list) | |||
| 29.Economic evaluation | |||
| 30. Cost effectiveness analysis | |||
| 31. Cost utility analysis | |||
| 32. Cost benefit analysis |
Quality assessment and grading of evidence
Techniques of quality assessment are commonly applied to gain insight into the credibility and reliability of studies being examined. Although the measurement of quality (in this case, the likelihood of the methods generating unbiased results) is inherently difficult,657 numerous techniques have been developed to enable the quality of research methodology to be gauged with better clarity (see Verhagen et al663 for discussion). Given the time frame and resources permitted for this study, it was decided that an extensive examination of quality would not be feasible. Nevertheless, it was deemed necessary that a tool be adopted to analyse and ensure that the literature used is of an acceptable level of quality during the synthesis of evidence. In addition to quality, there was also a need to establish a means of grading the evidence (for example, systematic reviews, cohort studies, and expert opinion) in order to measure the overall strength of recommendations. Used together, the two techniques allowed studies to be assessed independently irrespective of study design, and graded collectively for the purpose of formulating clear and evidence based recommendations for GI service delivery. The following two subsections outline the methods used in this study.
Quality assessment
The purpose of this exercise is to provide a quantitative assessment of the quality of studies irrespective of their study design. Given that some study types are generally considered to be more reliable than others (for instance, systematic reviews are generally regarded as more reliable evidence than, say, consensus opinion—see next section), it was important that each study included in this report be assessed for its individual quality, rather than its design. The benefit of this approach is that extra weighting (if indeed justified) can be assigned to studies which are regarded as of a lower level of evidence, but which are nevertheless carried out with sufficient rigour to justify the findings carrying greater significance. The result is that the true quality of the evidence can be captured with greater clarity and the interpretation of recommendations can be enhanced.
Possible instruments available for this purpose include the Maastricht, Delphi, and Jadad lists (designed predominantly for randomised controlled trials (RCTs) and experiments), and the AGREE tool (for the assessment of clinical practice guidelines developed by the Appraisal of Guidelines Research and Evaluation Collaboration664). These are typically measurement/rating scales which share broad themes in an examination of the appropriateness, transparency, relevance, and hence quality, of the chosen methodology for the research question. Of those instruments suitable for this report, the AGREE tool seemed to be the most appropriate. Designed as a generic and relatively compact scale, it measures the quality of reporting and recommendations of clinical guidelines, and has been used by a wide range of medical institutions for evaluative purposes. Similar to tools such as the Delphi and Jadad lists, it covers various aspects of quality concerning clinical research. Some of the advantages of this tool include a concise 23 question/item scale as compared with the 40‐plus items in the Delphi list665 and the comparatively simplistic three item Jadad list666, a wide range of general components (as opposed to strict requirements on specific study designs such as RCTs), and its easy modification to suit the requirements of this study. Statistical tests conducted by Cluzeau et al667 also found a good level of reliability for individual sections and of the scale as a whole (Cronbach's α between 0.64 and 0.88, which exceeds or is close to the recommended value of 0.7668).
The AGREE tool evaluates quality via questions within each the following sections:
Scope and purpose
Stakeholder/participant involvement
Rigour of methodological development
Clarity and presentation
Applicability and relevance
Editorial independence.
As can be elicited from the above, all parts of the tool can be made directly applicable through minor adjustments of terminology for a quality appraisal of the literature used in this report. Through a consultation with subject experts and those with relevant expertise, such as statisticians and questionnaire designers involved with the project, appropriate modifications, mostly involving minor changes to words and phrases to make them relevant to literature, were made: the final instrument consists of 22 questions/items, and can be found in Appendix 2. The decision was made to use a three point Likert scale to measure the agreement, disagreement, or undisclosed information (such as methodology) for each item: 0 = not specified (little or no evidence); 1 = disagree (some evidence); 2 = agree (good or strong evidence). A total score for each article was then calculated to obtain an indication of overall quality.
As a means of piloting the tool for validity and consistency, 20 articles were chosen at random and appraised by two project researchers. Scores for each item were assigned after reading the articles in detail and further discussion, and then total scores were calculated for each article included in the report; this score was simply a percentage calculated as the sum of scores for each of the 22 items divided by the maximum possible score, 44—thus, a paper with a total score of 22 obtained 50%. The next step was to determine how these scores could be usefully interpreted as an indication of quality. Although there are no clear guidelines for this, the general observation was that the higher the score, the greater the rigour and quality of the article, and hence the following intervals were used as a general indicator of quality (S = score):
S⩽45%—generally poor quality of evidence; falls short in a few key areas of quality (see (a) to (f) above)
45%<S<65%—generally reliable quality of evidence; falls short in one or more key areas of quality
S⩾65%—good quality of evidence; falls short only in a few items of quality.
Next, Cohen's κ669 was calculated in SPSS (Statistical Package for the Social Sciences) to determine the degree of agreement between the two assessors. Interrater reliability between two assessors was 0.894 for the 20 articles, indicating a strong degree of consistency. Because of resource limitations, the decision was then made for one assessor to appraise all the remaining articles in the study.
Grading of evidence
In addition to quality assessment, a means of classifying the evidence needed to be established in order to reflect the strength and type of evidence that has been used to formulate recommendations. The appropriateness of this approach, however, depends on the study question. Evidence hierarchies typically used for this purpose, being focused on effectiveness, may not fully acknowledge the validity of other studies which, despite taking into account a much wider range of issues, may be considered to be of a lower level.670 As this study focuses on service delivery, which encompasses a broad range of subject matter, which cannot always be measured or assessed easily by intervention studies of effectiveness, it would not be a complete surprise to find that the evidence collected for it reflects those studies which are placed lower down in the hierarchy, hence reducing the overall grades of recommendation. Nevertheless, it was thought that used in conjunction with quality appraisal, the grading of evidence would help to provide a wholesome reflection of existing research, and would provide a conventional framework within which to proceed.
The hierarchy used in this study is based on that documented in the Centre for Reviews and Dissemination (CRD)660 and NICE.671 But in light of the issues raised above, some minor modification (mainly to reduce the number of groups in the hierarchy) was made to allow for a wider range of studies to be graded with greater ease (table 5.1.4).
Table 5.1.4 Hierarchy of evidence.
| Level of evidence | Type of evidence |
|---|---|
| 1 | High quality or well conducted meta‐analyses, systematic reviews of randomised control trials (RCTs), or RCTs with a low risk of bias and direct topic relevance |
| 2+ | High quality or well conducted case‐control or cohort studies with a low risk of confounding, bias or chance and a good probability that the relationship is causal; RCTs without direct topic relevance |
| 2− | RCTs, case‐control, cohort studies, or surveys with a risk of confounding bias, or chance that the relationship is not causal |
| 3 | Non‐analytic studies (for example, case reports, case series) |
| 4 | Expert opinion, formal consensus, and policy documents |
| Guidelines | Guidelines set by clinical groups (for example, NICE, BSG, AUGIS)—see quality assessment for an appraisal of these |
Using this hierarchy, recommendations can be classified by the strength of evidence on which they were based (see table 5.1.5—adapted from CRD660; NICE671). However, in this document conclusions have been drawn, but recommendations have not specifically been made.
Table 5.1.5 Classification of recommendations.
| Class | Evidence |
|---|---|
| A | • At least one meta‐analysis, systematic review, or RCT rated as 1, and directly applicable to the target population, or |
| • A systematic review of RCTs or a body of evidence consisting principally of studies rated as 1, directly applicable to the target population and demonstrating overall consistency of results | |
| • Evidence drawn from a NICE technology appraisal. | |
| B | • A body of evidence including studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results, or |
| • Extrapolated evidence from studies rated as 1 | |
| C | • A body of evidence including studies rated as 2−, directly applicable to the target population and demonstrating overall consistency of results, or |
| • Extrapolated evidence from studies rated as 2+ | |
| D | • Evidence level 3 or 4, or |
| • Extrapolated evidence from studies rated as 2−, or | |
| • Formal consensus | |
| D (G) | • A body of evidence from guidelines published by clinical groups (for example, NICE, BSG, AUGIS). |
5.0.2 Focus group with patient and carer representatives
A discussion session with the Patient and Carer Network was held at the Royal College of Physicians, London, on 8 December 2004. Its aim was to highlight some of the key problems associated with the delivery of services in gastroenterology based on the review of evidence described above, and to get views on these from patients and carers which could be reflected in this report. Three researchers from the project team attended and designed the objectives for the session—JGW (observer/GI expert), HS (facilitator), and BI (scriber). After the review of evidence described above, four main topics for discussion were identified and presented by the facilitator for discussion. These were:
Greater self management by patients
Endoscopy outside hospitals
Should endoscopy be carried out by nurses or doctors?
Where should services be located—for example, primary, secondary, tertiary, specialist care?
A total of 11 patient and carer representatives were present on the day, and gave permission for the discussion to be recorded electronically for transcription. Two independent observers were also present, but did not partake in the debate. Discussions on each topic lasted for around 20 minutes, and participants were encouraged to raise their views. It was felt by the research team that a number of important concerns were raised in this session, and these have been incorporated into the results sections of this report.
5.1 Results
5.1.1 Literature search and synthesis
Using the methods described in section 5.0.1, a total of 5039 articles (1830 for search 1 and 3209 for search 2) were identified by the literature search for potential inclusion in the report. Further screening by two project researchers for inclusion and exclusion (see section 5.0.1) reduced the final number of articles included to 394. A further 38 articles and reports were identified through hand searching of related sources (such as reports, the internet, and periodicals), which gave a final total of 432 for the main sections of the document. Articles obtained but not used are given in table A.14 in Appendix 4.
Table A.14 Articles not used in this report.
| No | Reference | Reason for exclusion |
|---|---|---|
| 1 | Rakatansky H. Review article: gastroenterology and the pharmaceutical industry. Aliment Pharmacol Ther 2002;16:1859–66. | Too specific |
| 2 | Chaudhry F, Ashish K, Brant S. Saturday surgeries—do patients feel their needs can be met by alternative out‐of‐hours care? A questionnaire study. Br J Gen Practice 2003;54:46–9. | Too specific |
| 3 | Franks A. General practitioner with a special interest in dermatology—the dermatologist's perspective. Clin Med 2004;4:87–8. | Too brief, not gastroenterology |
| 4 | Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res 2004;56:271–8. | Too specific |
| 5 | Yacavone RF, Locke III GR, Provenzale D, et al. Quality of life measurement in gastroenterology: what is available? Am J Gastroenterol 2001;96:285–97. | Too specific |
| 6 | Morris JS. Laennec's stethoscope—the Welsh connection.; J Roy Soc Med 2004;97:137–41. | Too specific |
| 7 | D'Costa H, Taylor EW. Patient management following uncomplicated elective gastrointestinal operations. Br J Clin Pract 1990;44: 552–6. | Too treatment focused |
| 8 | Woolfson RG, Jennings K,Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997;132:1093–7. | Too treatment focused |
| 9 | Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer (Cochrane review). In: The Cochrane Library 2004, Issue 3. | Too treatment focused |
| 10 | Soares‐Weiser K, Brezis M, Tur‐Kaspa R, et al. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding (Cochrane review). In: The Cochrane Library 2004, Issue 3. | Too treatment focused |
| 11 | Selective Decontamination of the Digestive Tract Trialists' Collaborative Group. Meta‐analysis of randomized controlled trials of selective decontamination of the digestive tract. BMJ 1993;307:525–32. | Too treatment focused |
| 12 | Guenaga KF, Matos D, Castro AA, et al. Mechanical bowel preparation for elective colorectal surgery (Cochrane review). In: The Cochrane Library 2004, Issue 3. | Too treatment focused |
| 13 | Lewis SJ, Egger M, Sylvester PA, et al. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta‐analysis of controlled trials. BMJ 2001;323:1–5. | Too treatment focused |
| 14 | Logan AJ, Morris‐Stiff GJ, Bowrey DJ, et al. Upper gastrointestinal complications after renal transplantation: a 3‐yr sequential study. Clin Transplant 2002;16:163–7. | Too treatment focused |
| 15 | Henry DA, O'Connell DL. Effects of fibrinolytic inhibitors on mortality from upper gastrointestinal haemorrhage. BMJ 1989;298:1142–6. | Too treatment focused |
Given the broad nature of topics covered in the report, and that some articles cover many subjects, the next stage was to categorise each article into its main areas of relevance. This was performed by one researcher who, for each included article, skim‐read and recorded the topics covered (for example, incidence, mortality, quality of life). From here, the recording of evidence was compiled by assigning each of the articles to an appropriate section of the report, and then into summary tables. Details of each article (such as topics covered, quality score, grade of evidence, and key findings) were recorded so that an overall synopsis (or where appropriate, a recommendation) of the main topics could be derived.
5.1.2 Economics of GI services
A total of 153 articles were identified which dealt with some aspect of the economics of GI disease. The review was limited to studies undertaken in the UK, partly to keep the number of studies manageable but also because of recognised problems in transferring cost and cost effectiveness results between countries.672
The main overall message from the review is that there is a paucity of high quality economic studies in this area. The evidence for the economic burden of gastrointestinal disorders, and the cost effectiveness of treatment is summarised in section 5.5. Articles obtained but not used are given in table A.15 in Appendix 4.
Table A.15 Articles not used for economic analysis.
| No | Reference | Reason for exclusion |
|---|---|---|
| 1 | Sonnenberg A. Personal view: cost and benefit of medical rituals in gastroenterology. Aliment Pharmacol Ther 2004;20:939–42. | Too specific |
| 2 | Ladas SD, Malfertheiner P, Axon A. An introductory course for training in endoscopy. Dig Dis 2002;20:242–5. | Too specific |
| 3 | Dick A, Keady S, Mohamed F, et al. Use of unlicensed and off‐label medication in paediatric gastroenterology with a review of the commonly used formularies in the UK. Aliment Pharmacol Ther 2003;17:571–5. | Too specific |
| 4 | Cooper DL, Smith GE, O'Brien SJ, et al. What can analysis of calls to NHS direct tell us about the epidemiology of gastrointestinal infections in the community? J Infect 2003;46:101–5. | Too specific |
| 5 | Keys J, Beardon PHG, Lau C, et al. General practitioners' use of non‐steroidal anti‐inflammatory drugs in Tayside and Fife regions. J R Soc Med 1992;85:442–5. | No economics |
| 6 | Rembacken B, Fujii T, Kondo H. The recognition and endoscopic treatment of early gastric and colonic cancer. Best Pract Res Clin Gastroenterol 2001;15:317–36. | No economics |
| 7 | Dube MG, Lobo DN, Rowlands BJet al. Audit of acute pancreatitis management: a tale of two hospitals. J R Coll Surg Edinb 2001;46:292–6. | No economics |
| 8 | Langman M, Kahler KH, Kong SX, et al. Drug switching patterns among patients taking non‐steroidal anti‐inflammatory drugs: a retrospective cohort study of a general practitioners database in the United Kingdom. Pharmacoepidemiol Drug Saf 2001;10:517–24. | No economics |
| 9 | Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow‐up after curative resection for colorectal cancer:systematic review and meta‐analysis of randomized trials. BMJ 2002;324:813. | No economics |
| 10 | Pathmakanthan S, Murray I, Smith K, et al. Nurse endoscopists in United Kingdom health care: a survey of prevalence skills and attitudes. J Adv Nurs 2001;36:705–10. | No economics |
| 11 | O' Hanrahan T, Irving MH. The role of home parenteral nutrition in the management of intestinal failure—report of 400 cases. Clin Nutr 1992;11:331–6. | No economics |
| 12 | Thompson WG, Heaton KW, Symth GT, et al. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46:78–82. | No economics |
| 13 | Thomas‐Gibson S, Thapar C, Shah SG, et al. Colonoscopy at a combined district general hospital and specialist endoscopy unit:lessons from 505 consecutive examinations. J Roy Soc Med 2002;95:194–7. | Not relevant |
| 14 | Stanghellini V, Armstrong D, Monnikes H, et al. Systematic review: do we need a new gastro‐oesophageal reflux disease questionnaire? Aliment Pharmacol Ther 2004;19:463–79. | Non‐UK article |
| 15 | Spiegel BMR, Vakil NB, Ofman JJ. Dyspepsia management in primary care:a decision analysis of competing strategies. Gastroenterology 2002;122:1270–85. | Non‐UK article |
| 16 | Lin OS, Mannava S, Hwang KL, et al. Reasons for current practices in managing Barrett's esophagus. Dis Esophagus 2002;15:39–45. | Non‐UK article |
| 17 | Abuksis G, Mor M, Segal N, et al. Patient education program is cost effective for preventing failure of endoscopic procedures in a Gastroenterology department. Am J Gastroenterol 2001;96:1786–90. | Non‐UK article |
| 18 | Van Kouwen MC, Drenth JP, Verhoeven HM, et al. Upper gastrointestinal endoscopy in patients aged 85 years or more. Results of a feasibility study in a district general hospital. Arch Gerontol Geriatr 2003;37:45–50. | Non‐UK article |
| 19 | Lapane KL, Spooner JJ, Mucha L, et al. Effect of nonsteroidal anti‐inflammatory drug use on the rate of gastrointestinal hospitalizations among people living in long term care. J Am Geriatr Soc 2001;49:577–84. | Non‐UK article |
| 20 | Longobardi T, Jacobs P, Wu L, et al. Work losses related to inflammatory bowel disease in Canada:results from a National Population Health Survey. Am J Gastroenterol 2003;98:844–9. | Non‐UK article |
| 21 | Levy RL, Von Korff M, Whitehead WE, et al. Costs of care for irritable bowel syndrome patients in a health maintenance organization; Am J Gastroenterol 2001;96:3122–9. | Non‐UK article |
| 22 | Yim HB, Jacobson BC, Saltzman JR, et al. Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction; Gastrointest Endosc 2001;53:329–32. | Non‐UK article |
| 23 | Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology 2001;34:1089–95. | Non‐UK article |
| 24 | Pardo A, Durandez R, Hernandez M, et al. Impact of physician specialty on the cost of nonvariceal upper GI bleeding care; Am J Gastroenterol 2002;97:1535–42. | Non‐UK article |
| 25 | Fletcher DR. Peptic disease: can we afford current management? Aust N Z J Surg 1997;67:75–80. | Non‐UK article |
| 26 | Callahan CM, Buchanan NN, Stump TE. Healthcare costs associated with percutaneous endoscopic gastrostomy among older adults in a defined community. J Am Geriatr Soc 2001;49:1525–9. | Non‐UK article |
| 27 | Lang M, Niskanen M, Miettinen P, et al. Outcome and resource utilization in gastroenterological surgery. Br J Surg 2001;88:1006–14. | Non‐UK article |
| 28 | Richter JM, Wang TC, Fawaz K, et al. Practice patterns and costs of hospitalization for upper gastrointestinal hemorrhage; J Clin Gastroenterol 1991;13:268–73. | Non‐UK article |
| 29 | Parente F, Bargiggia S, Bianchi Porro G. Prospective audit of gastroscopy under the ‘three‐day rule': a regional initiative in Italy to reduce waiting time for suspected malignancy. Aliment Pharmacol Ther 2002;16:1011–14. | Non‐UK article |
| 30 | Quirk DM, Barry MJ, Aserkoff B, et al. Physician specialty and variations in the cost of treating patients with acute upper gastrointestinal bleeding; Gastroenterology 1997;113:1443–8. | Non‐UK article |
| 31 | The Burden of Gastrointestinal Diseases, The American Gastroenterological Association. Available at http://www.gastro.org/wmspage.cfm?parm1 = 669 (accessed 3 January 2006). | Non‐UK article |
| 32 | Delvaux M. Digestive health in the elderly: faecal incontinence in adults. Aliment Pharmacol Ther 2003;8(Suppl 3):84–9. | Non‐UK article |
| 33 | Marshall JK, Cawdron R, Yamamura DL, et al. Use and misuse of cost effectiveness terminology in the gastroenterology literature:a systematic review. Am J Gastroenterol 2002;97:172–9. | Non‐UK article |
| 34 | Provenzale D, Lipscomb J. A reader's guide to economic analysis in the GI literature. Am J Gastroenterol 1996;91:2461–70. | Non‐UK article |
| 35 | Gross CP, Canto MI, Hixson J, et al. Management of Barrett's esophagus: a national study of practice patterns and their cost implications. Am J Gastroenterol 1999;94:3440–7. | Non‐UK article |
| 36 | Froehlich F, Burnand B, Pache I, et al. Overuse of upper gastrointestinal endoscopy in a country with open access endoscopy: a prospective study in primary care. Gastrointest Endosc 1997;45:13–19. | Non‐UK article |
| 37 | Norum J, Olsen JA. A cost effectiveness approach to the Norwegian follow‐up programme in colorectal cancer. Ann Oncol 1997;8:1081–7. | Non‐UK article |
| 38 | Spiegel BMR, Targownik LE, DeRosa V, et al. The quality of published health economic analyses in digestive diseases:a systematic review and quantitative appraisal. Gastroenterology 2004;127:403–11. | Non‐UK article |
| 39 | Provenzale D, Wong JB, Onken JE, et al. Performing a cost effectiveness analysis:surveillance of patients with ulcerative colitis. Am J Gastroenterol 1998;93:872–80. | Non‐UK article |
| 40 | Maroun J, Ng E, Berthelot JM, et al. Lifetime costs of colon and rectal cancer management in Canada. Chronic Dis Can 2003;24:91–101. | Non‐UK article |
| 41 | Sonnenberg A, Inadomi JM, Becker LA. Economic analysis of step‐wise treatment of gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 1999;13:1003–13. | Non‐UK article |
| 42 | Loeve F, Brown ML, Boer R, et al. Endoscopic colorectal cancer screening:a cost‐saving analysis. J Natl Cancer Inst 2000;92:557–63. | Non‐UK article |
| 43 | Nietert PJ, Silverstein MD, Mokhashi MS, et al. Cost effectiveness of screening a population with chronic gastroesophageal reflux. Gastrointest Endosc 2003;57:311–18. | Non‐UK article |
| 44 | Sonnenberg A, Delco F, Inadomi JM. Cost effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000;133:573–84. | Non‐UK article |
| 45 | Baxter YC, Dias MCG, Maculevicius J, et al. Economc study in surgical patients of a new model of nutrition therapy integrating hospital and home vs the conventional hospital model. JPEN J Parenter Enteral Nutr 2005;29(Suppl):S96–105. | Non‐UK article |
| 46 | Chen SC, Rex DK. Review article:registered nurse‐administered propofol sedation for endoscopy. Aliment Pharmacol Ther 2004;19:147–55. | Non‐UK article |
5.2 Developments in service delivery
Shared care
Both the Department of Health673,674 and the British Society of Gastroenterology jointly with the Royal College of Physicians489 recommend that high quality services should be delivered locally whenever possible, with blurring of the traditional primary/secondary care divide, and should aim at promoting independence and self management when appropriate. An analysis of routine data suggests that more efficient use of services would result from greater integration between primary and secondary care,675,676 and this is recommended by the Royal College of Physicians of London, Royal College of General Practitioners, and the NHS Alliance for the improved management of chronic disease.677 The evidence for this, however, is from a study which examined the 11 leading causes of bed use—that is, it did not focus on GI disease. Two studies included an economic component for palliative care schemes for patients with cancer. Neither showed a difference between control and intervention groups.517,621
A rapid review of strategies to facilitate transferring specialised care into the community found some evidence to support moving diagnostic testing and outpatient follow‐up to primary care, but the studies did not deal with GI disorders.678
For inflammatory bowel disease, there is a strong evidence that patients benefit, and there is less demand on conventional services when comprehensive patient education is combined with easy and rapid access to specialist care when needed,679,680,681,682,683 although overall costs are broadly unaffected.679,682 Possibly, these findings could be extrapolated to other chronic gastrointestinal disorders, particularly irritable bowel syndrome.684 The particular problems faced by adolescent people with inflammatory bowel disease and the need for support in the transition from paediatric to adult care has been emphasised by the National Association for Colitis and Crohn's Disease (NACC).685
Total parenteral nutrition can be delivered at home686 and is a safe alternative to early surgery in complicated Crohn's disease. It can also be cheaper,687 but increased resources will be needed if it is to be implemented for inoperable cancer.688 There are no economic evaluations of home parenteral nutrition for malignant disease and AIDS.687
Treating patients at home is not a cheaper alternative to inpatient care. The evidence for this, however, is from studies that did not focus on GI disease. Further research is needed, and treatment at home as an alternative to inpatient care cannot, at the present time, be recommended.689
The Royal College of Pathologists set recommendations on specimens of limited or no clinical value, which might lead to a reduction of pathology workload,603 but the cost effectiveness of this remains to be assessed.
Table A.1 summarises the articles examined for shared care.
Table A.1 Developments in service delivery: summary of articles examined for shared care.
| ID and authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Level of evidence | Quality score (AGREE) (%) |
|---|---|---|---|---|---|---|---|
| Kennedy et al683 | UK (1999–2000) | RCT | 700 Patients | Impact of a guidebook for self care | A whole systems approach to self management using a guidebook developed with patients and with physicians trained in patient centred care improves clinical outcomes and leads to cost effective use of NHS services. This method should receive more widespread use in chronic disease management, and seems likely to improve patient satisfaction and reduce health expenditure without evidence of adverse effect on disease control. Evidence suggests that further attention needs to be placed on self referral and access arrangements and a redistribution of control to patients through increased adherence to patient centred norms on the part of consultants | 1 | 93 |
| Williams et al679 | UK (1995–96) | RCT | 180 Patients | Open access follow‐up for IBD | Open access follow‐up delivers the same quality of care as routine outpatient care and is preferred by patients and GPs. It uses fewer resources in secondary care, but total resource use is similar. Better methods of ensuring urgent access to outpatient clinics are needed | 1 | 73 |
| Robinson et al681 | UK (2001) | RCT | 203 Patients | Guided self management and patient directed follow‐up | Self management of ulcerative colitis accelerates treatment provision and reduces doctor visits, and does not increase morbidity. This approach could be used in long term management of many other chronic diseases to improve health service provision and use, and to reduce costs. Nursing staff, appointment clerks, and secretarial teams need to be willing to assist with implementation of changes, and one person from the medical team needs to be available for patients to contact for advice | 1 | 66 |
| Shepperd and Iliffe689 | International (1996–2001) | Systematic review | 16 Trials | Hospital versus home care | This review does not support the development of hospital at home (active treatment by healthcare professionals in the patient's home) as a cheaper alternative to inpatient care. Providing that the views of carers are taken into account, early discharge schemes for patients recovering from elective surgery and elderly patients with a medical condition may have a place in reducing the pressure on acute hospital beds | 1 | 66 |
| Kennedy et al682 | UK (1999–2000) | RCT | 700 Patients | Self management in IBD | Adoption of guided self management was generally popular with both patients and clinicians, reduced use of hospital services without burden to primary care, and increased quality of care without an adverse effect on disease control at the same time as reducing cost. More widespread adoption of this programme for patients with IBD and other chronic medical disorders, particularly those with relapsing remitting patterns, now seems indicated | 1 | 75 |
| Ham et al675 | Comparison between UK and USA practice (2000–01) | Analysis of routine data | Hospital episode data for 2000 and 2001; data from Kaiser and Medicare systems | Comparison of hospital bed use | There is scope for hospital beds to be used in a different way in the NHS; primary and secondary care should be integrated to give priority towards self care; the NHS can learn from the Kaiser approach | 2− | 66 |
| O'Hanrahan and Irving688 | UK (1992; data taken between 1977 and 1991) | Analysis of routine data | 400 Records | Role of HPN | Recent reports have highlighted the palliative benefits of HPN, and the way in which it facilitates compassionate home care for carefully selected patients with inoperable malignant bowel obstruction. However, it is unlikely that the current financial constraints within which the NHS operates could cope with the demand associated with HPN | 2− | 66 |
| Evans et al686 | Canada (1996–2000) | Analysis of routine data | 15 Patients | Home total parenteral nutrition (HPN) | Patients receiving HPN benefit from reduced stress on the family, increased independence, and ability to perform normal work and study activities. All patients preferred HPN to hospitalisation and reported good or excellent quality of life. HPN is a safe alternative to hospitalisation or early surgery of patients with the complication IBD | 2− | 61 |
| Kennedy et al684 | UK (2003) | Survey of patients | 147 Responses | Development of self help book for patients with IBS | Guided and practical ways of support are required for people with IBS who want to self manage their condition. Patient information is essential for shared decision‐making, but most information is not patient centred and often does not involve the patient at all. All information should include patients at each development stage | 2− | 57 |
| Robinson680 | UK (2004) | Expert commentary | NA | IBD and patient empowerment | Self care is a normal human function and accounts for the management of three quarters of all episodes of ill health. More formalised applications include patients and doctors working collaboratively to develop a set of guidelines which patients use to manage their chronic disease themselves. Clinicians may be reluctant to pass control of treatment changes to patients, particularly the use of steroids. There are indications that passing ownership of management back to patients may improve compliance as patients realise their own responsibilities for remaining well | 3 | 59 |
| DoH674 | UK, NHS (2004) | Strategy and commentary | NA | Improving chronic disease management | A key approach to managing chronic disease is to support people to take an active role in managing their own care, specific conditions, and approaches that prevent these conditions from getting worse. This is linked with the development of GPwSI to provide patient centred care | 3 | 39 |
| RCP and RCGP677 | UK, NHS (2004) | Report/commentary | NA | Service provision | There should be active support for the development of GPs with special interests | 4 | 67 |
| BSG and RCP489 | UK (2003) | Expert commentary and recommendations | NA | GI service provision | Services and high quality care for patients and their families should be delivered locally whenever possible. Emphasis should be placed on integrating primary and secondary care, and moving hospital services closer to the patient | 4 | 43 |
| DoH673 | UK, NHS (2004) | National framework and commentary | NA | Outline of plans for national standards | Emphasis on the importance of improving the whole experience of patients, with particular attention to tailoring services to patients with long term conditions, promoting independence for older people, and supporting self care and the expert patient | 4 | 36 |
Primary care
NHS policy supports the development of the concept of general practitioners with special interest,690 though endoscopy is the only facet of gastroenterology that is covered in the recommendations.691 However, although 16% of GPs were already providing specialist interest services in 1998,692,693 there is currently no evidence to support the cost effectiveness of these changes.693,694
Few studies have examined the economic implications for primary care of H pylori testing and eradication.695 It is unclear whether such a strategy would be cost effective as an initial management strategy in primary care.696
Outreach educational interventions have been shown to improve appropriateness of referral to secondary care of patients with dyspepsia.580 However, before it is more widely used, further investigation is required to assess the overall cost effectiveness of this expensive intervention.697 A self help guidebook has been shown to reduce the number of primary care consultations for IBS, with cost savings.681
There is a high level of patient satisfaction with those units that currently offer endoscopy in primary care.698 Currently, no evidence exists to support the clinical and cost effectiveness of such roles,693,699 and the professional view is that they should be additional to, rather than a substitute for, secondary care.699 More research is needed. The problem of training136,700,701 and governance702,703 will need to be examined carefully.
There is, as yet, no published evidence for the safety, clinical or cost effectiveness of undertaking endoscopy or minor gastrointestinal surgery in diagnosis and treatment centres. Research is needed.
Table A.2 summarises the articles examined for primary care.
Table A.2 Developments in service delivery: summary of articles examined for primary care.
| ID and authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Level of evidence | Quality score (AGREE) (%) |
|---|---|---|---|---|---|---|---|
| Rubin et al136 | Northern England; UK (2000) | Survey of clinical records | 135 723 Patients | Epidemiology of IBD | GPs have expressed interest in shared care with gastroenterologists; need for GP training in the management of IBD in primary care | 2− | 73 |
| Jones and Bartholomew701 | UK (2002) | Survey of clinicians | 398 Responses | Survey to elicit views on GPwSI | This survey indicates that substantially more GPs than are required in the NHS plan are already providing clinical specialist sessions. There is, however, something of a mismatch between the clinical topics listed in the plan and those in which GPwSI are currently delivering their services. Strategic thinking at regional and PCG/T level is patchy, making the recent policy initiatives of the RCGP and the RCP of London particularly timely. Many GPwSI are likely to have developed a particular skill or expertise during hospital training, others after vocational training. These activities provide an important source of variety and stimulation, and there is evidence that recruitment and retention of GPs is enhanced by offering “mixed portfolio” job descriptions, and that patient outcomes may be improved. For example, the Primary Care Society for Gastroenterology has demonstrated a level of safety comparable to hospital endoscopy, associated with better access and very high levels of patient satisfaction | 2− | 70 |
| Littlewood et al700 | UK (2000) | Survey of GPs | 153 Survey responses | Study of GPs' research interests | GPs show interest in updating their practice and carrying out research; there is a difference in emphasis on health issues and research interests between GPs in different inner city trusts | 2− | 64 |
| Nocon and Leese699 | UK (2004) | Commentary and recommendations | NA | The role of GPwSI | It is not clear that GpwSIs are cost effective. What is clear is that they are additional to, rather than a substitute for, secondary care; allocations to secondary care can rarely be reduced as a result of GPwSI provision. Were any such reductions to be considered, the objections raised by hospital consultants would probably be so strong as to jeopardise their support for GPwSI schemes. In any case, a key driving force behind GPwSI policy is the reduction of waiting times. Evidence of cost effectiveness may be less important than policy objectives and professional interests | 3 | 73 |
| Kernick693 | UK (2003) | Commentary and recommendations | NA | Developing intermediate care with GPwSI | Although the development of GPwSI services is being encompassed within formal governance and professional development frameworks, and 16% of GPs are already providing services outside their core commitments, there is currently no evidence to support the effectiveness or cost effectiveness of these changes. In many areas, GPwSI development will build on existing historical services that may have actually encouraged inefficient use of resources. Developing GPwSI services is one of a range of options open to PCOs for developing the NHS modernisation agenda. This initiative forms part of an overall process of healthcare integration that sees the patient at the centre of a pathway of care. Although the move towards unified PCO budgets may facilitate this development, GPwSI service shifts may have a better chance of success when additional resources are available, rather than financing them from the removal of existing resources | 3 | 73 |
| Gerada and Limber703 | UK (2003) | Commentary and recommendations | NA | The role of GPwSI | The development of GPwSI is an exciting opportunity for GPs to develop their interests and widen their clinical horizons. However, it is important that PCOs and clinicians understand that if patient safety is not to be jeopardised, these services should be underpinned by robust clinical governance frameworks. The RCGP together with the NHS Modernisation Agency is currently developing frameworks in a number of clinical areas together with guidance for PCOs in developing this service further | 3 | 66 |
| PCSG698 | UK (2001) | Survey, commentary, and recommendations | 27 Primary care units | Endoscopy in primary care | The data suggest a very high level of patient satisfaction' the overall assessment of 98% was very good or excellent. This also highlights how sensitive patients are about waiting for appointments or waiting within the unit as their answers here are clearly at variance with the rest of their assessments. No official body has yet pronounced on the thorny issue of the maintenance of endoscopy skills, and knowledge. Three areas need to be examined: (a) number of endoscopies performed each year; (b) supervision of practical skill level; (c) maintenance of knowledge base. Although an endoscopist may make the actual examination, the nursing team resource the process from beginning to end. This ensures continuity and support for the patient, a safe and efficient endoscopy room, and well cared for and reliable equipment | 3 | 55 |
| Gerada and Harris702 | UK (2003) | Commentary and recommendations | NA | GPwSI | The development of GPwSI requires clear clinical governance, which includes appraisal and revalidation criteria, in order to maintain a good standard of care | 3 | 53 |
| NHS690 | UK (2003) | National guidelines, commentary, and recommendations | NA | Setting up GPwSI services | This guide focuses on the provision of a clinical service to patients by GPwSI. However, we acknowledge that this is only one aspect of the role of GPwSI. Equally important are the roles of GPwSI as a trainer, educator, and coach of other healthcare professional colleagues in raising overall standards of care. The GPwSI may also play a significant part in the strategic planning of services across a health economy | Guideline | 59 |
| DoH and RCGP691 | UK (2002) | Commentary and recommendations | NA | Implementing GPwSI | Progress to date suggests the introduction of GpwSI brings real and sustainable benefits for patients and the NHS. They are providing localised services, in familiar surroundings, with easier access and speedier care for patients. In addition, this role will help support GPs in their professional development and allow GPs with specialist experience and expertise to apply their skills and knowledge to best effect for the benefit of patients and local services. It will also improve management of workload between primary and secondary care and enhance the quality of referrals to consultants | Guideline | 45 |
Secondary services
A review of the literature published between 1980 and 1998 found a paucity of high quality studies that dealt with the effectiveness of specialised care in general hospitals.704 However, there is some evidence that patients admitted with gastrointestinal bleeding, acute pancreatitis, and acute liver disorders fare better when looked after by appropriate specialists.704,705,706,707
Six studies looked at access to specialist care; there is a serious underprovision of a colonoscopy service in most NHS hospitals. Training in colonoscopy is often inadequate and improved practice should result from better training. Unless there is a dramatic increase in manpower and resources available for lower GI investigations, the introduction of a national screening programme would rapidly overburden already inadequate facilities.556 A shortage of resources for coloproctology also exists708; some assessments of resource needs have been performed in cancer services.709 Although there is very little evidence on the cost effectiveness of CT colonography, this technique is widely available in the UK, although experience and throughput vary considerably. Limited CT scanner facility is the major barrier to further dissemination.710 There is also some evidence that greater access to specialist paediatric gastroenterology services for children with a suspicion of IBD should be sought.576 None of the above literature included proper economic evaluation, apart from the MINuET study of nurse endoscopy, which concluded that there would be no cost benefit, when compared with doctors.491
Notwithstanding the absence of cost benefit, there is now strong evidence that there should be a shift from doctors to nurses for diagnostic upper and lower GI endoscopy in hospitals.491,560,711,712,713,714,715 Other studies with an economic component which have examined the role of the nurse in undertaking tasks traditionally performed by doctors include upper GI endoscopy,174,529 managing children with GI disease,103 screening for colorectal cancer,716 running dyspepsia clinics,542 and administering propofol during endoscopy.717 Again, none included a proper economic evaluation. Research to evaluate the clinical effectiveness of other professionals in roles traditionally filled by doctors, such as dietician‐led coeliac clinics, is needed. The need for governance and accountability issues to be examined as roles change has been emphasised by the BSG.718
Long term follow‐up of patients with extensive ulcerative colitis, or patients receiving immunomodulators, or patients with Crohn's disease is appropriate.584 Colorectal cancer complicating ulcerative colitis is most commonly identified in patients who have been lost to hospital follow‐up.719,720
Table A.3 summarises articles examined for secondary services.
Table A.3 Developments in service delivery: summary of articles examined for secondary services.
| ID and authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Level of evidence | Quality score (AGREE) (%) |
|---|---|---|---|---|---|---|---|
| Provenzale et al704 | International (1980–98) | Literature review | 2157 Articles; 10 included | Specialised and general GI care | Gastroenterologists may provide better care than other provider types for certain disorders | 1 | 80 |
| Williams et al491 | UK (2001–04) | RCT and cost effectiveness study | 1800 | Nurse endoscopy | Nurses are clinically as effective as doctors but preferred by patients | 1 | |
| Delaney et al724 | UK (1995–98) | RCT | 442 Patients | Cost effectiveness of endoscopy for patients over 50 | Initial endoscopy in dyspeptic patients over 50 years of age might be a cost effective intervention | 2+ | 77 |
| CRD560 | UK (2004) | Commentary; review of evidence | Around 190 articles | Management of colorectal cancers | Nurse endoscopy (predominantly flexible sigmoidoscopy) is not uncommon and levels of satisfaction among patients using nurse‐led endoscopy clinics are consistently high. Where accuracy of diagnosis is reported, GPs and nurses who have received appropriate training perform as well as surgeons and gastroenterologists. A survey found that nurses carried out endoscopy in 43% of 176 units. The comparison between endoscopy performed by doctors and nurses showed equally good outcomes. Complications were not reported in any of these studies | 2+ | 59 |
| Pathmakanthan et al711 | UK (2000) | Survey of clinicians | 176 Responses | Nurse endoscopists in the UK | Nurse endoscopy is widely practised in the UK and is not limited to one procedure or carried out solely for diagnostic purposes. Perceived benefits include the reduction of waiting lists, reported good patient acceptability, improved care and safety. Most clinicians foresee a role for nurse endoscopy in the provision of endoscopic services, albeit in a limited capacity | 2− | 70 |
| Aly et al705 | UK (1999) | Survey of clinicians | 538 Responses | Non‐compliance with guidelines | It was clear in this study that the practice of hepatobiliary and pancreatic (HBP) specialists was more in keeping with UK guidelines than the practice of non‐specialists. Non‐specialists for whom guidelines might have most to offer by providing an easily accessible source of accumulated evidence and conclusions seem to have taken least heed of the advice offered. These results have implications for the rationale of creating guidelines, and for the strategies associated with their introduction | 2− | 66 |
| Smale et al712 | UK (2000–01) | Analysis of routine data; survey | 3489 Patients | Upper GI endoscopy performed by nurses | Experienced nurses perform routine diagnostic gastroscopy safely in everyday clinical practice and with as little discomfort and as much patient satisfaction as medical staff | 2− | 66 |
| Quirk et al707 | USA (1994–95) | Retrospective study; analysis of routine data | 124 Patients | Physician specialty | Patients admitted to hospital under the care of a gastroenterologist had shorter hospital stays that were less costly than patients under the primary care of general internists or surgeons | 2− | 64 |
| Bohra et al706 | Dublin, UK (2000) | Analysis of routine data | 242 Cases | Analysis of GI services | We recommend that patients are seen at the initial consultation by a registrar/fellow in most cases, and at a follow‐up consultation before discharge. Specialisation helps to improve quality of care, stimulates thought, aids training of junior doctors, and leads to cost savings, but constitutes a substantial workload for the gastroenterologist owing to endoscopic procedures and patient follow‐up | 2− | 57 |
| McKinlay et al713 | UK (2003) | Expert commentary and recommendations; survey of clinicians | 28 GI units; 67 GI consultants | Modernisation of the gastroenterology service in Scotland | A rapid expansion of the specialist GI nurse numbers mix is required to include endoscopy training where locally important to case mix. A large number of units in Scotland would like to employ more specialist nurses, particularly in the management of IBD. There is no doubt that nurses already make a significant contribution, particularly to the provision of upper GI endoscopy, which frees consultant sessions for more technically difficult procedures such as colonoscopy and ERCP | 3 | 52 |
Tertiary services
The Senate of Surgery of Great Britain and Ireland recommends that surgical care should be provided locally, but that patients should be moved to a centre of excellence for further specialist care when appropriate.721 It is the view of professional societies that complex hepatology, hepatobiliary surgery, and liver transplantation should also be delivered in specialist, tertiary centres.214,722 For complex hepatology the expert opinion is that this should be supplemented by clinical networks of specialists in secondary care.214
Many individual studies suggest that complex surgery for cancer, including upper723,724 and lower gastrointestinal,559,560,725 hepatobiliary,373,726,727,728 and pancreatic malignancy,716,729,730,731,732 should be performed at specialist centres which look after larger numbers of patients with these diseases. A systematic review by the NHS Centre for Reviews and Dissemination confirms this view.557 However, an analysis, also from the NHS Centre for Reviews and Dissemination, points out that the evidence comes from methodologically weak studies which do not sufficiently take account of differences in case mix, and thus probably overestimate the impact of volume of activity on the quality of care. It also highlights the fact that there is very little research (and none in the UK) that directly evaluates the effects of mergers on costs.733 An international literature review suggests that it is not possible, on present evidence, to define the optimal configuration of services for oncology.734 A retrospective analysis of routinely collected data by surgeons in a district general hospital concluded that pancreatic surgery could be performed safely in such locations with good short and long term outcomes.735 Conversely, there is no clear evidence that distance from specialist services is associated with poorer outcomes.733
The implications for district hospitals of increasing concentration of specialist services in tertiary centres have not been formally modelled and a detailed review of concentration and choice warns of increasing costs without proven improvements in quality for all patients.733 Other evidence shows that after adjusting for prognosis and treatments, cost‐volume relationships become U‐shaped, reflecting more active intervention by higher volume doctors along with little activity and long stays for low volume doctors. This non‐linear relationship between cost and volume suggest that highly concentrated cancer care might lead to inefficient resource allocation.716 Furthermore, it may denude secondary care of the expertise needed to manage less serious gastrointestinal and hepatic disorders.733 A systematic review of more than 100 studies in the international literature found little evidence to suggest that merging hospitals will result in better patient outcomes.736
Thus the optimum configuration of secondary and tertiary services remains uncertain on present evidence, and it cannot be assumed that improvements in outcome or efficiency will be achieved by increasing the number of patients seen by a unit or individual practitioner through concentration of specialised skills on a single site. An equally valid conclusion is that improvements are derived from better training and experience of practitioners, with access to well trained colleagues in other disciplines, and supported by adequate facilities. An overwhelming conclusion is that high quality research is needed, and that either radical change should await the findings, or be rigorously evaluated as it is implemented.
Table A.4 summarises articles examined for tertiary services.
Table A.4 Developments in service delivery: summary of articles examined for tertiary services.
| ID and authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Level of evidence | Quality score (AGREE) (%) |
|---|---|---|---|---|---|---|---|
| Ferguson et al733 | UK, NHS (1997) | Review of evidence and recommendations | NA | Concentration and choice of hospital services | Specialisation has important implications for service configuration—a small trust may need to employ more consultants to provide the required skills. There is no compelling reason to believe that further concentration of hospital services will improve efficiency or clinical outcomes. In consideration of the negative effects of concentrated access and utilisation, the implications for disadvantaged groups (for example, smaller departments with low funding) should not be overlooked | 1 | 83 |
| Sowden736 | International (1995; literature taken between 1985 and 1994) | Systematic review | Over 100 studies | Relationship between volume and quality of health care | There is little evidence as to whether merging hospitals to create larger units will result in a change of outcomes. Owing to this uncertainty, caution should be exercised in using research literature to justify policies of reorganisation of healthcare delivery. The main recommendation is that policy makers should be cautious when invoking the assumed improvements in outcome achieved by volume as a key argument for centralisation of services | 1 | 75 |
| Grilli et al734 | International (1980 onwards) | Literature review | 47 Papers | The impact of specialised services | The impact of specialised cancer care has been poorly assessed. It is not possible through existing studies to define an optimal configuration of health services for oncology | 1 | 73 |
| Bachmann et al730 | England and Wales (1996–97) | Cohort follow‐up study | 782 Patients | Specialised care for pancreatic cancer | Specialisation of pancreatic cancer care in hospitals was associated with longer survival. Patients referred to less specialised doctors and hospitals were less likely to be investigated thoroughly. A concentration of pancreatic cancer care into higher volume hospitals is likely to improve survival even among patients with incurable disease | 2+ | 73 |
| Bachmann et al723 | Southwest England (1996–97) | Analysis of routine data | 1512 Patients | Analysis of specialised GI cancer care | Lower mortality is associated with more specialised care; the concentration of cancer care in the UK is supported | 2+ | 72 |
| CRD557 | UK, NHS (2000) | Commentary; review of evidence | Around 230 articles | Management of upper GI cancers | There is evidence for each type of GI cancer that treatment in hospitals which manage larger numbers of these patients, and/or by clinicians who see larger numbers, leads to better outcomes. There should be clearly documented policies for patient referral between hospitals, and for the processes by which clinicians seek advice from specialist treatment teams about the management of patients for whom referral may not be appropriate | 2+ | 66 |
| CRD559 | UK (1997) | Commentary; review of evidence | Around 130 articles | Management of colorectal cancers | An American study found that trained nurses were as likely to discover cancers by sigmoidoscopy as gastroenterologists; patients were more willing to return for a repeat procedure after examination by a nurse. There is some evidence that volume of activity and specialisation may be associated with better surgical technique or practice | 2+ | 59 |
| CRD560 | UK (2004) | Commentary; review of evidence | Around 190 articles | Management of colorectal cancers | Six systematic reviews and a number of more recent primary studies were consistent in showing evidence that for rectal cancer at least, higher patient volumes and greater specialisation among surgeons were associated with much better outcomes, lower surgical complication rates, decreased local recurrence, lower colostomy rates, and improved survival | 2+ | 59 |
| Senapati et al726 | UK (2003) | Survey of clinicians | 583 Survey responses | Surgical management of cholelithiasis | Management of cholelithiasis in patients with acute biliary pancreatitis in the UK remains suboptimal. Moreover, only a minority of surgeons offer patients presenting with acute cholecystitis the benefits of early laparoscopic cholecystectomy. The management of acute biliary disease might be improved if these cases were concentrated in the hands of surgeons with upper GI/hepato‐pancreato‐biliary interest and those who perform laparoscopic cholecystectomy regularly | 2− | 73 |
| Bachmann et al716 | Southwest England and south Wales (1996–97) | Cohort follow‐up study | 2294 Patients | Costs of GI cancer care and specialisation | A greater concentration of specialised hospital cancer care will cost more; doctors' specialisation is as important as hospital specialisation for the effectiveness of cancer care | 2− | 73 |
| Parks et al729 | Scotland (1993–97) | Analysis of routine data | 2794 Patients | Benefits (or otherwise) of specialised GI cancer care | Surgically treated patients with pancreatic cancer are likely to fare better when managed by specialist pancreatic surgeons, or clinicians with an interest in this field. Specialisation and concentration of cancer care has major implications for service delivery | 2− | 68 |
| Neoptolemos et al731 | UK (1995–96) | Survey | 1026 Cases | UK survey of specialist pancreatic units | The data argue strongly in favour of concentrating pancreatic surgery into specialised units, and a higher referral rate from gastroenterologists exclusively to such units should be encouraged | 2− | 64 |
| McKiernan et al373 | UK (1993–95) | Analysis of patient data; survey | 93 Cases | Biliary atresia | Biliary atresia is a rare but important condition and this study confirms that outcome is better if surgical management is done in centres with experience. Throughout the study period referral to surgical centres appeared to be on broadly geographical grounds without any other obvious selection criterion. With this information the major challenge in the future management of biliary atresia will be to ensure that surgical management is rationalised to fewer centres. The need to facilitate the concentration of medical and surgical expertise, junior surgical training, and development of support services has received general acceptance in other areas of paediatric specialist practice. The data presented here suggest to us that children with biliary atresia should be managed in centres with a caseload of more than five cases annually to ensure a better outcome | 2− | 64 |
| Davenport et al727 | England and Wales (1999–2002) | Analysis of routine data | 148 Patients | Management of biliary atresia | Early results indicate that improvements in treatment and overall survival of biliary atresia can be achieved nationally through a centralisation of care to supraregional centres | 2− | 59 |
| Hutchins et al735 | UK (1992–98) | Retrospective study; analysis of routine data | 65 Patients | Pancreatic surgery in a district general hospital | Compared with specialised pancreatic centres, pancreatic surgery can be performed safely in general district hospitals with low mortality and morbidity, and good long term outcomes. This workload should be undertaken with a dedicated surgeon with the necessary ancillary facilities | 2− | 55 |
| Williams722 | UK (2004) | Survey | 34 Liver centres | Provision of specialist liver services | The survey showed substantial deficiencies in staffing levels, particularly of consultant hepatologists and specialist nurses. Of the various bottlenecks identified in support services, lack of expansion in radiological facilities was the greatest. The failure to provide dedicated beds for hepatology services and sufficient numbers of general and specialist outpatient clinics, as well as the waiting times in many of the centres, contribute to major limitations in service provision. Increasing the number of transplant centres would be one way of enhancing the level of provision of liver services generally | 2− | 52 |
| Duxbury et al725 | UK (1999–2000) | Analysis of routine data | 211 Patients | Management of colorectal cancer | This study found striking variation in practice within one hospital in Devon. Colorectal specialists were more likely to conform to best practice guidelines, involve the colorectal nurse specialists to a greater extent with patients with rectal cancer, and perform more extensive lymphadenectomy in rectal cancer surgery. Formal involvement of the colorectal specialist nurse further improves the opportunity for patient information | 2− | 48 |
| Majeed and Price728 | UK (1997–2002) | Analysis of service delivery | 615 Patients | Resource and manpower calculations for hepatobiliary surgical services | A centralisation of hepatobiliary surgical services is supported. We estimate that a minimum of two full‐time specialist hepatobiliary surgeons with appropriate ancillary support is required for a typical population of two million people in the UK | 3 | 64 |
| Andren‐Sandberg and Neoptolemos732 | International, with emphasis on UK (2002) | Literature review | 96 Articles | Pancreatic cancer | In the UK, pancreatic surgery must be concentrated into regional centres ideally serving catchment areas of 2–4 million. Smaller district hospitals will have the role of determining provisional diagnosis and stages. It seems highly likely that regionalisation of pancreatic cancer surgery will be adopted | 3 | 45 |
| Senate of Surgery of GB and Ireland721 | UK, NHS (2004) | Expert commentary and recommendations | NA | Configuration of healthcare services | Where appropriate, clinical care should be provided locally, but patients should be moved to a centre of excellence for further specialised care | 4 | 32 |
| BASL, BSG, AUGIS214 | UK (2004) | Expert commentary and recommendations | NA | National plan for liver services in the UK | Best clinical outcomes for hepato‐pancreato‐biliary surgery can only be achieved at specialised centres. A planned approach is needed to develop expertise within specialised units. Many patients with liver disease are managed by GPs, but there is a gradual shift towards involving gastroenterologists and nurse specialists | 4 (Guideline) | 50 |
5.3 Patient perspectives on service delivery
Topics discussed at the patients' workshop centred on four subjects identified from the review of policy and research evidence as of current concern (the brief sent to the participants is attached in Appendix 3):
Greater self management by patients
Provision of endoscopy services outside hospitals
Changing roles: should nurses or doctors carry out endoscopies and care of patients with chronic conditions?
Location of services: specialisation versus local care.
Greater self management by patients
Workshop participants were generally positive about the idea of greater self management, which would bring the benefits of a greater sense of control, and reduce anxiety about wasting the time of health professionals. Information and access to services when needed were felt to be key.
“As long as people are informed…[they] are very happy to self manage their conditions; they don't want to keep going off to hospitals, GPs etc, which are all getting more difficult to see these days anyway (participant 14).
For the access to services, it's all very well saying “if you're unwell, give us a call and arrange an appointment”, but if you do that you may not be able to get in through the door.” (participant 21)
Ability to self care was seen as more than simply being informed, however, and concern was expressed about those people who may agree to look after themselves but may not actually be able to achieve this without some support.
“The consultation is 10 or 20 minutes in hospital, how are you able to assess if the person is suitable for self management?” (participant 21)
Flexibility and continuity of care were considered important, with different patients having varying levels of need for support, and a perception that in a self management model, patients may be more likely to be treated by new professionals when they seek care than in a traditional model.
“Continued care is totally lost and frustration comes in for the patient, especially endoscopies. You know, different people doing endoscopies at different visit and giving different information to the patients” (participant 27)
Further to this, concern was expressed that GPs or others coming into contact with a patient attempting to achieve a greater level of self management may not understand the patient's level of control and may undermine the model.
“I find that difficult because when you go into the hospital you have your plan of treatment and what your input is, and you go to your GP and you don't get the same level of interaction.” (participant 21)
Provision of endoscopy services outside hospitals
Primary concerns expressed by participants in the workshop centred around risk and safety.
“There's obviously a risk factor. The one concern is there's got to be the backup to deal with that and the safety issues that come with it.” (participant 12)
“I have great concerns about this. There are GPs with special interests operating and carrying out endoscopy in smaller hospitals and patients are not offered sedation because, leaving out whether it is safe or not, they don't have resuscitation facilities.” (participant 14)
Participants were quite cautious about this model, and questioned whether, again, continuity of care would be adversely affected. There was a feeling that it might take time for patients to develop confidence in a system outside hospital.
“just because when you go into an endoscopy unit… it gives you that little bit of reassurance. I suppose it's just that you're not used to going down to the GP to have that done, but maybe over time people will get more used to it. There's bound to be lots of hesitation.” (participant 21)
Some benefits were mentioned, related to local access and quality of facilities:
“I think location has quite a bit to do with it because there are some parts of the country where hospitals are a long way away, whereas the local centre may be down the road. That would affect you and your ability to get to hospital when there's no public transport and you don't have a car.” (participant 11)
“Most people that I talk to really accept the diagnostic centres and …think they are excellent because most people do not want to go into hospital and don't like the atmosphere of hospitals. They find the centres to be more accessible, attractive, comfortable to be in…” (participant 23)
A distinction was made between minor, routine procedures that could be done locally and more complex investigations that needed to be carried out by specialist staff. There was concern that the most important thing was that the operator had the appropriate training and expertise.
“Is there not a difference between what are fairly minor things and very major things where I think most people will travel to a centre of excellence but for a slightly lower level of access? If I had cancer I would want to go to a centre of excellence.” (participant 23)
“Things like screening could be done at GPs for convenience, but anything more than that… needs expertise…in an ideal situation, then yes, I would like the endoscopy done near my home…but under the prevailing conditions, I think it would be dangerous to have a blanket statement saying that it's safe to do endoscopies in GPs surgeries.” (participant 27)
Changing roles: should nurses or doctors carry out endoscopies and care of patients with chronic conditions?
There was cautious support for changing roles in relation to endoscopies and aftercare, with an emphasis again on training, safety, and continuity of care. The importance of management of the “whole” patient was emphasised, whether this be done by a nurse or doctor.
Although some participants were positive:
“I want to support the role of nurses because they have a good track record in specialist roles in diabetes, cancer.” (participant 25)
there was still some anxiety about the safety of care by nurses:
“Presumably the nurses would have a backup of a doctor within the vicinity while this was taking place should something go wrong? That would be my worry.” (participant 19)
One participant seemed to sum up the feelings of the group:
“At the end of the day the label's pretty irrelevant in some ways. I could go to a doctor and get really good care and say doctors are brilliant, but you go to the doctor and not be happy. Or you could go to a nurse and it's brilliant. The label is irrelevant so long as the standard of training is equal to what they are doing.” (participant 21)
Location of services: specialisation versus local care
Views on this topic were mixed, and participants referred to their own experiences of specialist or local care to illustrate associated problems. This was clearly a complex topic, with many considerations and varied personal preferences.
“I think local expertise is important to me… the family has to be involved, it should be easier for them to visit, and should be nearby. For those reasons I am prepared to put up with slightly less expertise, but adequate and safe enough.” (participant 27)
“I've had a complicated gastric operation that had to be in [remote specialist centre]. That causes me a lot of problems because I'm isolated from my family and friends and that worries me.” (participant 24)
On the other hand, specialist care was valued by others, even at a distance:
“On a personal level, I'd be happy to go to the specialist centre because I have a specialised condition and I have confidence in the unit that I go to. So I'm prepared to travel rather than go local.” (participant 19)
It was seen as an important subject that may be eventually decided through local and national policy rather than on the basis of research evidence.
“It's such a major debate, not just for GI… on the whole because the people for local hospitals are so vocal in their campaign, that I think it is going to happen. And that may mean that there won't be those centres of excellence that there should be. It's a huge issue which, at the end of the day, will be decided politically.” (participant 23)
Participants understood the complexity of the debate:
“I don't think taking expertise away from hospitals is a good thing because you are narrowing down the number of people who can get access. Locality is important.”
“I think I'd support that view in terms of access, because if you have to have emergency access and go by ambulance, you may not be able to go to the specialist centre… [but] to the [local] hospital. So if they didn't have that expertise, it would be a disadvantage, but I agree that complex surgery needs to be done at specialist centres.” (participants 21, 25)
There was concern that some may benefit at the expense of others, with increased specialisation of services.
“I think it's got the potential of affecting people differently. You take somebody who's got a diagnosis of mental health, learning, old age, whatever—there's less likelihood of early diagnosis. The issue of having somebody to support them in hospital may be more of an issue. My concern is that …selection out of particular groups because you have a highly specialised service which doesn't actually want them…is a price paid by the minority of the population for having a better service for other people.”
Summary of key findings from the patient workshop
Greater self management by patients
Cautious support was expressed for greater self management—as long as care was taken to assess the ability of patients to self manage, continuity of care could be maintained, and services could be accessed when required.
Provision of endoscopy services outside hospitals
Views were mixed, with benefits of local access being recognised but concerns expressed about safety and continuity of care.
Changing roles: should nurses or doctors carry out endoscopies and care of patients with chronic conditions?
Participants agreed that appropriate training and skill level were more important than who delivers care, and the policy was supported if nurses were able to manage the “whole” patient safely.
Location of services: specialisation versus local care
Little consensus was reached across the group, with some participants expressing a preference for local care and others valuing specialist care, even at a distance. The needs of minority groups were emphasised.
5.4 Economic burden of GI disease
Studies which attempted to estimate the burden of GI disease tended to focus on individual conditions or on specific elements of the total burden. Only one study483 attempted a comprehensive costing of GI disease. It estimated the total burden in 1997 to be roughly £8000m, which included £3000m to the NHS and personal social services.
A further 20 studies attempted specifically to cost GI conditions: IBD,737,738 IBS,739,740,741 GI cancer,324 upper gastrointestinal disease (UGI),697,742,743,744 traveller's diarrhoea,653 non‐specific abdominal pain,745 colic,746 GERD,16 and dyspepsia.747,748 A further paper examined the economic consequences of waiting time for gallbladder surgery.749 Estimated costs are not presented here because of wide variations in the methods used and in the quality of the studies. Even the better studies, such as that on dyspepsia,747 pointed out the limitations of the study's external validity.
Modelling exercises dominated. Studies which extrapolated local results to the UK as a whole failed to deal with the geographical differentiation across the country. Most used a prevalence based approach, whereas an incidence based approach would have sought to estimate the lifetime costs of managing a cohort of patients first diagnosed in a given year. Most studies were merely “snapshots” based on national statistics and aimed only at indicating the possible scale of the problem without claiming precision. Suggestions for future research focused on ways to improve the quality/accuracy of routinely collected data, and on the need for prospective cohort multicentre studies to confirm results from modelling exercises.
Table A.5 summarises the articles examined for economic burden of GI disease.
Table A.5 Summary of articles examined for economic burden of GI disease.
| ID and Authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Bassi et al708 | UK (2004) | A six month cohort study | 307 Cases of ulcerative or undetermined colitis and 172 cases of Crohn's disease | Profile, determinants and scale of cost of IBD in UK | Inpatient services were required by 14% of the sample but accounted for 49% of secondary care costs. Drug costs accounted for less than a quarter of total costs. Individual patient's costs ranged from £73 to £33 254. Mean cost per patient were £1256 (CI 988 to 1721) for colitis and £1652 (CI 1221 to 2239) for Crohn's disease. The corresponding average cost for the ambulatory was respectively £516 (CI 452 to 618) for colitis and £539 (CI 497 to 589) for Crohn's disease. For the hospitalised group the mean costs were £6923 (CI 5415 to 8919) for colitis and £7658 (CI 5693 to 10561) for Crohn's disease. The mean cost of an “incident” was respectively, £2662 (CI 1006 to 5866) for colitis and £2111 (CI 1488 to 3078) for Crohn's disease. Survey suggested that the average costs for six months were <£30 per patient for primary care visits, and the median loss of earnings was £239 for colitis and £299 for Crohn's disease. The mean (range) for out‐of‐pocket expenses, prescription charges, OTC drugs, etc, was £40 (0–250) and £66 (0–750) for colitis and Crohn's disease | Level and profile of healthcare costs for IBD in Britain is remarkably limited. Robust CEAs data for rival treatments are lacking as few studies incorporated the prospective collection of resource data. Furthermore, the cost inputs for modelling exercises have relied on subjective cost estimates rather than real patient data. Diagnostic information is not routinely collected for most ambulatory care episodes. Also, there is no requirement to record patient borne costs. |
| Wells et al739 | UK (1995) | Review | NA | The burden of IBS | The study used the prevalence approach to estimate the NHS resource use. The number of GP visits was estimated on the Office of Population Census and Surveys, study of morbidity in general practice in England and Wales (1995) and indicated that 846 349 visits a year in the UK are IBS related. This represents about 10% of the total primary care workload for GI diseases and results in an expenditure of £13.1m per year. Prescriptions for all medicines for IBS were estimated using the DIN‐LINK database; a total number of 1.7 million prescriptions were written for IBS in 1995. This figure multiplied by the average net ingredient cost for each prescription for gastrointestinal medicines (£5.1) and including an average of £1 per prescription yields a total expenditure on GP‐prescribed medication for IBS of £10.5m. This figure was then adjusted to £12.5m to account for the difference between the prevalence estimated from the database used and the general practice survey. Cost in secondary care (outpatient and inpatient stays) were estimated at £16.4m. In addition it was estimated that 3600 people are admitted to hospital, generating a total of 18 000 beds days for a total of inpatient costs of £3.4m. Hence, the total cost to the NHS of 45.4 million a year | The authors give a warning about the accuracy of the estimates—the prevalence approach method relies on ICD coding. In those diseases where an unambiguous definition of the condition exists and which have a unique identification number in the ICD, it is a straightforward process. This is not the case for IBS, although one ICD number does refer to irritable colon, it is uncertain how accurately the volume of resource utilisation coded under this heading reflects the true magnitude of IBS |
| Akehurst et al740 | UK (6 GP surgeries in the Trent Region) (1999) | A case‐control study | 374 | Impact of irritable bowel syndrome on time off from work, utilisation and cost of health services and HRQoL | Patients with IBS had considerably lower HRQoL than controls. They scored worse in all dimensions of the SF‐36 and the EQ‐5D. The IBS group had 0.2 (95% CI −2.1 to 2.6) more days off than the control group; this difference did not reach statistical significance. A significantly higher number in the control group had no time off from work in the previous three months, and more people in the IBS group had more than a week off in the previous three months. On average, the patients with IBS cost the NHS £123 (95% CI 35 to 221) more each year than people in the control group (p = 0.04). The cost figures show that even using a conservative estimate of 9% of IBS prevalence, the total cost for the UK exceeds £200m a year, and for a 25% prevalence rate would rise to £600m a year | It is estimated that only about 30% of these are referred on to specialists. Further research would be needed to determine the impact of IBS on the quality of life of those affected who do not present to their GP |
| Creed et al741 | UK (northern England) (1998) | Cross sectional survey | 257 Patients (from secondary and tertiary GI clinics) who did not respond to the usual treatment and were recruited for a trial of psychological treatment | To determine whether the severity of bowel symptoms and psychological symptoms directly influence HRQoL and healthcare costs | Global severity and somatisation contributed to the physical component score of the SF‐36, but only psychological scores were associated with disability due to ill health. These variables did not predict healthcare costs (R2 = 9.3%). Resource use costs were collected prospectively; these were $1743±2263 and included $1338±2107 for secondary care costs, $410±424 for primary care costs, $2.7±30.8 for alternative treatments; $63.5±260 in patient borne costs; and $334±1052 from loss of productivity | This study is only representative of patients with IBS whose management is difficult because of refractory symptoms, marked disability, and high care utilisation. Further work is needed to assess what leads to high healthcare costs in these patients |
| Lewison483 | UK (1997) | Use of existing data from ONS (and counterparts in Scotland, NI) and from the DSS on work related data | NA | Burden of GI disease study | Cost of lost productivity due to early death from GI disease = £2.2 billion, from long term disability = £0.8 billion, from short term disability = £2.0 billion, NHS costs = £3.0 billion (45% in patient cost, 27% drugs). Total burden of disease = £8 billion (1997) | Crude estimate. There is a great debate on how sickness absence should be costed (human capital v friction cost method) as these methods give very different figures. NHS costs, especially drug costs, are here more reliable. Much debate on value of burden of illness studies |
| Keighley324 | UK (2003) | Review | GI cancers in Europe | Crude statements about what the main cost components are for the treatment of a variety of GI cancers. Most detail given for colorectal cancer. Average cost of treating a case of colorectal cancer in the UK estimated at €12 630 | Crude estimates. Interesting for intercountry comparisons, but very inaccurate | |
| Belsey516 | UK (2001–02) | Modelling exercise based on Hospital Episode Statistics of England. | 109 000 | Prescribing practice | Of a population of 109 000 patients in the UK in 2001–02 waiting for knee and hip replacement, around 637 had upper GI bleeding linked to NSAID treatment, with between 51 and 89 deaths resulting. Based on the cost of £3000 (estimated by Moore et al, 1999) for every admission for an NSAID related GI bleed. The additional 637 events identified in this analysis will therefore account for an expenditure of £1.9m | A critical review of prescribing practice is required, in order to reduce reliance on agents with a tendency to trigger upper GI ulceration |
| Moore and Phillips709 | UK (1999) | Modelling exercise | NA | The burden of NSAID related gastrointestinal disease | The burden of NSAID adverse effects to the NHS was calculated for the UK population on the basis of an average PCG with a population of 100 000. The average cost per year was estimated as: (a) low cost mix = £241 (10% coprescriptions); (b) middle cost mix = £266 (15% coprescriptions), and high cost mix = £308 (20% coprescriptions). The UK inpatient costs related to UGI bleeding were estimated at £35m (£7.7 per patient). Because aspirin and ibuprofen are OTC drugs, the total burden is likely to be greater | Most of the information on presription rate was collected from the USA. There is a high variation of prescribing mix in the UK, which we could not account for in detail. Given the high cost of coprescribing, a systematic survey is needed urgently |
| Duggan743 | UK (1998) | Modelling exercise | NA | Cost of management of UGI disease | The cost per patient (prescription costs, GP surgery visits, and outpatient services) were as follows: IGPCG = £157, Hp testing for all = £175, Hp testing and endoscopy for all = £236, Hp testing for ulcer = £172, Hp testing and endoscopy for ulcer = £219, endoscopy for all = £404, endoscopy for patients aged over 45 = £335, and endoscopy for patients aged over 45 presenting for the first time = £218. All the guidelines modelled here would result in a significant increase in the number of patients presenting for endoscopy. The scenario involving endoscopy for all patients aged over 45 would require a 13‐fold increase in the provision of endoscopy services. A one year period was chosen because of the lack of studies with a longer follow‐up. Hospitalisation costs could not be included because of insufficient published data to quantify any differences between differing drug treatment regimens. Because the IGPCG did not include gastric cancer, this was also excluded in the five scenarios | Bearing in mind the limitations of any modelling exercise, this study used robust data sources |
| Morant et al744 | UK (Scotland) (1989–95) | Population based observational cohort study | 17 244 New users of aspirin, each with 10 matched comparators | To determine the cost to the NHS of prescribed low dose aspirin | Aspirin use cost an additional £49.86 a year, made up of £1.96 for aspirin tablet (4%), £5.49 for dispensing costs (11%), £24.60 for UGI complications (49%), and £17.81 for renal complications (36%). These results projected to Scotland would give an estimate of £3 m (fastidious analysis) or £11 m (pragmatic analysis) a year for newly treated patients. If we assume that the antiplatelet trial meta‐analysis is an accurate assessment of the benefits of aspirin, then the cost for preventing one vascular event lies between £62 500 (primary prevention) and £867 (secondary prevention—patients without history of UGI or renal problems) | A large number of sensitivity analyses were performed and they all confirmed the original results |
| Haycox et al697 | UK (1996) | Modelling exercise | NA | The resource demand placed on general practitioners by patients following the many different management strategies available for UGI disease | The average cost incurred in primary care by patients with symptoms of UGI is £165; however, there was a wide variation across patients (range 10 to 989). The IMS database indicated that an average of 4.4 prescriptions a year is currently provided to patients presenting to GPs with UGI complaints. Implementing the IGPCG algorithm it reduces the number of prescriptions to an average of 1.9, with 70% of patients treated in accordance with the algorithm receiving long term (more than six months) symptom relief from their course of treatment. This would save £70 m out of current total drug cost of £488 m. Such cost reductions arise mainly from improving diagnosis and symptomatic management of patients and the more intensive eradication of H pylori infection | Although the results were confirmed by an extensive number of sensitivity analyses, the authors consider the study a preliminary analysis because refinement of the model is still continuing |
| Haycox et al742 | Europe and USA (1996) | Modelling exercise | 4 Countries | The extent to which economic analyses can be transferred across national borders | Assuming UK = 100 (£174), the cost of managing UGI for some of the other countries was respectively: Sweden = 179(£311), Germany = 101(£176), Switzerland = 163(£284) In all major cost categories the cost of treating a patient in the UK and Germany is significantly lower than treating the patient in Sweden and Switzerland | The fact that the pound sterling was comparatively strong compared with the other currencies might have contributed to the lower comparative costs identified in the UK. Further research is required to identify in more detail the factors influencing such variations and their impact both upon patients and public expenditure |
| Thomson and Booth653 | UK (1996) | Modelling | NA | The resource implication of TD (traveller's diarrhoea) | The incidence approach is used, the incidence of TD is assumed to be 8, 15, and 56 in low, intermediate, and high risk countries, respectively. The cost varied from £78m (prophylaxis) to £17m (treatment) | These calculations are rough and reflect the poor state of knowledge in this area |
| Ellis768 | UK (1989/1990) | Observational study | 3975 Usable data | Assess the contribution of the most frequently performed procedures to surgical workload and evaluate the financial implications | The patients incurring the greatest costs were those who had undergone large bowel surgery, vascular reconstruction, or amputation. The top five procedures in order of frequency were upper GI endoscopy, inguinal hernia repair, cystoscopy, tranurethral resection of the prostate, and surgery for long saphenous varicosities. Upper GI endoscopy in 420 patients (352 as day cases) cost £149 630. The cost of hospital stay for patients with large bowel surgery for carcinoma was £38 529 for 12 patients | The accuracy of coded discharge data on inpatient care is still a problem; 18% of the records had to be excluded because they were imprecise, ambiguous, or not present at all. The article fails to compare its results with others and fails to assess the external validity of the findings |
| Beard et al769 | UK (Costing data) (1990–98) | Literature review and modelling exercise | NA | Cost of liver resection | Based on the average costs from the Royal Hallamshire Hospital, Sheffield, the total treatment costs with resection were £6402. The cost of the alternative pathway of care (chemotherapy, Gramont regimen) is estimated at £6669. Hence the marginal cost saving of resection is £267. However, if patients have recurrence, the resection would only delay the chemotherapy by one year. Hence it would be cost saving only with no recurrence (patient remained free of tumour). Excluding any savings that may be made through avoided chemotherapy, and assuming no differences in salvage treatment of relapses, the cost per LYG ranges from £2134 to £3945. Even if 50% of the patients were found unsuited for surgery, or only received palliative resection, the survival benefits for the remaining patients are likely to cost around £6078 per LYG (undiscounted five year survival) | The study results might be highly sensitive to Sheffield unit characteristics. Moreover, some of the data are based on anecdotal evidence. There are no RCTs which directly compare the role of liver resection against treatments for liver metastases |
| Sheridan et al745 | UK (1991) | Prospective cohort study. | 100 Patients aged between 15 and 35 admitted with lower abdominal pain to one general surgical firm | To audit the extent of the problem of NSAP (non‐specific abdominal pain). To assess resource implications | 67 Patients were disgnosed as having NSAP; GI disorders were one of them. The total cost to the NHS of these patients was £54 115. On the basis of this, it is calculated that NSAP might be responsible for a total of 7708 emergency general surgical admissions per year in Wales, using 31 757 bed days and costing the NHS in Wales 6.4 million. Extrapolating these figures to the UK as a whole, the annual cost of NSAP to the NHS may be over £100m a year | The study provides a crude estimate of the NHS costs, in particular the extrapolation from one DGH to the country as a whole |
| McLoughlin et al746 | Europe (2002) | Literature review | 10 Countries | The burden of coeliac disease | In England it was estimated that 120 000 patients are affected by the condition and the test and treatment costs were £120m and £216m, respectively | This is a short article to provide a “snapshot” of coelic disease. No formal assessment of the robustness of the data sources is offered |
| Mahmood and McNamara16 | UK (Ireland) (2003) | Modelling exercise. Using the Swedish model of cost analysis recently published in Pharmaeconomics 2002, the authors applied the same model to calculate the burden of the disease in different countries across Europe | 6 Countries | Burden of GERD (gastro‐oesophageal reflux disease) | The UK prevalence was estimated as 24% giving the following costs: direct costs (million euro) = 2591; indirect costs = 1610; drugs = 1532; cost of sick leave = 1358; and other costs = 1239 | It is difficult to calculate the economic burden of GERD or peptic ulcer disease as most studies have used the broader term “dyspepsia” in their calculations. The study uses the prevalence approach, and there is insufficient information on the data sources for costing and how the summary figures are arrived at |
| Moayyedi and Mason747 | UK (2001) | Article review | 8473 Individuals participating in the HELP study. To identify NHS costs, 5056 primary care notes were reviewed for 1992–94 | Clinical and economic impact of dyspepsia | Dyspepsia was costing £21 per person per year—hence about £1000m each year in the UK. Within primary care dyspepsia was costing £11.25 per person per year, which represents £500m each year in the UK. Although based on patients in the Leeds HELP study, the study is very robust. The authors do point out that the national figure is representative as long as the cohort is representative of all the population | Cost effectiveness management strategies and treatments are urgently required. Strategies for managing dyspepsia have focused on attempting to reduce the endocscopy workload, although this procedure accounts for only a small proportion of the total costs of dyspepsia. Future approaches should also examine the drug used, as this might result in more cost savings |
| Logan and Delaney748 | UK (1999) | Review article | NA | Implications of dyspepsia | At any one time 4% of the population are thought to be taking drugs prescribed for dyspepsia. Drugs for dyspepsia account for 10% of drug expenditure. The number of gastroscopies performed each year is also rapidly increasing; 450 000 tests were performed in the United Kingdom in 1996 | This is a short article and the author does not provide any information on the data sources |
| Somasekar et al749 | UK (Wales) (1999–2000) | Retrospective study and model exercise | 156 Patients who underwent elective cholecystectomy from a DGH | The economic burden of patients waiting for cholecystectomy admitted with recurrent gallstone related symptoms | The mean (SD) waiting time for surgery was 12 (3) months. 37 patients were admitted as an emergency owing to gallstone related symptoms and complications while waiting. The cost for each episode was £946 and the total cost of treating the 37 patients was calculated to be £44 462. Performing early laparoscopic cholecystectomy for acute cholecystitis may help to reduce costs by preventing recurrent emergency admissions of these patients | Crude estimate. A small analysis of the effect of comorbidities is also made |
5.5 Cost effectiveness of GI services
One hundred and twenty seven articles examined the cost effectiveness of alternative forms of service delivery. As with the burden of illness studies, these were also of varying quality.
Primary care
Fifteen studies looked at management of GI disease in primary care.
Few studies have examined cost effectiveness and no studies of sufficient power have yet been performed in general practice populations to investigate the role of H pylori and the implication for primary care.582 No attempt has been made to measure quality of life after eradication therapy in patients with peptic ulceration. Further research is needed to quantify the risks and to test the value of screening elderly patients for H pylori before using NSAIDs. To determine whether or not a subgroup of patients with H pylori related chronic gastritis and non‐ulcer dyspepsia would benefit from eradication therapy a longer follow‐up period is needed. Until this is determined, the treatment of non‐ulcer dyspepsia with eradication therapy should remain a research activity.582
The available clinical and economic information about NSAIDs is limited, and the publication of numerous poor quality studies has corrupted the knowledge base. However, there does seem to be enough evidence to indicate that expenditure on NSAIDs could be considerably reduced and adverse effects avoided if practitioners were persuaded to change their behaviour.514 A growing body of evidence suggests that information provision on its own does not lead to substantial changes in practice. More active strategies, such as “academic detailing” using evidence based educational outreach, show promise, but their cost effectiveness has not yet been evaluated rigorously.514
Educational intervention concerning GPs' management of patients with dyspepsia, to control dyspepsia costs without increasing demand for endoscopy, could lead to a £25m saving each year.750 However, proper multicentre RCTs are needed to support the cost effectiveness of this approach.
It is unclear whether a strategy to test for H pylori and then eradicate it is as cost effective as initial management strategy in primary care. Future trials should evaluate the cost effectiveness of this strategy compared with empirical prescribing.751
The remainder of the studies, although they investigated the management of GI patients in primary care, were mainly based on either qualitative or review work exploring the safety of endoscopy performed in primary care, the effect of guidelines for the management of IBD, the development of GPwSI (GPs with special interests in gastroenterology); a survey of GPs requirements for support from secondary care; the effect of bulletin findings on patient's management; and the effect of H pylori testing results on referral rate. None of the studies included an economic measurement.
Table A.6 summarises the articles examined for primary care.
Table A.6 Cost effectiveness of GI services: summary of articles examined for primary care.
| Authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Jones770 | UK (1996) | Review | NA | GI disease in primary care | Conclusions mainly in the form of a research agenda | Although only 1996, this report is already dated. Main conclusion is that the statement “there is little evidence on which guidelines can be based” is no longer true |
| Delaney582 | 1990s | Review | NA | This review aims to summarise the evidence on the role of H pylori in dyspepsia from the perspective of the primary care, to suggest a strategy for managing dyspepsia, including peptic ulcer disease | The two UK studies suggest that eradicating H pylori in patients with proven peptic ulceration who are receiving long term treatment (the entry criteria were conservative in defining “long term” treatment) might produce savings of up to £41 000 per 100 000 population a year, or save up to £20m a year in the UK spending of £90m a year on H2 antagonists, based on the figure for 1990 | The studies referred by the author have a small number and they do not seem to be proper cost effectiveness studies |
| Bloor and Maynard514 | UK (1994) | Review | NA | Prescribing of NSAIDs | NSAIDs accounted for about 1 in 20 prescriptions. Switching patients so that ibuprofen accounts for 50% of prescriptions and reducing all the other brands accordingly would reduce overall drug expenditure by £45m (26%). In addition, serious adverse reactions could be reduced by 12.5% (to 440 a year) and GI reactions by 16.5% (to 263 a year) | This is an excellent review. However, by the authors' own admission, it has to be considered an exploratory work |
| Delaney et al751 | UK (1998) | RCT | 478 Patients aged under 50 and presenting with dyspepsia for longer than four weeks | To determine the cost effectiveness of near patient testing for H pylori and endoscopy for managing dyspepsia | Costs effectiveness was determined from the NHS perspective, and based on improvements in symptoms and use of resources at 12 months. Quality. Costs were higher in the intervention group (£368 v £253) per patient. The test and endoscopy strategy was less cost effective than usual management | It is unclear whether a policy to test for H pylori and then eradicate it is cost effective as an initial management strategy in primary care. Future trials should evaluate the cost effectiveness of this strategy compared with empirical prescribing |
| Banait et al580 | UK (north west England) (1997) | RCT | 114 GP practices (57 control, 57 intervention group) | To test the effectiveness of “educational outreach” as a strategy for facilitating the uptake of dyspepsia management guidelines in primary care | The proportion of appropriate referral was higher in the intervention group in the six month post‐intervention period. In this study, the dissemination of clinical practice guidelines using educational outreach proved to be more effective than passive guideline dissemination alone. However, the intervention also produced unintended outcomes, notably an increase in prescribing expenditure | Before it is more widely used, this strategy requires further investigation to confirm that changes in GP behaviour do improve patient outcomes and to assess the overall cost effectiveness of this expensive intervention |
| Galloway et al519 | UK (2000) | Questionnaire based survey | 28 General practices performing endoscopy | To examine whether endoscopy in primary care can be considered a safe procedure | Endoscopy in primary care seems to be safe. This good safety record is probably attributable to careful case selection and minimal use of intravenous sedation | The economics of service provision have not been investigated within this survey, but data have been published showing that rigid sigmoidoscopy performed outside secondary care is not necessarily a cheaper option |
| Valori et al750 | UK (England) (1995) | Controlled trial | 123 GPs covering a population area of 325 000 and 250 000 for intervention and control group, respectively | To determine whether a multifaceted educational strategy for general practitioners aimed at improving quality of dyspepsia management can control dyspepsia costs without increasing demand for endoscopy | After the intervention, drug costs declined and then stabilised in the intervention group. The overall cost in the intervention group was reduced by 57.9 pence per head of population per half year in comparison with the control group. This difference was maintained for three consecutive years, resulting in a cumulative savings of £1.13m. The estimated cost of the intervention was £6600. If the intervention were carried out across the country the estimated national savings would be £25m a year | There are two limitations to this study: the study was not an RCT and important outcomes, such as symptoms and GP consultations, were not measured |
| Read et al771 | UK (England) (1995) | Before‐and‐after study | 487 GPs in Leicester HA and gastroenterology teams within the hospitals | To study whether the introduction of consensus guidelines would encourage a movement towards care in the community for patients with stable disease, and hence speed up new consultation rates | The guidelines did not reduce the period between initial referral and first consultation in outpatients | Response rate was quite low: 106 (21%) and a further 52 (total 32%) after a reminding letter. In an ideal world such guidelines should be evidence based, but when this is unavailable a consensus view on diagnosis and management can be valuable, although it may not produce the best answers |
| Kernick693 | UK (2002) | Review | NA | The economic perspective of intermediate care in GI | Although 16% of GPs are already providing specialist interest services,692 there is currently no evidence to support these changes for increased effectiveness or cost effectiveness | This article is a good review, but it does not include a proper economic evaluation. |
| Moody et al772 | UK (Leicestershire) (1991) | Postal questionnaire survey | 259 (41% response rate) | Care of chronic GI disorders and patients with IBD. What do GPs want from local GI units? | GPs desire a more specialist education (that is, regular bulletin on the management of both IBD and chronic GI disorders). 60% wanted a telephone hot line to senior GI personnel, with direct dialling for immediate advice. 80% wanted shared care with hospital consultants | The article includes no economics |
| Parry et al,564 | UK (2002) | Retrospective case series | 51, mean age 60 (range 31–84) | To determine the number of patients referred to a DGH, the indication, enteroscopy with or without histological diagnosis, and to compare findings with other series from tertiary referral centres or outside the UK | Indications (obscure GI bleeding), most common findings (small bowel AVMs), and “missed” lesions within reach of a gastroscope were in keeping with other series. The current need for push enteroscopy in a DGH is small (about 1 per 8000 population a year). Criteria for enteroscopy should be developed and refined. It may be that enteroscopy does have a place in the routine procedures carried out in selected DGHs, but this will have resource and training implications that need to be measured | The study does not include a proper economic evaluation |
| Hungin et al773 | UK (south Tees DHA) (1990) | Cohort study. Patient management in the year before open access was compared with the year after | 715 | To determine the impact of open access gastroscopy in general practice and in particular, the value of a normal result | Open access is associated with a rationalisation of drug treatment, reduced consultations, and a low hospital referral rate | The study did not include a proper economic evaluation of the resource consequences. The authors point out that one of the main limiting factors of the study design was the accuracy of the general practice records. However, although details of clinical symptoms were variable and only briefly recorded, recording of drug prescribing was consistently good |
| Parry et al578 | UK (northwest region of England) (1998) | GP survey | 177 | To describe how awareness of patient H pylori status changes the practice of GPs who do not currently use H pylori testing and/or eradication in their management of dyspepsia | Until the use of H pylori tests in primary care has been evaluated in appropriate RCTs, advocates of testing as a means to reduce endoscopy referrals should be cautious about its potential impact on service workload | This article is dated as more RCTs have been performed since. However, it did not include any economics |
| Parry et al774 | UK (1999) | RCT | 136 GP randomised to receive effectiveness matters and 126 who did not receive effectiveness matters (control group) | To investigate the impact of distribution of a printed summary of research findings on GPs' self reported management of peptic ulcer disease and dyspepsia | Distribution of a single, printed summary of research findings in isolation from other interventions is unlikely to have an impact on patient management | The study did not include an economics component |
| Smith et al775 | UK (2004) | Cross sectional observational study | 486 Patients (recruited via an advert in the newspaper) | Frequency, duration, and severity of symptoms. HRQoL of patients managed in primary care compared with patients managed in secondary care | Patients managed solely in primary care do not have less “severe” IBS. Thus the overall impact of IBS on society may be much greater than currently estimated | There might have been problems of self selection. Further study to evaluate the effects of patients' symptoms and HRoQL over time is also merited. The study did not include an economic component |
Specialist care
Nine studies looked at access to specialist care; there is a serious underprovision of colonoscopy service in most NHS hospitals. Training in colonoscopy is often inadequate and improved practice should result from better training. Unless there is a dramatic increase in manpower and resources available for lower GI investigations, the introduction of a national screening programme will rapidly overburden already inadequate facilities.752 There is a shortage of resources for coloproctology753; some assessment of resource needs has been performed in cancer services.754 Although there is very little evidence on the cost effectiveness CT colonography, this techniques is widely available in the UK; however, experience and throughput varies considerably. Limited CT scanner facility is the major barrier to further dissemination.755 There is also some evidence that greater access to specialist paediatric gastroenterology services for children with suspected IBD should be sought.756
None of the above publications included a proper economic evaluation.
Table A.7 summarises the articles examined for access to specialist care.
Table A.7 Cost effectiveness of GI services: summary of articles examined for access to specialist care.
| Authors | Research setting and year of study | Study design | Sample size | Topic of document | Results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Association of Coloproctology of Great Britain and Ireland753 | UK (2000) | Postal survey | Replies from 75 centres covering 26.5 million | The number of staff needed for coloproctology | Extra staff (full time equivalent) required for the UK were: 170 surgeons; 18 histopathologists; 72 oncologists; 42 radiologists; 60 palliative care nurses; 98 colorectal cancer nurses; 140 stomatherapy nurses; and 760 other colorectal specialist nurses. Some solution to this shortage can be offered by involving more GPs and endoscopy nurses | A good mapping of needs; it does deal with the most cost effective ways to reach the target |
| South Wales Cancer Network754 | UK (Wales) (2002) | Review | NA | Service development plan | By end of 2004: Reflect local workforce and skill mix required. Examine the training requirements Undertake a mapping exercise to establish current availability and future needs of specialist nursing workforce. By end of 2005: Agree long term service model in response to NICE guidelines | Mainly a research agenda document |
| Guillou et al776 | UK (1996) | Review | NA | Minimal access GI surgery | Evidence on cost effectiveness of laporoscopic v conventional cholesystectomy too thin to report | Most evidence is from the USA, where charges rather than costs are used. Authors (quite rightly) warn about this |
| MacKenzie et al597 | UK (1999–2001) | RCT | 552 Intervention group; 565 control Group | Method of investigating large bowel symptoms | NHS and patient borne costs were included. The study had a three month follow‐up. Total costs per patient were £307 (consultant led) and £203 (open access), respectively. The difference was not statistically significant | SD and ranges of cost figures were not reported. Further research is required to identify the reasons for non‐attendance |
| Bowles et al752 | UK (England) (2002) | Cross sectional study (4 months follow‐up) | 9223 colonoscopies across 68 hospital units (5 teaching hospitals, 18 DGH, 7 private hospitals, and 1 paediatric unit) | To study the availability and the quality of adult and paediatric colonoscopy in three NHS regions | There is a serious underprovision of colonoscopy service in most NHS hospitals. Only 17% of colonoscopists had received supervised training for their first 100 coloscopies and only 39.3% had attended a training course | The study did not include an economic component |
| Burling et al755 | UK (2003) | Observational study | 138 Departments | The provision of CT colonography in UK radiology departments | CT colonography is widely available in the UK, with about one third of respondents offering a service. Experience and throughput varies considerably. Limited CT scanner facility is the major barrier to further dissemination | The paper does not include an economic dimension. Although there is little evidence on the cost effectiveness of the technique, this is widely available |
| Sawczenko et al756 | UK (1998–99) | Prospective population based survey | 739 new IBD cases across 3247 paediatricians, adult gastroenterologists, and surgeons in the UK | Variation in the management of children with newly diagnosed IBD | This study suggests that in many, if not the majority, of institutions there is no designated care pathway for the management of childhood IBD. Current specialist provision, and initial investigation and treatment of IBD, are heterogeneous | There is no economic analysis |
| Kennedy et al683 | UK (2003) | RCT | 700 Patients (297 intervention, 403, control) across 19 hospitals in northwest England | Explore models for training health professionals in methods to promote and support self care, study long term effect, and assess whether faster treatment reduces the duration of relapses in IBD | After one year, the intervention resulted in fewer hospital visits, without change in the number of primary care visits (2.01 v 3.22). The total average costs for the groups were respectively £922 and £1070. Patients felt more able to cope with their condition. The intervention did not reduce quality of life and did not raise anxiety. The intervention group reported fewer symptom relapses, and 74% of the patient wanted to continue the system. CE analysis favoured self management over standard care. Standard care was associated with slightly better QALYs profile (QALYs gain of 0.00022) and an increase in cost per patient of £148. This is likely to be far in excess of values currently deemed acceptable to healthcare founders. The authors also estimated that the burden of IBD ranges between £75m and £85m a year | An excellent study. Future research is recommended to evaluate the operating systems within secondary and primary care that would allow self managers to self refer and to keep them informed of new treatments, also to explore self care methods, to study long term effects of self management in chronic disease. There is little evidence on long term effects. This study looked at one year, but it is likely that significant morbidity and mortality effects will take several years to determine. There is a need to establish how well open access works over a long period and whether clinic and patients revert to a system of fixed appointments |
| Moayyedi et al777 | UK (1999) | Discrete Choice Experiment | 354 Patients (mean age 47) | Eliciting patient preferences for gastroenterology clinic reorganisation | Four key attributes were identified: waiting time from the GP's referral, waiting time in the clinic, consultation time with the specialist, and waiting time for the investigation. Our data suggest that patients value waiting for investigations as highly as time spent on a waiting list, as a reduction in either will lead to a more rapid diagnosis. | Discrete Choice Experiment (DCE) is a technique recently introduced to ascertain patients' preferences in the delivery of health care; hence, the results need to be confirmed by other studies |
Role of nurses
Four studies with an economic component examined the role of nurses in performing a variety of GI services, including upper GI endoscopy,712 managing children with GI disease,757 screening for colorectal cancer,758 running dyspepsia clinics,759 administering propofol during endoscopy.717 Again, none included a proper economic evaluation. A multicentre RCT comparing nurses and doctors undertaking diagnostic upper and lower GI endoscopy has shown that doctors are more cost effective than nurses in carrying out these procedures.491
Table A.8 summarises the articles examined for the role of nurses in GI services.
Table A.8 Cost effectiveness of GI services: summary of articles examined for the role of nurses in GI services.
| Authors | Research setting and year of study | Study design | Sample size | Topic of document | Results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Maule758 | UK (1994) | Prospective cohort study | 1881 Patients (examined by nurses) and 730 (examined by physicians | Screening of colorectal cancer by nurse endoscopists | Nurses can carry out screening by flexible sigmoidoscopy as accurately and safely as experienced gastroenterologists | The paper did not include a proper economic evaluation |
| Melleney and Willoughby759 | UK (England) (1999–2000) | Case series study | 100 Patients | To audit a nurse endoscopist based one stop clinic | The system was popular with patients as most of them (70%) were dealt with at a single hospital attendance. However, the one stop clinic as currently formulated is open to the criticism that its productivity is low, and moreover, the overload of minor disorders increased the waiting time | The study does not include a proper economic evaluation |
| Smale et al712 | UK (2000) | Retrospective and prospective cohort study | 3009 Patients (retrospectively) 480 (prospectively) | To determine the effectiveness, patient comfort, and attitude towards future development of endoscopy performed by nurses | Experienced nurses perform routine diagnostic gastroscopy safely and as with as little discomfort for patients and as much patient satisfaction as medical staff | A good study, but it did not include any economic evaluation |
| Burnett et al757 | UK (1998–2000) | RCT | 102 Patients | To evaluate the effectiveness of a nurse led clinic (NLC) compared with a consultant led paediatric GI clinic (PGL) in the management of chronic constipation | Results suggest that an NLC can significantly improve follow‐up of children with intractable constipation, and highlight the important role for clinic nurse specialist in management of children with GI disease | The study suffers from small numbers and it did not include any economic evaluation |
Home parenteral nutrition
Two studies760,761 looked at the cost effectiveness of home parenteral nutrition (HPN). There is some evidence that home parenteral nutrition (HPN) is a cheaper alternative than hospital care. No economic evaluations of HPN for malignant disease and AIDS have been made.760
Table A.9 summarises the articles examined for HPN.
Table A.9 Cost effectiveness of GI services: summary of articles examined for home parenteral nutrition.
| Authors | Research setting and year of study | Study design | Sample size | Topic of document | Results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Richards et al760 | NA (1980–85) | Systematic review | NA | What evidence exists for the cost effectiveness of HPN? What questions could be answered with additional research? | Two cost utility analyses (CUAs) were identified (one in Canada and one in the UK). The marginal cost per QALY varied from Canadian $14 600 to UK £69 000. The most recent estimate of cost to the NHS was £45 000 for the first year and £36 000 for subsequent years. The studies showed that HPN was 65–80% cheaper than the alternative hospital treatment. However, patients and community costs were not measured. There are no economic evaluations of HPN for malignant disease and AIDS | Patient referral patterns for HPN treatment are inconsistent; some regions in the UK have very few patients receiving HPN. However, there are several large centres in the UK where HPN is considered as an essential, life‐saving treatment. What methodological issues need to be dealt with in future: 1. Up‐to‐date registries 2. A more in‐depth description of episodes of care should be in place 3. patient long term monitoring should be laid down 4. Large multicentre RCTs should be carried out to evaluate alternative models of care |
| Puntis761 | UK (1998) | Literature review | NA | Cost‐utility appraisal of HPN | The effectiveness of HPN is about 65% greater than hospital care | This article reviewed most of the articles included in the previous article760 |
Surveillance programmes
Nine articles with an economic component were identified. Intensive follow‐up after resection for colorectal cancer was shown to be more effective and more cost effective than conventional follow‐up,601 producing an incremental cost for each life year saved of £3402 over conventional follow‐up. This is very low compared with other life extending interventions, indicating that on economic grounds, intensive follow‐up after curative resection for colorectal cancer should become normal practice. Large RCTs are needed to evaluate the cost effectiveness of specific surveillance tools.
A number of economic modelling exercises have examined population screening/eradication programmes for H pylori. One modelling exercise estimated that a programme to screen for and eradicate H pylori in a population of one million 45 year olds would produce an incremental cost for each life year saved of £14 200,614 which again is low compared with other life extending programmes. Another619 showed that population screening for H pylori was a cost effective way of preventing gastric cancer and peptic ulcer disease, producing an incremental cost for each life year saved at age of 40 of £5860, but this result was sensitive to H pylori prevalence, the degree of opportunistic eradication, the discount rate, the efficacy of eradication on gastric cancer risk, the risk of complicated peptic ulcer disease and gastric cancer associated with H pylori infection, and the duration of follow‐up. Many assumptions are required in modelling exercises of this type. However, when these assumptions were varied in sensitivity analyses, the incremental cost for each life year saved rarely exceeded £20 000 over an 80 year follow‐up, although it did for shorter periods. Population H pylori screening may be cost effective in the long term (over 25 years). However, before it can be recommended further evidence is needed to resolve some of the uncertainties, particularly about the efficacy of eradication on risk of gastric cancer, the risk associated with complicated peptic ulcers, and the effect of more widespread opportunistic testing of patients with dyspepsia. The long duration between the age of screening and the incidence of gastric cancer means that screening does not become cost effective for several decades. Before screening can be recommended on economic grounds further evidence is needed to resolve some of the uncertainties, particularly with regard to the time horizon and the discount rate.
Table A.10 summarises the articles examined for surveillance programmes.
Table A.10 Cost effectiveness of GI services: summary of articles examined for surveillance programmes.
| Authors | Research setting and year of study | Study design | Sample size | Topic of document | Results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Renehan et al618 | UK NHS perspective but based on five studies from Finland, Denmark, Italy, Sweden, and Australia (2004) | Modelling exercise based on the results of a previous meta‐analysis of five RCTs performed by the authors | 1342 Patients | Patient follow‐up | Based on the five trials the life years gained were 0.73, and the adjusted net (extra) cost for each patient was £2479 and for each life year gained was £3402. NHS perspective. The main predictor of incremental cost effectiveness ratios was surveillance cost. Based on the available data and current costs, intensive follow‐up after curative resection for colorectal cancer should be normal practice | Large RCTs are needed to evaluate the efficacy of specific surveillance tools. These studies should include CEAs and quality of life assessments. Also, costs beyond the five years' initial treatment need to be considered as a proportion of patients with recurrences undergoing salvage treatment will have delayed second recurrences. Also a societal perspective should be taken as travel and time off work might be relevant in cancer surveillance |
| Maule758 | UK (1994) | Prospective cohort study | 1881 Patients (examined by nurses) and 730 (examined by physicians | Screening of colorectal cancer by nurse endoscopists | Nurses can carry out screening by flexible sigmoidoscopy as accurately and safely as experienced gastroenterologists | The paper did not include a proper economic evaluation. |
| Mathew et al778 | UK (England) (2000–02) | Case series study | 2382 Patients | This study aimed to identify the percentage of patients aged <45 who undergo flexible sigmoidoscopy for rectal bleeding and compare the incidence of colorectal cancers and polyps above and below this age | The incidence of colorectal cancers and adenomatous polyps in patients aged <45 years with rectal bleeding is very low | There is no real economic analysis |
| Atkin622 | UK (1998) | Review | NA | Flexible sigmoidoscopy as mass screening tool | Flexible sigmoidoscopy as mass screening tool No economics evaluation. The authors simply hypothesise that FS screening “would cost only marginally more than is currently spent on treating the disease” and describe a continuing trial set up to test that hypothesis. Nothing in here about cost for case detected (cf FOB test, RS, etc) or cost for each life year gained | The economic analysis of the study is flawed |
| Pignone et al619 | USA, UK, and others (1993–2001) | Systematic review | – | To assess cost effectiveness of colorectal cancer screening for the US Preventive Services Task Force | Seven articles were identified. Compared with no screening, cost effectiveness ratios for screening with any of the commonly considered methods were generally between $10 000 and $ 25 000 for each life year saved. No one strategy was consistently found to be the most effective or to have the best ICER. Currently available models provided insufficient evidence to determine optimal starting and stopping ages for screening. | No studies considered patient time cost associated with attendance for screening, diagnostic, or surveillance procedures or for treatment of cancer |
| Smith et al573 | UK (1997) | Postal questionnaires to clinicians (members of the society of gastroenterology) | 152 | To determine the practices that clinicians employ in the management of Barrett's oesophagus in the UK | Wide disparity in surveillance for Barrett's oesophagus. A recent UK study quoted £120 for each examination (£80 for endoscopy with £40 for historical assessment), giving a cost of about £49 000 per each detected cancer | No proper economic analysis. A prospective trial is needed to determine whether screening is beneficial |
| Mason et al614 | UK (2002) | Markov model extrapolating results of an RCT | n = 8407 in an RCT | Population screening for gastric cancer using H pylori testing and eradication | Cost difference overall favoured intervention group but did not reach statistical significance. Cost difference was statistically significant for men. For women there was no difference. Modelling estimated that population screening/eradication for population of one million 45 year olds would save £6m and 1300 life years. Cost for each life year saved was £14 200, which is good value for money | Very useful subject to usual caveats about modelling, but the “base case” (producing figures in the previous column) was conservative—that is, a lower limit of 95% CI for cost savings and only 10% efficacy of H pylori eradication in reducing mortality from distal gastric cancer and peptic ulcer disease |
| Duncan616 | UK (1992) | Case study | 9 Patients | The importance of identifying self induced diarrhoea by self administration of laxatives | For eight patients in whom the diagnosis was unsuspected, an average of £2807 (range £60–10 709) was spent on investigations, which would have been avoided had an early laxative screening been performed. In comparison, the cost of performing a laxative screen on all patients presenting with diarrhoea can be estimated at £600 for each laxative abuser identified (assuming a prevalence of 4% and a laxative screen cost of £24). | The economic analysis is not fully described |
| Roderick et al779 | UK (2002) | A discrete event simulation model | NA | To evaluate the cost effectiveness of population screening for H pylori | The cost/life years saved (LYS) at age of 40 was £5860 at a discount of 6%. The outcomes were sensitive to H pylori prevalence, the degree of opportunistic eradication, the discount rate, the efficacy of eradication on gastric cancer risk, the risk of complicated peptic ulcer disease and gastric cancer associated with H pylori infection, and the duration of follow‐up. In sensitivity analysis the cost/LYS rarely exceeded £20 000 over an 80 year follow‐up, but did for shorter periods. Two critical determinants of cost effectiveness are time horizon and discount rate. Screening does not become cost effective for several decades, which largely reflects the long duration between the age of screening and the incidence of gastric cancer. A lower discount rate for benefits makes screening appear more cost effective | A very good study |
Dyspepsia and endoscopy
Quite a few studies examined endoscopy. One used modelling to examine a wide range of different situations in which endoscopy is given for patients with dyspepsia.34 Results showed that endoscopy is not cost effective in patients with low risk of malignancy, but targeting had major impacts on cost effectiveness ratios. Restricting endoscopy to those with continuous epigastric pain or symptoms of less than one year's duration, or both, improved the incremental cost for each life year saved from £50 000 to £8400. Estimates of incremental cost per life year saved for men of various ages ranged from £454 000 at age 40 to £15 779 at age 70. Results for women showed similar reductions at older ages, which provides good evidence of the need to restrict endoscopy in younger age groups.34 When the initial strategies for managing dyspepsia were examined, a comparison of early endoscopic investigation with acid suppression showed that the cost of additional endoscopies was offset by a significant reduction in the number of PPIs prescribed and outpatient attendance. The overall management cost of prompt endoscopy was £420 compared with £340 for empirical management.697
Table A.11 summarises the articles examined for dyspepsia and endoscopy.
Table A.11 Summary of articles examined for dyspepsia and endoscopy.
| Authors | Research setting and year of study | Study design | Sample size | Topic of document | Results and conclusions | Comments |
|---|---|---|---|---|---|---|
| Delaney et al579 | UK (2004) | Review and modelling exercises | Dyspepsia | Endoscopy not shown to be cost effective in patients with low risk of malignancy. Restricting endoscopy to those with continuous epigastric pain and/or symptoms of <1 year's duration would reduce cost/life year gained from £50 000 to £8400. Discrete event simulation used to compare 70 combinations of investigation and prescribing strategies. 61 eliminated by dominance—including all strategies involving endoscopy. Extra cost for each extra month dyspepsia‐free shown for remaining 9. Conclusion is that endoscopy needs to be targeted. Markov modelling used to estimate cost/life year saved from endoscopy in men by age (£454 000 at 40 to £15 779 at 70, but by restricting endosopy for those over 70 to those at high risk it falls to £8398). Incremental cost effectiveness ratios for men over 55 and at high risk are all favourable. For women aged 40 = £158 823 and aged 70 = £22 062. Strong conclusion of need to restrict demand for endoscopy in younger age groups | Very good economics. Assumptions spelled out. Sensitivity analyses conducted | |
| Moayyedi et al780 | UK and non‐UK studies | Cochrane review | To review the effectiveness of six classes of drugs in the improvement of both the individual or global dyspepsia symptom score and quality of life scores. | It is estimated that £450m is spent on dyspepsia drugs in the UK each year. There is evidence that anti‐secretory treatment is effective in a small proportion of patients with NUD. The evidence is strongest for PPI as the studies were generally of higher quality and the funnel plot did not show any publication bias | We did not identify any economic analysis; this information is important as patients will often need to take drugs long term | |
| Bodger et al596 | UK (1994) | Prospective observational study (4 month period) | 257 Consultations (150 patients) | Prescribing patterns for symptomatic dyspeptic patients. | The drug cost for each patient varied from £2 to £60 a month. Management guidelines may help to promote a more consistent and selective use of newer treatments, and promote more cost effective patient care | The practice sampled represented a reasonable cross section of doctors, though arguably biased towards more “rational” prescribers |
| Paterson et al781 | UK (1998–99) | RCT pilot study | 60 | The effects of adding treatment of acupuncture or homeopathy to current treatment | Total mean (SD) cost of acupuncture per patient was £175 (52) and total mean cost for homeopathy per patient was £105 (33) | This study suggested important changes for the design of a full scale study |
| Bate et al782 | UK (England) (1998) | Cohort study | 90 Patients with symptoms suggestive of GERD | To assess the diagnostic value of a therapeutic trial of omeprazole 40mg in a dyspeptic population | We conclude that omeprazole can be used as a clinically effective tool in the initial management of GERD and that it is of diagnostic value in patients who present with typical symptoms, such as heartburn, when the diagnosis is based on assessment of symptoms alone | The cost of patients who might have been misdiagnosed was not part of the study. The quality of the economic evaluation was poor |
| Delaney et al579 | UK, USA, Canada, and others (2004) | Cochrane review | 19 RCTs | Management strategies (combination of initial investigation and empirical treatment) for dyspeptic patients | It is unlikely that early endoscopy would reduce overall economic costs of managing dypspsia over only one year. It is more likely that an initial excess cost would be incurred that might be recouped in some prescribing and consultation reduction in subsequent years. The point at which early endoscopy might become cost neutral cannot be determined from these trials. Delaney reported a full exploration of costs. The additional endoscopies were offset by a significant reduction in PPI prescribing (equivalent to a month's prescribing), outpatient attendance was also reduced from 0.45 to 0.22 per patient. Overall management by prompt endoscopy cost £420 compared with £340 for empirical management | The rest of the studies did not include a proper economic evaluation |
This review shows that economic evidence on the delivery of GI services is patchy and of variable quality. Very few studies were full economic evaluations and the limited economic evidence they produced—for example, of potentially large cost savings to be gained by changing from one model of service delivery to another—emphasises the need for comprehensive economic evaluations in this area.
Summary points
Multicentre studies are needed
Studies should take the societal perspective
Methodological problems/challenges of economic evaluations of primary/secondary care interphases have been highlighted
Insufficient evidence is available to support a positive correlation between volume and patients outcomes. This relationship needs further assessment.
Table A.15, Appendix 4, lists the references not used for the economic review and the reason why.
5.6 Information infrastructure
The requirements for information and IT support for gastroenterology have been published by the British Society of Gastroenterology.762 This describes the need for patient focused records that will provide access to appropriate information in the increasingly wide variety of contexts in which patients will receive health care, including self management. The most pressing immediate need is for universal support for the widespread introduction of systems to support gastrointestinal endoscopy. The requirements include booking, patient information, consent, results, reporting, and quality assurance. A survey of gastrointestinal units in 2001 found that one third of respondents from the UK were still using paper reporting systems.763 Many other aspects of gastroenterology need better information support, including all contacts with professionals and specific clinics, where acquisition of data should be used to monitor quality of care. It is hoped that patient focused systems will be developed in the future, which will enable support for patient care through a wide variety of situations in which the patient receives care.
There is presently no national dataset to enable comparative monitoring of activity and performance in gastroenterology. There are concerns about the quality of routinely captured data.11 Common standards for records and for data collection are needed to improve this,764 and to enable performance monitoring, monitor quality and training, inform service developments, and enable high quality clinical and health services research.765 There is evidence that routinely captured clinical data would enable health technology assessment by RCT if the data were more widely available and of improved validity.766,767 The data required to support gastrointestinal endoscopy are available on the British Society of Gastroenterology website (http://www.bsg.org.uk, accessed 26 December 2006), and requirements are being developed for other areas of the specialty.
6. Discussion
6.1 Strengths and weakness of methods used
As detailed in the methods chapter and at the start of each chapter, four main methods of data collection were used to gather evidence for this review: review of published evidence; use of routinely available data; patient workshop; and consultation with professionals in gastroenterology. The strengths and weaknesses of each of these methods are considered below, with implications for strength of recommendations made in this report assessed in section 6.2.
Review of published evidence
As we applied currently defined and accepted standards to the review of effectiveness of service delivery, this section of the report is comprehensive and systematic. Validated tools were used to assess the quality of papers and level of evidence provided, with more than one reviewer independently grading papers. Full details of the search and of papers retrieved are presented through search results and tables. Other sections of the report are comprehensive and have retrieved key data and publications, although the methods used to identify sources have relied to some extent on existing knowledge and collections of materials. With extensive feedback sought from a variety of specialist professional and patient groups it is unlikely that key sources have been either overemphasised or overlooked.
Routine data
Several main sources of routine data were used in compiling this report: cancer surveillance and registry units across the UK; the Office of National Statistics (ONS) and its predecessor, the Office of Population Censuses and Surveys (OPCS); the Department of Health; and communicable disease surveillance units across the UK. In addition to well described generic limitations of routine data, the different data sources have their own particular strengths and weaknesses.
Limitations of cancer surveillance and registry data include concerns about variability in case ascertainment and completeness of registrations over time and between different registry regions. The major limitation of mortality data from the ONS and the OPCS is that it is based on underlying cause of death alone, and therefore underreports true mortality for many gastrointestinal diseases; major concerns have also been raised about the accuracy and completeness of hospital episode statistics from the Department of Health. The main limitation of data on hepatitis B and C infections from communicable disease surveillance units is that they are based on reported laboratory diagnoses only. As most people who are infected with these viruses are undiagnosed, the reported laboratory data are thought to account for only about one quarter of all cases.
Despite these limitations, these are the best data that are available for portraying the burden of gastrointestinal disease in the UK. Coverage is national, with standardised definitions and inclusion criteria agreed. They have provided the empirical basis for many publications in scientifically acclaimed international clinical journals, as well as National Service Frameworks and other policy documents.
Patient workshop
The limitations of the focus group carried out with patient representatives recruited from the RCP volunteers are acknowledged. The views reported in this document can only be taken to represent a flavour of the views of patients. Participants included patients and patient representatives, who were perhaps unusually articulate and able to interact as members of a group. Nevertheless, the findings complement the review findings, presenting a different side of the picture on the problems of service delivery arrangements that are currently undergoing change, which were discussed at the workshop.
Consultation with professionals from within the specialism of gastroenterology
Feedback has been sought through the BSG membership, and other societies, and has been collated from individual responses as well as from a wide range of specialty and patient groups that support the care of patients with GI disorders. Only that feedback which was supported by further evidence has been incorporated.
6.2 Strengths and weaknesses of evidence presented in report
Using a mixed methods approach in this review has allowed any weaknesses inherent in one method to be complemented by the strengths of another approach.
The systematic review of effectiveness of models of service delivery has been enriched through contextualisation, with the national policy agenda described; presentation of data describing burden of disease, current activity, economic costs, and workforce implications; and the views of patients and professionals represented. This has allowed a comprehensive document to be developed. Some aspects of the review—such as the perspective of patients to current developments in service delivery—would be more comprehensively and rigorously pursued through primary research, and the data presented in this report can only be taken as a taster of views. This has resulted in recommendations for further research, as existing evidence is thin. Indeed, an overwhelming conclusion of the report is that the evidence base for the development of services needs to be strengthened before further investments are made in shaping the delivery of services.
6.3 Research in gastroenterology
Although over 900 references have been used to inform this review, the evidence identified has often been weak and there are many gaps in areas where evidence is needed. The annual reports of the Health Technology Assessment and NHS Service Delivery and Organisation Research Programmes document relatively few studies in gastroenterology.
A coordinated approach to clinical and health services research in gastroenterology, such as the one being introduced for cancer, in gastroenterology would improve the identification of research questions and priorities, funding strategies, patient and carer involvement, and the research infrastructure. It is hoped that the UK Clinical Research Collaboration will promote and enable more research into the diagnosis, treatment, and care of patients with GI and hepatic disorders.
7. Conclusions
7.1 Burden of disease
The burden of gastrointestinal and liver disease is heavy for patients, the NHS, and the economy (sections 3.2–3.4, 3.7, 4.3–4.5, 4.7.2, 4.7.3, 5.5).
Gastrointestinal disease is the third most common cause of death, after circulatory and respiratory disease (section 3.3).
Gastrointestinal cancer is the leading cause of death from cancer (section 3.3).
Gastrointestinal disease is the most common cause of admission to hospital for both the total number of people admitted and the total number of episodes of care (section 4.3).
There have been large increases in the incidence of liver diseases, such as alcoholic liver disease, non‐alcoholic fatty liver disease, biliary cirrhosis, and hepatitis C infection, which have major implications for future healthcare needs (section 3.2).
There have also been increases for most other gastrointestinal diseases—in particular, for oesophageal and colorectal cancers, acute and chronic pancreatitis, gallstone disease, upper gastrointestinal haemorrhage, diverticular disease, and Barrett's oesophagus (section 3.2).
Chronic gastrointestinal disorders such as dyspepsia, gastro‐oesophageal reflux disease, and irritable bowel syndrome are highly prevalent; and coeliac disease is far more common than previously considered (section 3.2).
Socioeconomic deprivation is linked to a number of gastrointestinal diseases, including increased risks of gastric and oesophageal cancers, hepatitis B and C infections, liver cirrhosis, peptic ulcer, upper gastrointestinal haemorrhage, and poorer prognosis for colorectal, gastric, and oesophageal cancers (section 3.6).
There is substantial variation in the incidence and prevalence of many gastrointestinal disorders in the UK. For example, peptic ulcer, Helicobacter pylori infection, upper gastrointestinal haemorrhage, alcoholic liver disease, acute pancreatitis, and oesophageal cancers are all more common in Scotland and northern England than in southern regions (section 3.5).
Impact on patients is neither fully nor accurately reflected in statistics describing mortality and activity (sections 3.3, 4.3).
The burden on patients health related quality of life has been found to be substantial for their symptoms, activities of daily living, and employment (section 3.4).
Conditions with a high level of disruption to patients' lives include: gastro‐oesophageal reflux disease (GORD), dyspepsia, irritable bowel syndrome, anorectal disorders, GI cancers, and chronic liver disease (section 3.4).
Overall, the burden of GI disease on health related quality of life (HRQoL) in the general population seems to be high, although the burden is not systematically nor comprehensively described (section 3.4).
7.2 Service delivery
An extensive and systematic study of the problem of access for the delivery of GI services has yet to be carried out (section 4.6.1; level of evidence: 2− at best).
There is a lack of significant literature relating to inequalities in the delivery of GI services (section 4.6.2; level of evidence: 4).
Waiting times form the bulk of patients' concerns. There seems to be great difficulty in meeting government standards for referral and treatment (section 4.6.3; level of evidence: 2− at best, and guidelines by the Association of Coloproctology of GB and Ireland).
Most studies show that GI related drugs and procedures are safe. There is a need for more research on the safety of patient initiated drugs and procedures for the treatment of GI disease (section 4.6.4; level of evidence: 1).
There is a need to increase awareness and the implementation of initiatives aimed at improving the information flow between patients and practitioners (section 4.6.5; level of evidence: 2− at best).
There is a strong body of evidence on diagnostic services, and the need to develop and implement appropriate training and stringent assessment to ensure patient safety (section 4.6.6; level of evidence: 2+).
There is a substantial amount of work detailing guidelines for care, but there is a distinct paucity of rigorous, evidence based studies dealing with service provision (section 4.7.1; level of evidence: 1).
There is strong support for the development and use of widespread screening programmes for a wide variety of GI diseases. These need to be properly researched to determine how they are managed, their effectiveness, and their cost effectiveness (section 4.7.4; level of evidence: 1, section 5.5).
Emphasis should be given to developing interventions to increase preventative activities in primary care, and more research to determine their effectiveness and cost effectiveness (section 4.7.6; level of evidence: 1).
More research is needed to establish a robust evidence base for models of service delivery (section 5.2).
Overall there remains a paucity of cost effectiveness evidence particularly from multicentre studies in GI service delivery (section 5.5).
There is strong evidence for a shift in care towards greater patient self management for chronic disease in appropriate circumstances, and supported by adequate circumstances and access to services (section 5.2; level of evidence: 1).
The development of GPs with a special interest in gastroenterology is supported in primary care but the clinical and cost effectiveness needs to be researched (section 5.2).
In hospital, patients with gastrointestinal disorders should be looked after by specialists (section 5.2; level of evidence: 2+).
More diagnostic endoscopy should be undertaken by trained nurses, although such procedures are not more cost effective than when carried out by doctors (section 5.2; level of evidence: 1).
Complex surgery for gastrointestinal and hepatobiliary cancer should be performed by specialists who operate on large numbers of patients (section 5.2; level of evidence: 2+).
There is insufficient evidence to support a greater concentration of specialists in tertiary centres. More research is needed, especially on the impact on secondary services, before further changes are implemented (section 5.2; level of evidence: 2+).
The solution proposed for hepatology is to combine tertiary specialist centres for complex liver disease and transplantation with a network of specialists in secondary care, but we found no evidence for the clinical or cost effectiveness of this approach (section 5.2; level of evidence: 4).
There is an urgent need for better IT and information support for clinical care in gastroenterology (section 5.6; level of evidence: 2−).
7.3 Workforce
Consultant gastroenterologist numbers need to increase to about 1900 posts (1625 WTE). Six consultants are required to provide full services and emergency cover for a typical district general hospital population of 250 000 (section 4.2; level of evidence: 2−).
Gastroenterology teams led by consultants, but including appropriate non‐consultant career grade staff, dieticians, and specialist nurses, need to be developed in all hospitals, with integrated specialist training where appropriate (section 4.2; level of evidence: 4).
More nurses should be trained to undertake upper and lower diagnostic endoscopy (section 5.2; level of evidence: 1).
7.4 Future research
More research is needed into delivery and organisation of services for patients with gastrointestinal and liver disorders, in particular:
The clinical and cost effectiveness of GPs with a special interest in gastroenterology and endoscopy (section 5.2).
The clinical and cost effectiveness of undertaking endoscopy or minor gastrointestinal surgery in diagnostic and treatment centres (section 5.2)
The reconfiguration of specialist services and the potential impact on secondary and primary care and on patients (section 5.2)
The clinical and cost effectiveness of clinical networks (section 5.2).
The relationship between volume and patient outcome needs further assessment (section 5.2).
To account for geographical differences, future research should be based mainly on multicentre studies.
The establishment of the UK Clinical Research Collaboration will provide an opportunity to increase clinical and health services research in gastroenterology. It is important that the investment that is being made supports the growth of research into the care of diseases which are responsible for high morbidity and mortality, and are a significant burden on the patient, the NHS, and the economy (section 6.3).
8. Annex: Summary of articles used
Note on methodology – rationale for presentation of results in tables
The review of evidence of effectiveness of service delivery arrangements followed the CRD methods for systematic reviewing, with the primary literature search designed to identify papers concerned with service delivery. Results of the search are presented in section 5.2. All papers identified through this search were screened, and those that were relevant to any section of the report were summarised and graded. Papers cited in section 5.3, concerned with effectiveness of models of service delivery, are matched with tables (A.1–A.11) which provide further details of the research setting, study design, and key results, as well as their AGREE score and grading for level of evidence where relevant.
Additional papers for other sections of the report were identified through topic‐specific searches (burden of disease; quality of life; health economics of GI) and through existing collections of publications. These papers are included, where relevant, in various sections of the report, and where identified through the primary literature search are matched in summary table A.12. Table A.13 summarises the articles examined after consultant feedback.
Table A.12 Summary of articles identified through systematic search by section.
| ID and authors | Research setting and year of study | Study design | Sample size | Topic of document | Level of evidence | Quality score (AGREE) (%) |
|---|---|---|---|---|---|---|
| Section 3.1 Spectrum of disease | ||||||
| Jenkins541 | UK (2001) | Commentary, review of evidence | NA | Paediatric IBD | 3 | 52 |
| Cumberland et al358 | UK (not specified—circa 2003) | Survey and follow‐up | 85 Practices; over 1000 cases | Infectious intestinal disease in England | 2− | 73 |
| Dominitz et al783 | USA (not specified—circa 2002; data between 1987 and 1996) | Analysis of routine data | Sample taken from 746 130 births | Infants born to mothers with IBD | 2+ | 66 |
| Sheridan et al745 | UK (1990) | Analysis of routine data; survey | 100 Patients; 52 clinicians | Abdominal pain and resource implications | 2− | 73 |
| De Lillo and Rose784 | International (2000) | Commentary; appraisal of evidence | Around 50 articles | Bowel disorders in geriatric patients | 3 | 57 |
| Hislop and Heading785 | UK (2000–01) | Survey and analysis of routine data | 53 clinicians | Impact of alcohol related disease | 2− | 61 |
| Lunniss et al359 | UK (data taken between 1995 and 2002) | Analysis of patient data | 629 Patients | Faecal incontinence | 2− | 66 |
| McKiernan et al373 | UK (1993–95) | Analysis of patient data; survey | 93 Cases | Biliary atresia | 2− | 64 |
| Plevris et al786 | International, with emphasis on UK (1998) | Commentary; review of evidence | Around 120 articles | Management of acute liver failure | 3 | 61 |
| Morris787 | International (1991) | Commentary; review of research | Around 80 articles | Non‐ulcer dyspepsia | 3 | 59 |
| AGA788 | USA (2001) | Review of evidence; expert commentary | Over 125 articles | Prevalence and costs of GI diseases | 2+ | 68 |
| De Dombal544 | International; emphasis on UK (1994) | Commentary; review of evidence | Around 7 articles | Acute abdominal pain | 3 | 57 |
| Section 3.2 Incidence (includes prevalence) | ||||||
| Jenkins541 | UK (2001) | Commentary; review of evidence | NA | Paediatric IBD | 3 | 52 |
| Cumberland et al358 | UK (Not specified—circa 2003) | Survey and follow‐up | 85 Practices; over 1000 cases | Infectious intestinal disease (IID) in England | 2+ | 73 |
| Bodger737 | International, with emphasis on UK (2002) | Commentary; appraisal of evidence | Around 60 articles | Cost of illness of Crohn's disease | 3 | 66 |
| Hislop and Heading785 | UK (2000–01) | Survey and analysis of routine data | 53 Clinicians | Impact of alcohol related disease | 2− | 61 |
| Gut Week103 | UK (2004) | Public information leaflets; commentary | NA | Digestive health in the UK | 3 | 36 |
| Lunniss et al359 | UK (data between 1995 and 2002 taken) | Analysis of patient data | 629 Patients | Faecal incontinence | 2− | 66 |
| Ghanchi and Rembacken789 | UK (2003) | Review of evidence; commentary | Around 130 articles | IBD | 3 | 70 |
| Morris787 | International (1991) | Commentary; review of research | Around 80 articles | Non‐ulcer dyspepsia | 3 | 59 |
| Mamula et al790 | International; emphasis on USA (data between 1977 and 2000 taken) | Analysis of routine data | 82 Patients | IBD in children under 5 years of age | 2− | 70 |
| AGA788 | USA (2001) | Review of evidence; expert commentary | Over 125 articles | Prevalence and costs of GI diseases | 2+ | 68 |
| McNamara et al581 | International; emphasis on USA (2000) | Review of evidence; expert commentary | Around 70 articles | Non‐ulcer dyspepsia (NUD) | 2− | 64 |
| Wong et al791 | China and Hong Kong (2002) | Survey of Chinese population | 2209 Survey responses | Gastro‐oesophageal reflux disease (GERD) | 2− | 80 |
| Pimentel et al792 | USA (not specified—circa 2000) | Survey; analysis of patient data | 448 Patients | Small intestinal bacterial overgrowth (SIBO) and IBS | 2+ | 66 |
| De Dombal544 | International; emphasis on UK (1994) | Commentary; review of evidence | Around 7 articles | Acute abdominal pain | 3 | 57 |
| Loftus102 | International (2004) | Review of evidence | Around 170 articles | IBD | 2− | 59 |
| Russel793 | International (2000) | Review of evidence; commentary | Around 50 articles | Incidence of IBD | 3 | 55 |
| Moum and Ekbom794 | International (2002) | Review of evidence | Around 90 articles | Incidence of IBD | 3 | 55 |
| Wilson et al153 | International (2003) | Systematic review | 15 Articles | Prevalence of IBD | 1 | 66 |
| Farrokhyar et al795 | International (literature between 1950 and 1999 taken) | Review of evidence | Around 200 articles | Epidemiology of IBD | 1 | 66 |
| Lapane et al796 | USA (data between 1992 and 1996 taken) | Analysis of patient data | 133 839 Patient records | Effect of NSAID use | 2+ | 68 |
| Chiang et al797 | Australia (2001) | Analysis of patient data | 167 Patients | Acute pancreatitis management | 2+ | 68 |
| Fass et al798 | USA (1992–95) | Analysis of patient data; survey | 505 Patients | Functional bowel disorders (FBD) and sleep disorders | 2− | 73 |
| Parry et al799 | UK (2000–01) | Case‐control study | 482 Patients | IBS and bacterial gastroenteritis | 2+ | 75 |
| Bernstein et al800 | Canada and USA (data between 1984 and 1996 taken) | Analysis of data | Not specified, but a large number | Extra‐intestinal diseases in IBD | 2− | 59 |
| Payne and Saul801 | UK (data between 1994 and 1998 taken) | Survey and analysis of data | 12 239 Responses | Common disorders in long term illnesses | 2− | 61 |
| Ruigomez et al802 | UK (1994) | Analysis of data | 2956 Patients | Follow‐up of patients with IBS | 2− | 50 |
| Sanders et al174 | UK (1999–2001) | Cross sectional intervention | 1200 Participants | Diagnosis of coeliac disease | 2+ | 70 |
| Waddell and Hislop357 | UK (not specified—circa 1999 onwards) | Analysis of patient data | 390 Patients | Impact of alcohol related disease | 2− | 59 |
| BSG574 | UK (2002) | Guidelines | NA | Guidelines for dyspepsia management | 2− | 45 |
| ONS803 | UK (2001) | National data | Cancer trends between 1950 and 1999 | Cancer trends in England and Wales | 2+ | 66 |
| Kennedy and Jones22 | UK (Not specified—circa 1997–2000) | Cross sectional survey | 3179 Survey responses | Prevalence of gastro‐oesophageal reflux symptoms | 2− | 64 |
| Watson et al144 | UK (data between 1980 and 1999 taken) | Retrospective study of patient data | 107 Patients | IBD in children | 2− | 57 |
| Rockall et al91 | UK (1993) | Analysis of patient data | 4185 Cases | Incidence and mortality from GI haemorrhage | 2− | 57 |
| Metcalf et al243 | UK (data between 1987 and 1994 taken) | Analysis of patient data | 160 Cases | Incidence and prevalence of primary biliary cirrhosis in Newcastle Upon Tyne | 2− | 61 |
| Blower et al804 | UK (1990–91) | Analysis of routine data | 620 Cases | Upper GI disease and NSAID use | 2+ | 57 |
| Sawczenko et al805 | UK (1999) | Survey | 739 Responses | Childhood IBD | 2− | 50 |
| James et al238 | UK (data between 1987 and 1994 taken) | Collection and analysis of patient data | 770 Cases | Primary biliary cirrhosis | 2− | 52 |
| McKay et al306 | UK (data between 1984 and 1995 taken) | Analysis of patient data | Over 10 000 cases | Acute pancreatitis | 2− | 57 |
| Griffin et al806 | UK (2002) | Analysis and summary of patient data | 3361 Cases | Summary of incidence and mortality rates for upper GI cancers | 3 | 43 |
| Cooper et al807 | UK (2001) | Analysis of NHS Direct data | Over 150 000 calls | Calls to NHS Direct and GI diseases | 2− | 55 |
| CSCG808 | UK (2003) | Commentary | NA | Response to NICE service guidance on upper GI cancers | Guideline | 41 |
| Jones809 | International (1999) | Review of evidence | Around 20 articles | Methodological considerations | 3 | 68 |
| Feuer595 | International (1999) | Commentary; review of evidence | Around 20 articles | Management of intestinal obstruction | 3 | 50 |
| NHS, NICE810 | UK (2004) | National commentary; guidelines; recommendations | NA | Improving the outcomes in colorectal cancer | Guideline | 70 |
| South Wales Cancer Network754 | UK (2003) | Service guidelines; recommendations | NA | Configuration of services | 3 | 45 |
| Aerts and Penninckx266 | Europe (2003) | Review of evidence; commentary | Around 30 articles | Burden of gallstone disease | 2− | 52 |
| Ashorn811 | Europe (2003) | Review of evidence; commentary | Around 20 articles | Paediatric GI disease | 3 | 43 |
| Delvaux210 | Europe (2003) | Review of evidence; commentary | Around 30 articles | Diverticular disease of the colon | 3 | 48 |
| Delvaux812 | Europe (2003) | Review of evidence; commentary | Around 40 articles | Faecal incontinence | 3 | 55 |
| Delvaux813 | Europe (2003) | Review of evidence; commentary | Around 30 articles | Functional bowel disorders and IBS | 3 | 50 |
| McNamara319 | Europe (2003) | Review of evidence; commentary | Around 40 articles | Pancreatic disease | 3 | 50 |
| Burroughs and McNamara227 | Europe (2003) | Review of evidence; commentary; | Around 30 articles | Liver disease | 3 | 50 |
| Talley et al53 | USA (1987–90) | Survey of random sample of population | 328 Survey responses | Prevalence of gastrointestinal symptoms in the elderly | 2− | 70 |
| Section 3.3 Mortality | ||||||
| Gut Week103 | UK (2004) | Public information leaflets; commentary | NA | Digestive health in the UK | 3 | 36 |
| Munkholm814 | International (2003) | Review of evidence; commentary | Around 25 articles | Incidence and prevalence of colorectal cancer | 3 | 57 |
| AGA788 | USA (2001) | Review of evidence; expert commentary | Over 125 articles | Prevalence and costs of GI diseases | 2+ | 68 |
| de Dombal544 | International; emphasis on UK (1994) | Commentary; review of evidence | Around 7 articles | Acute abdominal pain | 3 | 57 |
| Davis et al815 | International (1990) | Analysis of data | Data from 1968 to 1987 | International cancer mortality trends | 2− | 64 |
| Stanley et al816 | International (1988) | Review of trends | Around 20 articles | Mortality trends | 2− | 59 |
| Khan et al817 | International (data between 1979 and 1998 taken) | Analysis of mortality data | Not specified, but a large amount of data | Mortality trends | 2− | 57 |
| Cucino and Sonnenberg818 | USA (data between 1991 and 1996 taken) | Analysis of mortality data | Data of around 5000 patient deaths | Occupational mortality | 2− | 57 |
| Farrokhyar et al795 | International (literature between 1950 and 1999 taken) | Review of evidence | Around 200 articles | Epidemiology of IBD | 1 | 66 |
| Maroun et al819 | Canada (not clear—circa late 1990s) | Analysis of data; review of evidence | NA; cases taken from databases | Costs of cancer management | 2− | 73 |
| Fernandez et al820 | Europe (data between 1955and 1989 taken) | Analysis of data | Not specified (but a large amount) | Trends in pancreatic cancer mortality | 2+ | 61 |
| La Vecchia et al821 | Europe (data between 1970 and 1996 taken) | Analysis of data | Not specified (but a large amount) | Trends in primary liver cancer mortality | 2+ | 55 |
| Maheswaran et al822 | UK (1993–95) | Analysis of mortality data | Over 10 000 cases | Trends in stomach cancer | 2+ | 61 |
| Taylor‐Robinson et al446 | UK (data between 1968 and 1996 taken) | Analysis of mortality data | A large amount | Mortality rates from intrahepatic cholangiocarcinoma | 2− | 57 |
| ONS803 | UK (2001) | National data | Cancer trends between 1950 and 1999 | Cancer trends in England and Wales | 2+ | 66 |
| Payne et al823 | UK (1991) | Cross sectional survey | 3877 Survey responses | Comparison of prevalence rates | 2− | 73 |
| Rockall et al91 | UK (1993) | Analysis of patient data | 4185 Cases | Incidence and mortality from GI haemorrhage | 2− | 57 |
| Sharp et al824 | UK (data between 1968 and 1992) | Analysis of cancer data | Not specified, but a large amount | Cancer incidence and mortality | 2− | 50 |
| Blower et al804 | UK (1990–1991) | Analysis of routine data | 620 Cases | Upper GI disease and NSAID use | 2+ | 57 |
| Pye et al825 | UK (1995–1996) | Analysis of patient data | 910 Patients | Carcinoma of the oesophagus and stomach | 2− | 57 |
| James et al238 | UK (data between 1987 and 1994 taken) | Collection and analysis of patient data | 770 Cases | Primary biliary cirrhosis | 2− | 52 |
| McKay et al306 | UK (data between 1984 and 1995 taken) | Analysis of patient data | Over 10 000 cases | Acute pancreatitis | 2− | 57 |
| Bray et al328 | Europe (1995) | Analysis of mortality data | Not specified, but a large amount | Cancer incidence and mortality | 2+ | 66 |
| AUGIS826 | UK (2002) | Analysis and summary of patient data | 3361 Cases | Summary of incidence and mortality rates for upper GI cancers | 3 | 43 |
| Rockall et al398 | UK (1993–94) | Collection and analysis of patient data | Over 5000 cases | Outcomes after acute upper GI haemorrhage | 2− | 57 |
| Tekkis et al827 | UK (1999–2001) | Analysis of patient data | 8077 Patients | Operative mortality in colorectal cancer | 2− | 70 |
| NHS, NICE671 | UK (2004) | National commentary; guidelines; recommendations | NA | Improving the outcomes in colorectal cancer | Guideline | 70 |
| South Wales Cancer Network754 | UK (2003) | Service guidelines; recommendations | NA | Configuration of services | 3 | 45 |
| Burroughs and McNamara227 | Europe (2003) | Review of evidence; commentary | Around 30 articles | Liver disease | 3 | 50 |
| Section 3.4 Morbidity | ||||||
| Morris787 | International (1991) | Commentary; review of research | Around 80 articles | Non‐ulcer dyspepsia | 3 | 59 |
| Waddell and Hislop357 | UK (not specified—circa 1999 onwards) | Analysis of patient data | 390 Patients | Impact of alcohol related disease | 2− | 59 |
| Payne et al828 | UK (1991) | Cross sectional survey | 3877 Survey responses | Comparison of prevalence rates | 2− | 73 |
| McCulloch et al829 | UK (1999–2002) | Cohort study; analysis of patient data | 955 Patients | Mortality and morbidity in gastro‐oesophageal cancer surgery | 2− | 64 |
| Smith et al775 | UK (not specified—circa 2003) | Survey of people with IBS symptoms | 486 Cases | Management of IBS in primary and secondary care | 2− | 66 |
| Spechler20 | International (1992) | Review of research | Around 30 articles | Epidemiology of GERD | 3 | 66 |
| Section 3.5 Geographical variation | ||||||
| Talley et al830 | International (not specified—circa 1995 to 2000) | Survey of communities | Over 5000 survey responses | Classification of GI symptoms | 2− | 70 |
| Wong et al791 | China and Hong Kong (2002) | Survey of Chinese population | 2209 Survey responses | Gastro‐oesophageal reflux disease (GERD) | 2− | 80 |
| Loftus102 | International (2004) | Review of evidence | Around 170 articles | IBD | 2− | 59 |
| Russel793 | International (2000) | Review of evidence; commentary | Around 50 articles | Incidence of IBD | 3 | 55 |
| Moum and Ekbom794 | International (2002) | Review of evidence | Around 90 articles | Incidence of IBD | 3 | 55 |
| Farrokhyar et al795 | International (literature between 1950 and 1999 taken) | Review of evidence | Around 200 articles | Epidemiology of IBD | 1 | 66 |
| Maheswaran et al822 | UK (1993–95) | Analysis of mortality data | Over 10 000 cases | Trends in stomach cancer | 2+ | 61 |
| Bray et al328 | Europe (1995) | Analysis of mortality data | Not specified, but a large amount | Cancer incidence and mortality | 2+ | 66 |
| Levenstein et al831 | International (data between 1991 and 1996 taken) | Survey of patients | 2002 Patients | Cross‐cultural variation in patients with IBD | 2− | 75 |
| Section 3.6 Socioeconomic factors | ||||||
| Dominitz et al783 | USA (not specified—circa 2002; data between 1987 and 1996) | Analysis of routine data | Sample taken from 746 130 births | Infants born to mothers with IBD | 2− | 66 |
| Hislop and Heading785 | UK (2000–01) | Survey and analysis of routine data | 53 Clinicians | Impact of alcohol related disease | 2− | 61 |
| Neumann and Cooper32 | UK (data between 1989 and 1996 taken) | Analysis of patient data | 1101 Patients | Ethnic differences in gastro‐oesophageal disease | 2− | 68 |
| Wong et al791 | China and Hong Kong (2002) | Survey of Chinese population | 2209 Survey responses | Gastro‐oesophageal reflux disease (GERD) | 2− | 80 |
| Loftus102 | International (2004) | Review of evidence | Around 170 articles | IBD | 2− | 59 |
| Longobardi et al832 | USA (1999) | Analysis of data | 23 649 Records | Work losses due to IBD | 2− | 66 |
| Longobardi et al833 | Canada (1999) | Analysis of data | 23,649 Records | Work losses due to IBD | 2− | 66 |
| Fass et al798 | USA (1992–95) | Analysis of patient data; survey | 505 Patients | Functional bowel disorders (FBD) and sleep disorders | 2− | 73 |
| Danese et al834 | International (2004) | Expert commentary; summary of evidence | Around 40 articles | IBD and environmental factors | 3 | 48 |
| Payne and Saul801 | UK (data between 1994 and 1998 taken) | Survey and analysis of data | 12 239 Responses | Common disorders in long term illnesses | 2− | 61 |
| Kennedy and Jones22 | UK (not specified—circa 1997–2000) | Cross sectional survey | 3179 Survey responses | Prevalence of gastro‐oesophageal reflux symptoms | 2− | 64 |
| McKinney et al835 | UK (data between 1960 and 1990) | Analysis of cancer data | Not specified, but a large number | Oesophageal and gastric cancer incidence | 2− | 52 |
| Bray et al328 | Europe (1995) | Analysis of mortality data | Not specified, but a large number | Cancer incidence and mortality | 2+ | 66 |
| Dean et al836 | USA (1999) | Survey of patients | 11 604 Responses | Work productivity and gastro‐oesophageal reflux disease (GERD) | 2− | 75 |
| Bernstein et al837 | Canada (1995–96) | Survey and analysis of patient data | Not clear, but a large number | Socioeconomic factors associated with IBD | 2− | 55 |
| Sands et al552 | USA (patients between 1991 and 1997) | Analysis of patient data | 345 Patients | Risk of early surgery for Crohn's disease | 2+ | 64 |
| Vaughn et al838 | UK (not specified—circa 1998) | Survey of patients and their relatives | Not clear—around 29 patients | Expressed emotion during the course of IBD | 2− | 59 |
| Crane and Martin839 | UK (not specified—circa 2002) | Survey of patients | 58 Patients | Social learning, affective state, and passive coping in IBD and IBS | 2− | 68 |
| Sewitch et al534 | Canada (Not specified—circa 2001) | Survey of patients and physicians | 10 Gastroenterologists and 200 patients | Patient‐physician correlates in IBD | 2− | 75 |
| Casati and Toner840 | International (2000) | Review of evidence; commentary | Around 70 articles | Psychosocial aspects of IBD | 3 | 55 |
| Sewitch et al841 | Canada (1999) | survey of patients | 200 Patients | Psychosocial aspects in IBD | 3 | 70 |
| Soo et al842 | International (studies from 1966 to present) | Systematic review | 4 Studies | Psychological interventions for non‐ulcer dyspepsia | 2− | 80 |
| Guthrie843 | International (2002) | Review of evidence | 3 Studies | Psychodynamic‐interpersonal therapy for functional bowel disorders | 3 | 52 |
| Kisely844 | UK (1996–97) | Analysis of patient data | 65 204 Patient records | Multiple readmissions | 2− | 59 |
| Moum585 | International (2000) | Review of evidence | Around 80 articles | Medical treatment for IBD | 3 | 64 |
| Tojek et al845 | USA (not specified—circa 2000) | Survey of patients | 62 Patients | Health status in IBD | 2− | 75 |
| Jahnsen et al846 | Norway (not specified—circa 2003) | Population based study | 60 Patients | Body composition in patients with IBD | 2+ | 66 |
| de Rooy et al687 | Canada (not specified—circa 2001) | Survey of patients | 259 Patients | Concerns of patients with IBD | 2− | 68 |
| Ward et al738 | International (circa 1998) | Review of evidence | Around 40 articles | Management of IBD | 2− | 70 |
| Hatch847 | Not specified—appears to be USA (circa 1996) | Case study | 2 Patients | Treatment of bowel obsessions | 3 | 70 |
| Guthrie et al848 | UK (not specified—circa 2003) | Survey of patients; analysis of patient data | 107 Patients | Cluster analysis to define patient subgroups for IBS | 2− | 75 |
| Drossman849 | International (1999) | Review of evidence | Around 40 articles | Psychosocial factors in IBS | 3 | 66 |
| Payne et al823 | UK (1991) | Survey of population in Rotherham | 3877 Survey responses | Deprivation and morbidity | 2− | 70 |
| Section 3.7 Quality of life | ||||||
| Pachler and Wille‐Jorgensen850 | International (2002–03 (no restriction on date of studies) | Systematic review | 8 Studies | Quality of life (QoL) after rectal resection for cancer | 1 | 75 |
| Yacavone et al851 | International (review of evidence: 1966 to 1999) | Review of evidence | Around 80 articles | QoL in gastroenterology | 2+ | 68 |
| Simren et al852 | Sweden (not specified—circa 2002) | Survey of patients | 83 Patients | QoL in IBD | 2− | 66 |
| Rubin et al676 | UK (not specified—circa 2003) | Survey of patients | 409 Responses | QoL in IBD | 2− | 68 |
| Halder et al450 | USA (not specified—circa 2003) | Case‐control study | 112 Patients; 110 controls | GI disorders and QoL | 2+ | 66 |
| Hahn et al853 | UK and USA (not specified—circa 1999) | Survey of patients | Around 600 patients | Impact of IBS on QoL | 2− | 66 |
| Blondel‐Kucharski et al854 | France (not specified—circa 2001) | Survey of patients | 231 Patients | QoL in Crohn's disease | 2− | 66 |
| Akehurst et al740 | UK (1998) | Survey of patients | 161 Patients | QoL and cost impact of IBS | 2+ | 77 |
| Gonsalkorale et al855 | UK (not specified—circa 2002) | Survey of patients | 78 Patients | Cognitive change in patients during IBS | 2− | 70 |
| Moayyedi and Mason747 | UK (1992–94) | Analysis of patient data; survey of patients | Not clear; over 8000 patients | Economic consequences of dyspepsia | 3 | 82 |
| Borgaonkar and Irvine447 | International (2000) | Review of evidence between 1966 and 1999 | Around 140 articles | QoL measurement in gastrointestinal and liver disorders | 2+ | 80 |
| El‐Serag et al856 | International (2001) | Systematic review of evidence between 1980 and 2001 | 17 articlesArticles | QoL of people with IBS | 1+ | 77 |
| Koloski et al449 | Australia (not clear—circa 1996–2000) | Survey of random sample of population | 2910 Survey respondents | Impact of functional GI disorders on QoL | 2− | 70 |
| O'Keefe et al857 | USA (1987–90) | Survey of random sample of population | 530 Survey responses | Bowel disorders and its impact on QoL in the elderly | 2− | 75 |
| Gralnek et al858 | USA (1994–1998) | Survey of patients | 877 Patients | Impact of IBS on health related QoL | 2− | 73 |
| Section 4.1 Care pathways | ||||||
| McNamara et al581 | International; emphasis on USA (2000) | Review of evidence; expert commentary | Around 70 articles | Non‐ulcer dyspepsia (NUD) | 2− | 64 |
| Association of Coloproctology of GB and Ireland505 | UK (2001) | Specification of guidelines | NA | Guidelines for the management of colorectal cancer | Guideline | 61 |
| CSCG808 | UK (2003) | Commentary | NA | Response to NICE service guidance on upper GI cancers | Guideline | 41 |
| Pfau et al859 | USA (1997–1999) | Implementation of care pathway | 421 patients | Clinical care pathway for the management of acute nonvariceal upper GI bleeding | 2− | 77 |
| Kisely844 | UK (1996–1997) | Analysis of patient data | 65204 patient records | Multiple readmissions | 2− | 59 |
| Feuer595 | International (1999) | Commentary; review of evidence | Around 20 articles | Management of intestinal obstruction | 3 | 50 |
| Section 4.2 Workforce | ||||||
| Garvican607 | UK (1998) | Commentary; cost analysis | NA | Colorectal cancer screening | 3 | 70 |
| Duff et al506 | UK (2002) | Audit | 65 Patients | Waiting times for treatment of rectal cancer in northwest England | 3 | 52 |
| Slade et al860 | UK (not specified—circa 1998) | Survey of patients; treatment intervention | 232 Patients | Serological testing for H pylori to reduce workload | 2− | 61 |
| Lamy and McNamara861 | Europe (data between 1996 and 2001) | Survey of experts | Not clear—around 25 European countries | Gastroenterology and hepatology services in Europe | 3 | 59 |
| Association of Coloproctology of GB and Ireland753 | UK (2001) | Analysis of audit data; expert commentary and recommendations | 8 Main sources of audit data | Resources for coloproctology | 3 | 52 |
| Section 4.3.1 Primary care | ||||||
| Cumberland et al358 | UK (not specified—circa 2003) | Survey and follow‐up | 85 Practices; over 1000 cases | Infectious intestinal disease (IID) in England | 2+ | 73 |
| Section 4.3.2 Inpatients | ||||||
| Hislop and Heading785 | UK (2000–01) | Survey and analysis of routine data | 53 Clinicians | Impact of alcohol related disease | 2− | |
| Waddell and Hislop357 | UK (Not specified—circa 1999 onwards) | Analysis of patient data | 390 patients | Impact of alcohol related disease | 2− | |
| Section 4.3.3 Outpatients | ||||||
| Rayner et al862 | UK (1997–98) | Analysis of patient data | 1203 Patients | Outpatient review practices | 2− | 57 |
| Section 4.3.4 Procedures | ||||||
| Westbrook et al863 | Australia (data between 1986 and 1990 taken) | Analysis of patient data | Unclear—over 17 000 cases | Upper GI tract investigations in the elderly | 2− | 61 |
| Grassi864 | Italy (data between 1991 and 1999) | Commentary on data | Not clear, but a large number | Endoscopy activity | 4 | 36 |
| BSG865 | UK (1987) | Commentary; review of evidence | Around 20 articles | Requirements for colonoscopy | 3 | 57 |
| Section 4.6.1 Access | ||||||
| Froehlich et al500 | Switzerland; comparisons with UK (1994–95) | Review of evidence; analysis of patient data | 8135 Patients | Overuse of upper GI endoscopy in primary care | 2− | 61 |
| Gralnek501 | International (2002) | Commentary | NA | Outpatient management of low risk non‐variceal upper GI haemorrhage | 4 | 50 |
| Silcock and Bramble502 | UK (1994) | Survey | 333 Responses | Survey of current practice in open access gastroscopy (OAG) | 2− | 68 |
| Section 4.6.2 Inequalities | ||||||
| Watt503 | UK (2002) | Commentary | NA | The inverse care law | 4 | 39 |
| Section 4.6.3 Waiting lists | ||||||
| Association of Coloproctology of GB and Ireland505 | UK (2001) | Specification of guidelines | NA | Guidelines for the management of colorectal cancer | Guideline | 61 |
| Flashman et al507 | UK (2000–01) | Audit | 249 Patients | Two week standard for bowel cancer | 2− | 61 |
| Duff et al506 | UK (2002) | Audit | 65 Patients | Waiting times for treatment of rectal cancer in northwest England | 3 | 52 |
| Parente et al866 | Italy (1999–2000) | Audit | 142 Patients | Audit of gastroscopy | 2− | 61 |
| Dunnill and Pounder504 | UK (articles between 1966 and 2002) | Literature review | Around 40 articles | Outpatient services | 2− | 70 |
| Hellier508 | UK (1999–2001) | Survey of GI units | 210 GI units | Two week target for investigation cancer patients | 3 | 55 |
| Section 4.6.4 Patient safety | ||||||
| Van Kouwen et al522 | Netherlands (1994–98) | Analysis of endoscopy services and patient data | 218 Patients | Upper GI endoscopy for older patients | 2− | 57 |
| Navarro and Hanauer509 | International (2003) | Review of evidence; commentary | Around 70 articles | Safe treatment for IBD | 3 | 59 |
| Akehurst and Kaltenthaler510 | International (trials between 1987 and 1998) | Review of RCTs | 45 RCTs | Treatment of IBS | 1 | 66 |
| Dick et al511 | UK (2002) | Analysis of prescriptions | 308 Patientsand 777 prescriptions | Use of unlicensed and off‐label drugs in paediatric GI diseases | 2− | 61 |
| Chan and Graham512 | International (2004) | Review of evidence | Around 60 articles | Prevention of NSAID GI complications | 3 | 73 |
| Sheen and Colin‐Jones513 | International; emphasis on UK (2001) | Review of evidence | Around 100 articles | Over‐the‐counter drugs for GI diseases | 3 | 68 |
| Abbas et al520 | Not specified—circa 2003) | Survey of patients; analysis of patient data | 1287 Patients | Outpatient upper GI endoscopy | 2− | 64 |
| Bloor and Maynard514 | UK (1996) | Review of evidence | Around 40 articles | Prescription of NSAIDs | 3 | 73 |
| Feagan515 | International (2003) | Review of evidence | Around 90 articles | Maintenance treatment for IBD | 2+ | 61 |
| Ripamonti et al526 | International (1993) | Literature review | Around 40 articles | Management of bowel obstruction in patients with cancer | 2− | 75 |
| Cook et al517 | International (articles from 1966 onwards) | Meta‐analysis | 30 RCTs | Endoscopic therapy for acute non‐variceal upper GI haemorrhage | 1 | 73 |
| Page et al524 | USA (Patientsbetween 1989 and 1999) | Analysis of patient data | Over 100 patients | Surgical risk in elderly patients with IBD | 2− | 59 |
| Moorthy et al525 | UK (data between 1991 and 2001) | Analysis of patient data | 48 Patients | Patients undergoing laparoscopic surgery for Crohn's disease | 2− | 64 |
| Yim et al527 | Not specified—probably USA (1996–99) | Analysis of patient data | 29 Patients | Enteral stents for patients with upper GI obstruction | 2+ | 64 |
| Heuschkel et al518 | USA and UK (2000) | Survey of patients | 208 Survey responses | Complementary medicine used by children for IBD | 2− | 80 |
| Quine et al521 | UK (1991) | Survey of clinicians | 39 Hospitals; 383 clinicians | Audit of upper GI endoscopy | 2− | 64 |
| Section 4.6.5 Information to patients and practitioners | ||||||
| Dick et al511 | UK (2002) | Analysis of prescriptions | 308 Patients and 777 prescrip‐tions | Use of unlicensed and off‐label drugs in paediatric GI diseases | 2− | 61 |
| Sheen and Colin‐Jones513 | International; emphasis on UK (2001) | Review of evidence | Around 100 articles | Over‐the‐counter drugs for GI diseases | 3 | 68 |
| Sewitch et al534 | Canada (not specified—circa 2001) | Survey of patients and physicians | 10 Gastroenterologists and 200 patients | Patient‐physician correlates in IBD | 2− | 75 |
| Stone et al141 | UK (2002) | Analysis of patient data | Over 86 000 patients | Management of IBD | 2− | 73 |
| Sewitch et al535 | Canada (1999) | Survey of patients and clinicians | 10 Clinicians; 153 patients | Patient non‐adherence to medication in IBD | 2− | 75 |
| Mansfield et al537 | UK (not specified—circa 1997) | Survey of clinicians and patients | 732 Patients; 6 gastroenterology clinics | Information for patients about IBD | 2− | 64 |
| Thompson et al532 | UK (not specified—circa 2003) | Survey of patients and nurses | 402 Patients; 62 nurses | Information for patients undergoing gastroscopy | 2− | 73 |
| Hawkey and Hawkey530 | UK (not specified—circa 1989) | Survey of patients | 751 Survey responses | Information for patients with GI diseases | 2− | 70 |
| NICE671 | UK (2004) | Summary of guidelines | NA | Information leaflet for patients | 3 | 30 |
| Greiner et al536 | USA (2002) | Survey of patients | Not clear—around 800 patients | Barriers to colorectal cancer screening | 2− | 70 |
| Institute of Food Research529 | UK (2004) | Information and advice to the public | NA | Diet and health | 3 | 41 |
| Mukherjee et al528 | UK (not specified—circa 2001) | Qualitative study | 24 Patients | Parents' experiences of IBD | 2− | 75 |
| Section 4.6.6 Diagnosis and complications in care | ||||||
| Jenkins541 | UK (2001) | Commentary, review of evidence | NA | Paediatric IBD | 3 | 52 |
| Mamula et al790 | International; emphasis on USA (data between 1977 and 2000 taken) | Analysis of routine data | 82 Patients | IBD in children under 5 years of age | 2− | 70 |
| de Dombal544 | International; emphasis on UK (1994) | Commentary; review of evidence | Around 7 articles | Acute abdominal pain | 3 | 57 |
| Gatta et al561 | Europe (not specified—data collected between 1988 and 1999) | Analysis of cancer data | 2720 Patients | Colorectal cancer in Europe | 2− | 59 |
| Camilleri543 | USA (2001) | Review of evidence; commentary | Around 170 articles | Management of IBS | 3 | 64 |
| Limpert et al554 | USA (patients between 1992 and 2002 were used | Analysis of patient data | 181 Patients | Colon and rectal cancer in the elderly | 2− | 61 |
| Sanders et al174 | UK (1999–2001) | Cross sectional intervention | 1200 Participants | Diagnosis of coeliac disease | 2+ | 70 |
| Spray et al540 | UK (patients between 1994 and 1998 were used) | Analysis of medical records | Around 100 patients | IBD in children | 2+ | 52 |
| Hamilton and Sharp556 | UK (evidence between 1966 and 2002 taken) | Assessment of clinical guidelines | Around 100 articles | Guidelines for the diagnosis of colorectal cancer | 2+ | 66 |
| Ofman et al545 | USA (not specified—circa 2002) | Application of decision analysis | NA | Management strategies for gastro‐oesophageal reflux disease | 2− | 75 |
| Brignoli et al548 | Switzerland (not specified—circa 1997) | Case‐control study | 1078 Patients | Diagnostic strategies for dyspepsia | 2+ | 70 |
| Summerton and Paes549 | UK (1997–98) | Survey; audit | 275 Responses | Clinical assessment of patients with large bowel symptoms | 2− | 70 |
| Farmer et al539 | USA (not specified—circa 2000) | Evaluation of practice; analysis of patient data | 119 Patients | Diagnostic accuracy for IBD | 2− | 61 |
| Shah et al562 | USA (1995–98) | Review of patient data | 168 Patients | Use of colonoscopy and biopsy | 2− | 61 |
| Erickson and Glick563 | International (studies between 1974 and 1982 taken) | Review of evidence | Around 80 articles | Benefits of oesophagogastroduodenoscopy | 2− | 61 |
| Drossman et al551 | USA (1996–98) | Survey of patients | 211 Patients | Factors pointing to the severity of functional bowel disorders | 2− | 70 |
| Sands et al552 | USA (patients between 1991 and 1997) | Analysis of patient data | 345 Patients | Risk of early surgery for Crohn's disease | 2+ | 64 |
| Lang et al566 | Finland (1996–97) | Observational study | 503 Patients | Resource use in gastroenterological surgery | 2− | 70 |
| Tunaci558 | International (2002) | Commentary | NA | Imaging of GI cancers | 3 | 52 |
| Quine et al867 | UK (1991) | Audit | 15 Hospitals in East Anglia and northwest England | Audit of upper GI endoscopy | 2− | 59 |
| Parry et al564 | UK (not specified—circa 2001) | Retrospective study of patient data | 52 Patients | Push enteroscopy | 2− | 61 |
| Diamanti et al550 | Italy (not specified—circa 2002) | Analysis of patient data | 6 Patients | Patients with ultra‐short bowel disease | 2− | 52 |
| Yasui et al565 | International (cancer cases between 1993 and 1998) | Analysis of cancer cases | 9241 Cases | Molecular‐pathological diagnosis of GI tissues for cancer histopathology | 2− | 68 |
| Schofield567 | UK (data between 1994 and 1998 taken) | Review of medical cases | 245 Cases | Medical negligence in coloproctology | 3 | 57 |
| Hansen et al547 | Denmark (1991–92) | Interview of patients | 612 Patients | Management of dyspeptic patients in primary care | 2− | 70 |
| Talley et al546 | International (1991) | Review of evidence | Around 180 articles | Classification and guidelines for the diagnosis and management of functional dyspepsia | 3 | 80 |
| Mulcahy et al868 | UK (data between 1989 and 1998 taken) | Retrospective review of patients data | 9795 Patients | Patterns of sedation use for diagnostic gastroscopy | 2− | 55 |
| Hin et al378 | UK (1996–97) | Case finding study | 30 Patients | Coeliac disease in primary care | 2− | 66 |
| Schmulson and Chang542 | International (1999) | Review of evidence | Around 50 articles | Diagnostic approach to IBS | 3 | 70 |
| Martin et al555 | UK (1994) | Analysis of patient data; survey | 115 Patients | Delays in the diagnosis of oesophagogastric cancer | 2− | 61 |
| Angelelli et al553 | International (2003) | Review of evidence; commentary | Around 40 articles | Computed tomography and acute bowel ischaemia | 3 | 66 |
| Berg et al538 | International (2001) | Review of evidence; commentary | Around 40 articles | Acute surgical emergencies in IBD | 3 | 64 |
| Rockey et al869 | USA (2000–04) | Comparison of imaging tests | 614 Patients | Comparison of colon imaging tests | 2+ | 82 |
| Halligan and Atkin870 | UK (2005) | Commentary | NA | Virtual colonoscopy | 4 | 55 |
| Section 4.7.1 Guidelines for care | ||||||
| Chiang et al797 | Australia (2001) | Analysis of patient data | 167 Patients | Acute pancreatitis management | 2+ | 68 |
| BSG574 | UK (2002) | Guidelines | NA | Guidelines for dyspepsia management | Guideline | 45 |
| Hamilton and Sharp556 | UK (evidence between 1966 and 2002 taken) | Assessment of clinical guidelines | Around 100 articles | Guidelines for the diagnosis of colorectal cancer | 2+ | 66 |
| Association of Coloproctology of GB and Ireland505 | UK (2001) | Specification of guidelines | NA | Guidelines for the management of colorectal cancer | Guideline | 61 |
| Flashman et al507 | UK (2000–01) | Audit | 249 Patients | Two week standard for bowel cancer | 2− | 61 |
| Kubba and Whyman606 | UK (1994) | Survey of clinicians | 81 Responses | Survey of practice amongst Scottish gastroenterologists | 2− | 59 |
| DoH605 | UK (2004) | Guidelines | NA | Renal services implementation strategy | Guideline | 45 |
| Rockall et al398 | UK (1993) | Analysis of patient data | 2531 Patients | Management of upper GI haemorrhage | 2− | 57 |
| Limburg and Ahlquist569 | International; emphasis on USA (2002) | Commentary | NA | Management of colorectal cancer | 4 | 45 |
| Ahmed et al571 | International (studies from 1996 onwards) | Systematic review | Four RCTs | Supportive care for patients with GI cancer | 1 | 75 |
| McNamara et al581 | International; emphasis on USA (2000) | Review of evidence; expert commentary | Around 70 articles | Non‐ulcer dyspepsia (NUD) | 2− | 64 |
| Meineche‐Schmidt871 | Denmark (1999–2000) | RCT and follow‐up | 829 Patients | Healthcare consumption | 2+ | 57 |
| Wexner et al600 | USA (2001) | Commentary; consensus | NA | Principles for privileging and credentialing for endoscopy and colonoscopy | 4 | 52 |
| Bardou et al572 | USA (1999–2002) | Case‐control study | Over 80 000 patients | Treatment of oesophageal cancer | 2+ | 70 |
| Bodger et al596 | UK (not specified—circa 1996) | Analysis of physicians' practice data | 9 GPs | Prescriptions in primary care | 2− | 66 |
| Delaney et al579 | International (2003) | Systematic review | 20 RCTs | Management strategies for dyspepsia | 1 | 82 |
| Fisher et al599 | USA (1995–1996) | Analysis of patient data | 3546 Patients | Mortality and follow‐up colonoscopy after colorectal cancer | 2+ | 61 |
| Moum585 | International (2000) | Review of evidence | Around 80 articles | Medical treatment for IBD | 3 | 64 |
| Feuer595 | International (1999) | Commentary; review of evidence | Around 20 articles | Management of intestinal obstruction | 3 | 50 |
| Conio et al591 | International (2001) | Commentary; review of evidence | Around 80 articles | Endoscopic treatment of pancreatico‐biliary malignancies | 3 | 57 |
| Talley et al546 | International (1991) | Review of evidence | Around 180 articles | Classification and guidelines for the diagnosis and management of functional dyspepsia | 3 | 80 |
| Drossman et al551 | International (not specified—circa 1994) | Survey of patients | 270 Patients | Measuring health status and severity of illness for functional bowel disorders | 2− | 77 |
| Jeffery et al568 | International (studies from 1966 onwards) | Systematic review | 5 RCTs | Follow‐up strategies for colorectal cancer | 1 | 75 |
| Axon et al602 | UK (not specified—circa 1995) | Audit; survey of clinicians | 350 General physicians, 400 surgeons, 477 gastroenterologists, 70 GPs | Guidelines on appropriate indications for upper GI endoscopy | 2+ | 77 |
| Allum et al570 | UK (2002) | Review of evidence; recommendations | Around 340 articles | Guidelines for the management of oesophageal and gastric cancer | Guideline | 75 |
| Raine et al593 | UK (2002) | Qualitative research into GPs' beliefs | 46 GPs | GPs' perceptions of chronic disease syndrome and IBS | 2− | 73 |
| Carter et al584 | UK (2004) | Review of evidence; recommendations | Around 150 articles | Guidelines for the management of IBD | Guideline | 75 |
| Ryder594 | UK (2003) | Review of evidence; recommendations | Around 150 articles | Guidelines for the diagnosis and treatment of hepatocellular carcinoma | Guideline | 75 |
| Verma and Giaffer588 | UK (not specified—circa 2001) | Survey of patients; analysis of patient data | Around 1000 patients | H pylori eradication | 2− | 77 |
| Milne et al872 | UK (1993) | Survey of gastroenterologists | 670 Survey responses | H pylori and upper GI disease | 2− | 64 |
| Parry et al578 | UK (1995) | Survey of GPs | 140 GPs | GPs' management of dyspepsia in primary care | 2− | 73 |
| Probert et al604 | UK (not specified—circa 1993) | Survey of gastroenterologists | 236 Survey responses | Gastroenterologists' care profile for patients with IBD | 2− | 61 |
| Whitaker et al589 | UK (1997) | Commentary; review of guidelines for care | Around 50 articles | Management of GI disease in primary care | 3 | 59 |
| Paton590 | UK (1992–93) | Economic analysis; RCT | 255 Patients | Comparison of two treatments for gastro‐oesophageal reflux disease | 2+ | 64 |
| Wright et al586 | UK (not specified—circa 2001) | Analysis of patient data | 6037 Patients | Implementation of H pylori eradication therapy | 2− | 61 |
| NICE577 | UK (2003) | Guidelines | NA | Managing adult patients with dyspepsia | Guideline | 57 |
| Spiegel et al575 | International; emphasis on USA (2002) | Review of guidelines; economic modelling | Around 120 articles | Competing strategies for dyspepsia management | 2− | 75 |
| Bodger et al576 | UK (1995–1996) | Observational study | 340 Patients | Implications of BSG dyspepsia guidelines | 2− | 68 |
| Association of coloproctology of GB and Ireland505 | UK (1999) | Guidelines for coloproctology | NA | Guidelines for coloproctology | Guideline | 45 |
| Manes et al601 | Italy (1998) | Analysis of patient data | 706 Patients | Appropriateness and diagnostic yield of upper GI endoscopy in an open access system | 2− | 64 |
| Tremaine et al583 | USA (1997) | Survey and analysis of patient data | Not clear—around 100 patients | Practice guidelines in IBD | 2+ | 61 |
| Section 4.7.2 Incidence of cancer | ||||||
| Forman et al873 | UK (data up to 1992; published 2003) | Analysis of data | Over 1.5 million cases | Cancer prevalence in the UK | 2− | 66 |
| Munkholm814 | International (2003) | Review of evidence; commentary | Around 25 articles | Incidence and prevalence of colorectal cancer | 3 | 57 |
| AGA788 | USA (2001) | Review of evidence; expert commentary | Over 125 articles | Prevalence and costs of GI diseases | 2+ | 68 |
| Sant et al874 | Europe (2001) | Analysis of routine data | 1 836 287 Patient records | Cancer survival rates | 2− | 57 |
| Stanley et al816 | International (1988) | Review of trends | Around 20 articles | Mortality trends | 2− | 59 |
| Askling et al875 | Sweden (data between 1952 and 1995 taken) | Analysis of patient data | 114 102 Records | Colorectal cancer rates | 2+ | 52 |
| Lynch and de la Chapelle876 | International; emphasis on USA (2003) | Commentary; review of evidence | Around 100 articles | Hereditary colorectal cancer | 3 | 52 |
| ONS441 | UK (data between 1991 and 2001) | Update on cancer survival rates | NA | Cancer survival rates | NA | NA |
| Sharp et al824 | UK (data between 1968 and 1992) | Analysis of cancer data | Not specified, but a large amount | Cancer incidence and mortality | 2− | 50 |
| McKinney et al835 | UK (data between 1960 and 1990) | Analysis of cancer data | Not specified, but a large amount | Oesophageal and gastric cancer incidence | 2− | 52 |
| Pye et al825 | UK (1995–96) | Analysis of patient data | 910 Patients | Carcinoma of the oesophagus and stomach | 2− | 57 |
| Bray et al328 | Europe (1995) | Analysis of mortality data | Not specified, but a large amount | Cancer incidence and mortality | 2+ | 66 |
| Bardou et al572 | USA (1999–2002) | Case‐control study | Over 80 000 patients | Treatment of oesophageal cancer | 2+ | 70 |
| Rhodes and Campbell877 | International (2002) | Review of evidence | Around 70 articles | Inflammation and colorectal cancer | 3 | 61 |
| AUGIS826 | UK (1999) | Commentary and recommendations | NA | Service provision | 4 | 35 |
| Section 4.7.4 Screening (includes surveillance) | ||||||
| Mpofu et al611 | International; emphasis on UK (2004) | Systematic review | 9 Key reports | Strategies for detecting colon cancer | 1 | 84 |
| Rozen et al608 | International (2002) | Expert commentary; recommendations | NA | Worldwide cancer screening | 3 | 73 |
| Mandel et al609 | USA (longitudinal study—results from 70s, 80s, and 90s) | Analysis of patient data | 46 551 Participants | Screening for colorectal cancer | 2+ | 64 |
| Garvican607 | UK (1998) | Commentary; cost analysis | NA | Colorectal cancer screening | 3 | 70 |
| Doria‐Rose et al633 | USA (data between 1994 and 2000 taken) | Analysis of patient data | Over 70 000 participants | Screening intervals | 2− | 70 |
| Lin et al613 | USA (1999) | Survey of clinical practice | 103 Responses | Current practices in Barrett's oesophagus | 2− | 73 |
| UK Colorectal Cancer Screening Pilot Group610 | UK (2000–03) | Pilot/audit | 271 646 Participants | Screening for colorectal cancer | 2− | 64 |
| Hill et al628 | UK (not specified—circa 2000) | Assessment of practice | 109 Families | Screening for colorectal cancer | 2− | 57 |
| Kronborg627 | Europe (1992) | Assessment of guidelines | Around 50 articles | Screening guidelines for colorectal cancer | 2− | 68 |
| Dubinsky et al623 | USA (2000) | Economic and statistical analyses | Not specified | Economic issues for competing diagnostic strategies | 2− | 75 |
| Gerson et al615 | USA (2003) | Economic modelling | Not specified, but a large amount | Cost effectiveness of endoscopic screening and surveillance for GERD | 2− | 73 |
| Lewis878 | USA (2000) | Commentary | NA | Screening of colorectal cancer | 4 | 43 |
| Macafee and Scholefield632 | UK (2002) | Commentary; review of evidence | Around 40 articles | Screening of colorectal cancer | 3 | 57 |
| Ganz et al626 | USA (1999) | Survey; RCT | 36 Provider organisations | Screening of colorectal cancer | 2+ | 68 |
| Gross et al612 | USA (1998) | Survey of gastroenterologists | 279 Survey responses | Management of Barrett's oesophagus | 2− | 80 |
| Loeve et al621 | USA (circa 2000—projected data between 1993 and 2023) | Data simulation | Baseline data from routine sources and expert opinion | Colorectal cancer screening | 2− | 77 |
| Rae630 | Australia (data between 1987 and 1996 taken) | Analysis of patient data | 3845 Patients | Community screening for colorectal cancer | 2− | 61 |
| Nietert et al620 | USA (circa 2002) | Economic modelling | NA | Cost effectiveness of screening for chronic gastroesophageal refulx disease | 2− | 75 |
| Ramsey et al617 | USA (data between 1993 and 1999 taken) | Analysis of patient data | 206 Patients | Costs of screening for colorectal cancer | 2− | 70 |
| Sonnenberg et al879 | USA (1998) | Economic modelling | NA | Costs of screening for colorectal cancer | 2− | 70 |
| Renehan et al618 | UK (2002) | Review of evidence | 5 RCTs | Cost effectiveness of surveillance for colorectal cancer | 2+ | 80 |
| Helm et al625 | International; emphasis on USA (not specified—circa 2003) | Review of evidence; commentary | Around 60 articles | Strategies for colorectal cancer screening | 3 | 80 |
| Taylor et al629 | UK (not specified—circa 2000) | Survey of patients | 4153 Survey responses | Acceptability of flexible sigmoidoscopy screening | 2− | 64 |
| Bejes and Marvel624 | USA (not specified—circa 1992) | Case‐control study | 546 Patients | Offering colorectal cancer screening to patients | 2− | 61 |
| Cotton et al635 | USA (2000–01) | Non‐randomised trial | 615 Patients | Virtual colonoscopy | 2− | 64 |
| Hur et al634 | USA (2004) | Mathematical modelling | Data from 1998 to 2002 | Computed tomographic colonography | 2− | 70 |
| Section 4.7.5 Genetics | ||||||
| Lynch and de la Chapelle876 | International; emphasis on USA (2003) | Commentary; review of evidence | Around 100 articles | Hereditary colorectal cancer | 3 | 52 |
| Polito II et al880 | USA (data between 1985 and 1991) | Analysis of patient data | 552 Patients | Genetic anticipation in Crohn's disease | 2− | 55 |
| Yasui et al565 | International (cancer cases between 1993 and 1998) | Analysis of cancer cases | 9241 Cases | Molecular‐pathological diagnosis of GI tissues for cancer histopathology | 2− | 68 |
| Morris‐Yates et al881 | Australia (participants between 1984 and 1986) | Structured interviews of patients; genetic modelling | 686 Individual twins | Genetic contribution to functional bowel disorders | 2− | 70 |
| Faybush et al882 | Canada (patient data between 1984 and 1995) | Analysis of patient data | 315 Patients | Generational differences in IBD: genetic, bias, and temporal effects issues | 2− | 59 |
| Section 4.7.6 Prevention | ||||||
| Chan and Graham512 | International (2004) | Review of evidence | Around 60 articles | Prevention of NSAID GI complications | 3 | 73 |
| Muller and Sonnenberg652 | USA (1988–93) | Analysis of patient data | Over 16 000 patients | Prevention of colorectal cancer | 2+ | 55 |
| Hulscher et al651 | International (review of studies from 1966 onwards) | Systematic review | 55 Studies | Prevention in primary care | 1 | 77 |
| O'Connor and Sebastian654 | Europe (2003) | Review of evidence; commentary | Around 60 articles | Burden of H pylori in Europe | 3 | 50 |
| Section 4.7.6 Devolution | ||||||
| Greer883 | UK (2004) | Commentary; review of evidence | Around 30 articles | Devolution and the NHS | 3 | 52 |
| Section 4.7.7 Managed cancer networks | ||||||
| CSCG808 | UK (2003) | Commentary | NA | Response to NICE service guidance on upper GI cancers | Guideline | 41 |
| South Wales Cancer Network754 | UK (2003) | Service guidelines; recommendations | NA | Configuration of services | 3 | 45 |
| Section 4.7.8 Quality assessment | ||||||
| Garvican607 | UK (1998) | Commentary; cost analysis | NA | Colorectal cancer screening | 3 | 70 |
| Association of Coloproctology of GB and Ireland505 | UK (2001) | Specification of guidelines | NA | Guidelines for the management of colorectal cancer | Guideline | 61 |
| Lilford et al884 | UK (not specified—circa 2004) | Review of evidence | Around 90 articles | Managing performance in acute medical care | 3 | 73 |
| Rockall et al885 | UK (1993–94) | Collection and analysis of patient data | Over 5000 cases | Outcomes after acute upper GI haemorrhage | 2− | 57 |
| Johansen et al886 | USA (applicable internationally) (2000) | Commentary | NA | Quality and outcomes assessment in GI endoscopy | 3 | 45 |
| Dougall et al887 | UK (1997) | Survey of patients | 84 Patients | Patient experiences of an open access flexible sigmoidoscopy service | 2− | 77 |
| Feuer and Broadley888 | International (studies from 1966 onwards) | Systematic review | 25 Articles | Surgery for malignant bowel obstruction in GI cancers | 2+ | 73 |
| Fletcher889 | Australia (1997) | Review of evidence | Around 40 articles | Management of peptic disease | 3 | 64 |
| Bodenheimer et al890 | USA (2002) | Case study | Four healthcare organisations | Improving primary care for chronic illness | 3 | 61 |
| van der Eijk et al891 | International (2000) | Literature review | Around 80 articles | Quality of care in IBD | 3 | 68 |
| Gilmore et al892 | UK (1991) | Audit | 1500 Completed audit forms | Audit of liver biopsy | 2− | 61 |
| Jones809 | International (1999) | Review of evidence | Around 20 articles | Methodological considerations | 3 | 68 |
| McCulloch et al829 | UK (1999–2002) | Cohort study; analysis of patient data | 955 Patients | Mortality and morbidity in gastro‐oesophageal cancer surgery | 2− | 64 |
| Kurlberg et al893 | Sweden (2004) | Commentary | NA | Access to patient information | 3 | 43 |
| Bass et al695 | UK (1994) | Analysis of patient data | 762 Patients | Frequent attendants at a gastroenterology clinic | 2− | 61 |
| Palmer and Morris894 | UK (2004) | Commentary | NA | Colonoscopy practice in England | 4 | 41 |
| Bowles et al752 | UK (not specified—circa 2003) | Survey of clinicians and patients | 9223 Cases | Colonoscopy practice in the UK | 2− | 66 |
| Macarthur et al895 | UK (1993) | Audit of deaths from large bowel surgery | 187 Cases | Deaths from large bowel surgery | 2− | 61 |
| van der Eijk et al896 | International (1998) | Observational study/survey | Physicians and health workers from 8 countries | Best practice in IBD | 2− | 73 |
| Eaden et al897 | UK (not specified—circa 1998) | Survey of clinicians | 341 Responses | Screening for colonic cancer by gastroenterologists | 2− | 64 |
| Section 5.1 Where should services be provided (primary, secondary, tertiary—includes patient roles)? | ||||||
| Scott et al898 | Canada (not specified—circa 2003) | Survey of patients | 14 Patients | Use of complementary therapies for IBD | 2− | 55 |
| Hinton899 | UK (not specified—circa 1994) | Survey | 415 Patients; focus on 77 of these | Transfer of care from home to hospital | 2− | 45 |
| Shepperd and Iliffe689 | International (1996–2001) | Systematic review | 16 Trials | Hospital versus home care | 1 | 66 |
| Barrett et al900 | UK (1998–99) | Retrospective study; analysis of routine data | 903 Patients | Description of intermediate care service | 2− | 57 |
| Gill and Martin901 | New Zealand (1995–97) | Analysis of patient data | 3351 Patients | Distance from hospital | 2− | 59 |
| South Wales Cancer Network754 | UK (2003) | Service guidelines; recommendations | NA | Configuration of services | 3 | 45 |
| Whynes and Thornton902 | UK (not specified—circa 1998) | Economic/statistical analysis | Not clear—data for around 18 wards | Concentration in primary care | 2− | 61 |
| Section 5.2 What types of services should be provided (includes current provision)? | ||||||
| Gonsalkorale et al903 | UK (not specified—circa 2002) | Survey of patients | 232 Patients | Hypnotherapy in IBS | 2− | 75 |
| van Dam et al904 | Not specified—probably international (not specified—circa 2004) | Review of clinical trials | Review of around 10 papers | Review of computerised tomographic colonography (CTC) | 2+ | 70 |
| Podolsky905 | Not specified—probably international (2004) | Expert commentary | NA | CTC | 4 | 41 |
| Silcock and Bramble502 | UK (1994) | Survey | 333 Responses | Survey of current practice in open access gastroscopy (OAG) | 2− | 68 |
| Baron et al906 | USA (2002–03) | Cohort study | 498 Patients | Demand for colonoscopy | 2+ | 66 |
| Cheung et al907 | UK (1999) | Survey and structured interviews | 160 GPs | Shared care in gastroenterology | 2− | 61 |
| Moody et al772 | UK (not specified—circa 1993) | Survey of GPs | 259 Responses | GPs views on requirements for gastroenterology services | 2− | 70 |
| Smith et al908 | UK (not specified—circa 2000) | Survey of patients | 100 Responses | Impact of a nurse‐led counselling service | 2− | 68 |
| Ilnyckyj et al909 | Canada (1996–1997) | Observational study | 70 Patients | Gastroenterology consultation | 2− | 64 |
| Pasricha910 | Not specified—probably international (2004) | Expert commentary | NA | Future of therapeutic endoscopy | 3 | 52 |
| Axon911 | UK (1998) | Expert commentary | NA | Open access endoscopy in Britain | 4 | 50 |
| DoH912 | UK (2001) | National commentary; recommendations | NA | Upper GI cancers | Guideline | 48 |
| Richards et al760 | International (1997) | Systematic review | 256 Studies | Home parenteral nutrition (HPN) | 1 | 77 |
| Talley and Spiller913 | International (not specified—circa 2002) | Review of evidence | Around 140 studies | IBS | 3 | 70 |
| Nord914 | USA (1999) | Expert commentary | NA | Developments in Endoscopy | 4 | 57 |
| Jones770 | UK (1996) | Expert commentary | NA | GI disease in primary care | 3 | 59 |
| van der Eijk et al896 | International (1998) | Observational study/survey | Physicians and health workers from 8 countries | Best practice in IBD | 2− | 73 |
| Abuksis et al915 | Israel (1998) | RCT | 142 Patients | A patient education programme | 2+ | 68 |
| Lewin van den Broek et al916 | Netherlands (1995–97) | RCT | 349 Patients | Management strategies for dyspepsia | 2+ | 66 |
| Delaney and Moayyedi917 | Predominantly UK (not specified—circa 2003) | Evidence‐based assessment | Around 160 articles | Dyspepsia management | 2− | 57 |
| Heaney et al918 | UK (1993–96) | Analysis of patient and GP data | 1872 Cases | Open access gastroscopy in Royal Victoria Hospital, Belfast | 2− | 55 |
| Pye et al825 | UK (1995–96) | Analysis of patient data | 910 Patients | Carcinoma of the oesophagus and stomach | 2− | 57 |
| Association of Coloproctology of GB and Ireland505 | UK (2001) | Specification of guidelines | NA | Guidelines for the management of colorectal cancer | Guideline | 61 |
| Hansen et al919 | Denmark (1987–88) | Survey of patients and GPs | 436 Patients | Efficacy of open access endoscopy | 2− | 68 |
| Section 5.3 Who should deliver/perform services and/or procedures (for example, endoscopies)? | ||||||
| Eaden et al897 | UK (not specified—circa 1998) | Survey of clinicians | 341 Responses | Screening for colonic cancer by gastroenterologists | 2− | 64 |
| Paisley et al920 | UK (1994–96) | Analysis of patient data | 222 Patients | Role of the surgical trainee | 2− | 59 |
| Chin and Newton921 | UK (not specified—circa 1996) | Survey | 167 Surgical trainees | Training in minimal access surgery | 2− | 66 |
| Pardo et al922 | Spain (1998–99) | Retrospective study; analysis of patient data | 620 Patients | Impact of physician speciality | 2− | 61 |
| Waye et al923 | International (2001) | Expert commentary | NA | Who is permitted to do endoscopy? | 4 | 36 |
| Bini et al924 | USA (1998–99) | Analysis of routine data | 197 Patients | Impact of specialists | 2− | 68 |
| Eaden et al925 | UK (data between 1985 and 1999 taken) | Survey of clinicians | 13 Clinicians | Variation between generals and specialists | 2− | 68 |
| Barrison et al926 | UK (2001) | Expert commentary and recommendations | NA | Provision of endoscopy services in general hospitals | 4 | 45 |
| Quine et al521 | UK (1991) | Survey of clinicians | 39 Hospitals; 383 clinicians | Audit of upper GI endoscopy | 2− | 64 |
| NHS CRD560 | UK (2004) | National commentary; guidelines; recommendations | NA | Improving the outcomes in colorectal cancer | Guideline | 70 |
| Lim et al927 | UK (1995) | Survey of clinicians | 453 Responses | H pylori serology and management | 2− | 68 |
| Cockel et al928 | UK (1976–79) | Survey | 173 Responses | GI endoscopy services | 2− | 66 |
| Meyer et al710 | USA (1993) | Analysis of patient data | Over 1.3 million cases | Differences between generalists and specialists | 2− | 68 |
| Knight‐Davis et al929 | UK (1996) | Survey of clinicians | 350 Responses | Cross‐cover for physicians | 2− | 59 |
| Association of Coloproctology of GB and Ireland753 | UK (2001) | Analysis of audit data; expert commentary; recommendations | 8 Main sources of audit data | Resources for coloproctology | 3 | 52 |
| Chen and Rex717 | International (2004) | Commentary; recommendations | NA | Nurse administered sedation for endoscopy | 3 | 59 |
| Nightingale and Hogg930 | UK (2003) | Review of evidence; commentary | Around 40 articles | GI advanced practitioners | 3 | 64 |
| Section 5.4 What are the key issues concerning changing roles and general practitioners with a special interest (GPwSI)? | ||||||
| Farthing et al931 | UK (1993) | Peer reviewed expert commentary and recommendations | NA | Service provision | 3 | 59 |
| Colin‐Thome932 | UK, NHS (2002) | Commentary | NA | GPwSI | 4 | 43 |
| Birch933 | UK, NHS (2004) | Commentary; guidelines | NA | GPwSI | 3 | 52 |
| AUGIS826 | UK (1999) | Commentary; recommendations | NA | Service provision | 4 | 35 |
| Pearson et al934 | Boston, USA (1996) | Survey of general internists and gastroenterologists | 91 Survey responses | Study of consultations provided to general internists by gastroenterologists. | 2− | 75 |
| Williams et al935 | UK (2002) | Commentary; recommendations | NA | The role of GPwSI | 3 | 66 |
| DoH and RCGP691 | UK (2003) | Commentary; recommendations | NA | Implementing GPwSI | Guideline | 45 |
| DoH and RCGP936 | UK (2002) | National guidelines | NA | GPwSI roles | Guideline | 25 |
| Ryan937 | UK (2002) | Commentary | NA | Role of GPwSI in respiratory disease | 4 | 30 |
| Gruffydd‐Jones938 | UK (2003) | Brief commentary | NA | Framework for GPwSI | 3 | 55 |
| Kernick939 | UK (2003) | Commentary | NA | Economic perspectives on GPwSI | 4 | 52 |
| Richardson940 | UK (2002) | Commentary; interviews | NA | Rise of GPwSI | 4 | 50 |
Table A.13 Summary of articles examined after consultation feedback.
| ID and authors | Research setting and year of study | Study design | Sample size | Topic of document | Key results and conclusions | Level of evidence | Quality score (AGREE) |
|---|---|---|---|---|---|---|---|
| Barry et al941 | UK (data between 1995 and 2000) | Comparative study | 110 Patients | Cancer staging | Special interest radiology improves the perceived preoperative stage of gastric cancer | 2+ | 57% |
| Bassi et al708 | UK (2000) | Single centre retrospective study | 479 Patients | Cost of IBD treatment | The study represents the first detailed characterisation of the scale and determinants of costs of illness for IBD. Hospitalisation affected a minority of patients but accounted for half the total direct costs | 2+ | 66% |
| Carter et al584 | International (2004) | Guidelines | NA | Management of IBD | Guidelines commissioned by BSG for the management of IBD in adults | Guidelines | 55% |
| Rubin et al*136 | UK (NA) | Retrospective case reviews | 568 Patients | Epidemiology and management of IBD | Prevalence rates, but not incidence rates, for IBD are substantially higher than described in UK populations. GPs make a significant contribution to meeting the healthcare needs of these patients | 3 | 66% |
| Axon*719 | International (NA) | Review of evidence | NA | Cancer surveillance in ulcerative colitis | Regular clinical follow‐up is important. At 8–10 years after their first attack, total colonoscopy should be performed with multiple biopsy specimens to check for colitis | 4 | 41% |
| Lim et al*720 | UK (data between 1978–1990) | Retrospective cohort study | 128 Patients | Follow up of patients with ulcerative colitis | Low grade dysplasia diagnosis is not sufficiently reliable to justify prophylactic colectomy. Conservative management of established low grade dysplasia cases should not be rules out | 2+ | 61% |
| Fullerton942 | International (projections for 2000) | Economic evaluation | NA | Economic impact of functional digestive disorders | The economic impact of functional GI disease is large. Economic estimates are useful in policy decision making for the allocation of healthcare resources | 3 | 50% |
| Robinson et al943 | UK (NA) | RCT | 458 Patients | Self help interventions for IBS | Introducing a self help guidebook results in a reduction in primary care consultations, a perceived reduction in symptoms, and significant health service savings | 2+ | 68% |
| Provenzale et al704 | International (1980–1998) | Literature review | 2157 Articles; 10 included | Specialised and general GI care | Gastroenterologists may provide better care than other provider types for certain disorders. | 1 | 80% |
| Norton and Kamm944 | UK 2002 | Discussion | N/A | Specialist nurses in gastroenterology | Specialist nurses can take on some tasks traditionally carried out by doctors, although evidence concerning safety and effectiveness is lacking. It is not necessarily cheaper to substitute nurses for doctors. A multidisciplinary approach is advocated, in which the skills of one professional group are complemented by the skills of the other | 5 | N/A |
| Robinson et al*681 | UK (NA) | RCT | 203 Patients | Ulcerative colitis care | Self management of ulcerative colitis accelerates treatment provision and reduces doctor visits, and does not increase morbidity. This approach could be used in long term management of many other chronic diseases to improve health service provision and use, and to reduce costs | 2+ | 68% |
| Wade945 | UK 1983 | Observational comparative interview follow‐up study | 215 Patients, 142 in district health authorities with stoma care nurses, 73 in districts without stoma care nurses | Psychological symptoms in colostomy patients after surgery and the benefits of stoma care nurses | Short term outcomes were improved in the stoma care district patients, although there were no differences at one year. 10% of patients who reported that they were well were anxious or depressed. Physical symptoms were associated with psychiatric morbidity. Psychiatric referral was suggested to be inappropriate, as medical referral may be more helpful in resolving problems | 2− | 45% |
| Erwin‐Toth and Spencer946 | USA, not given, published 1991 | Questionnaire follow‐up of patients after ostomy surgery, convenience sample | 52 Volunteers were recruited, 39 completed forms were received | Patient assessed quality of care | High satisfaction but results limited by methodological weaknesses, acknowledged by authors | 2− | 29% |
| Maule758 | USA 1994 published | Prospective non‐randomised controlled study | 1881 Intervention patients; 730 control patients | Effectiveness of screening for colorectal cancer by nurses compared with doctors | Depth of insertion of sigmoidoscope was greater in those examined by doctors. There was no difference in the proportion of examinations that were positive for adenomas or cancer. A higher proportion of patients whose examination was normal and were examined by nurses returned for follow‐up | 2+ | 57% |
| Moshakis et al*714 | UK Published 1996 | Comparative study | 50 Trainer and 50 pupil cases | Competence of nurses with training to undertake endoscopies | Quality and accuracy were assessed as equal between groups, with 60 cm insertion achieved in a similar number of cases. Nurses can be taught to practise flexible sigmoidoscopy efficiently and safely. | 2− | 23% |
| Schoenfeld et al*715 | USA Published 1999 | Randomised controlled trial | 162 Patients intervention group; 166 patients control group | Accuracy of polyp detection, depth of insertion and complication rate for flexible sigmoidoscopy: comparison of nurses and doctors | No differences in detection of polyps or frequency of complications were found, suggesting nurse endoscopists may perform screening flexible sigmoidoscopy as safely and as effectively as gastroenterologists | 1 | 59% |
*These articles were cited in the text.
9. Appendices
Appendix 1: Charities with an interest in the care of patients with gastroenterological and liver disorders (through patient support or research or both)
| 1 | Bardhan Research and Education Trust of Rotherham (BRET) |
| 2 | Barrett's Oesophagus Foundation |
| 3 | British Association for the Study of the Liver (BASL) |
| 4 | British Liver Trust (BLT) |
| 5 | Children's Liver Disease Foundation |
| 6 | Coeliac UK |
| 7 | Colon and Rectal Disease Research Foundation of GB and Ireland |
| 8 | Colon Cancer Concern |
| 9 | CORE (new name for the Digestive Disorders Foundation) |
| 10 | Crohn's in Childhood Research Association (CICRA) |
| 11 | Foundation for Liver Research |
| 12 | Guildford Undetected Tumour Screening (GUTS) |
| 13 | The Ileostomy and Internal Pouch Support Group (IA) |
| 14 | IBS Network |
| 15 | National Association for Colitis and Crohn's Disease (NACC) |
Appendix 2 AGREE tool for quality assessment
Score each of the following questions using a three‐point Likert scale:
0—Not specified (little or no evidence)
1—Disagree (some evidence)
2—Agree (good or strong evidence)
Scope and purpose:
The overall objective(s) of the research is (are) clearly described.
The research question(s) covered by the methodology is (are) clearly described.
The recipients to whom the research is meant to apply are clearly described.
Stakeholder/participant involvement:
The research is carried out by relevant professional groups.
The participants' views and preferences have been sought.
The target users of the research are clearly defined.
The research has been piloted among participants.
Rigour of development:
Methods to search for evidence have been specified (for example, systematic review, unbiased screening, search strategy).
The techniques for formulating the results have been specified.
The advantages, disadvantages, and risks are considered in the results.
There is an explicit link between the results and the supporting evidence (sufficient relevant references).
The research has been externally reviewed before its publication, or published in peerb reviewed sources.
A procedure for updating the research is provided, or for primary studies, a clear indication of what further research is needed.
Clarity and presentation:
The results are clear and unambiguous.
The different options for conducting the research are clearly presented, or for primary studies, a description of the pros and cons of each method.
Key points in the results are easily identifiable.
The research is supported with tools for application (for example, computer support, documentation, reference guide for reviews/guidelines), or for primary studies, a clear path for dissemination and potential implementation.
Applicability:
The potential barriers in applying the results have been discussed.
The potential cost implications of applying the results have been considered.
The research presents key (review) criteria for monitoring and/or audit purposes (for example, cost should be <£100; time <7 days).
Editorial independence:
The research is editorially independent from the funding body.
Conflicts of interest among research members have been recorded.
Appendix 3: Brief sent to participants for the patient workshop
Specific questions to be answered
(a) Greater self management by patients
There is good evidence to show that if patients with chronic illnesses such as inflammatory bowel disease or irritable bowel syndrome are given enough information and are supported by expert services that are easy to reach, they can manage with fewer hospital and GP appointments. This could be used for a wide variety of chronic illnesses, which would reduce demand on NHS services.
What is your view on such an approach?
(b) Endoscopy outside hospitals
It has been suggested that more endoscopies (internal examination of the gut through a tube) should be carried out in special centres or in local GP surgeries instead of hospital, which may be easier for patients but may mean that these tests would be less available in hospital. Research is needed to find out whether these tests would be safe and effective, if undertaken outside hospitals.
What is your view on such tests being carried out in places other than hospitals?
(c) Nurses or doctors?
There is good evidence that nurses do a good job when undertaking endoscopy to help make a diagnosis. Also, patients prefer nurses doing the test to doctors. This suggests that nurses should take over routine diagnostic endoscopy from doctors. There is some evidence (that is less strong) that nurses should also take over the continuing care of certain patients with chronic gastrointestinal problems.
What would you feel about seeing a nurse rather than a doctor for these tests and appointments?
(d) Where should services be located?
There is some evidence that major operations for gastrointestinal problems should be performed at specialist centres that serve large populations because the results may be better. However, this would take away expertise from local hospitals, where specialist care will still be needed, particularly for emergency problems.
What is more important to you—having services available locally or being treated by specialists, even if it is further away?
Open discussion
What else would be important to you about the way services for gastrointestinal problems are provided?
Appendix 4: Articles not used in this report
Table A.14 lists the articles not used in this report, and table A.15 the articles not used for economic analysis.
Appendix 5: Glossary
Table A.16 Glossary.
| AUGIS | Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland |
| BASL | British Association for the Study of the Liver |
| BSG | British Society of Gastroenterology |
| CLO | Columnar‐lined oesophagus |
| CRD | Centre for Reviews and Dissemination |
| CT | Computed tomography |
| DoH | Department of Health |
| FCE | Finished consultant episode |
| GERD | Gastro‐esophageal reflux disease |
| GI | Gastrointestinal |
| GORD | Gastro‐oesophageal reflux disease |
| HES | Hospital Episode Statistics |
| HPN | Home parenteral nutrition |
| HRQoL | Health related quality of life |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| ICD‐10 | International Classification of Diseases‐10th revision |
| ICD‐9 | International Classification of Diseases‐9th revision |
| JAG | Joint Advisory Group |
| NACC | National Association for Colitis and Crohns |
| NAFLD | Non‐alcoholic fatty liver disease |
| NASH | Non‐alcoholic Steatohepatitis |
| NCCGs | Non‐Consultant Career Grades |
| NHS | National Health Service |
| NICE | National Institute for Clincal Excellence |
| NSF | National Service Framework |
| OHE | Office of Health Economics |
| ONS | Office for National Statistics |
| OPCS | Office of Population Censuses and Surveys |
| PEDW | Patient Episode Database Wales |
| RCP | Royal College of Physicians |
| RCT | Radomised Controlled Trial |
| SF12 | Short Form‐12 |
| SF36 | Short Form‐36 |
| SIGN | Scottish Intercollegiate Guidelines Network |
| SMR | Standardised mortality ratio |
| SPSS | Statistical Package for the Social Sciences |
| UGIH | Upper gastrointestinal haemorrhage |
Appendix 6: Specialists who provided comments and feedback
Table A.17 Specialist who provided comments and feedback.
| Professor Qasim Aziz | Professor of gastroenterology |
| Professor Andrew Burroughs | Consultant physician and hepatologist |
| Shona Campbell | Consultant GI radiologist |
| Dr John de Caestecker | Consultant gastroenterologist |
| Professor RM Charnely | Professor of surgery |
| Dr Ian Forgacs | Consultant gastroenterologist |
| Professor Ian Gilmore | Consultant gastroenterologist |
| Dr Barry Jones | Consultant gastroenterologist |
| Norma McGough | Dietetic services manager |
| Professor Paul Moayyedi | Professor of gastroenterology |
| Professor Christine Norton | Professor of gastrointestinal nursing |
| Dr KR Palmer | Consultant gastroenterologist |
| Lynne Smith | Chair of the Association of GI Physiologists |
| Dr Richard Stephens | General practitioner |
| Professor Robert Sutton | Professor of surgery |
| Dr Simon Travis | Consultant gastroenterologist |
| Dr Kevin Wedgwood | Consultant surgeon |
10. Acknowledgements
We are grateful to the British Society of Gastroenterology (BSG) for funding this project and to the BSG Steering Group (Dr Mike Hellier, Professor Elwyn Elias, Dr Tony Morris, and Dr Jeremy Sanderson) for providing additional commentary and feedback throughout its development. We are also grateful to the many other colleagues who have given constructive feedback on the draft report. We thank Professor Michael Goldacre and the Unit of Health‐Care Epidemiology, University of Oxford, for the provision of linked hospital admission data, and Lori Havard and other members of the UWS Library and Information Services who obtained references for us. We would like to acknowledge the contribution of Professor Robert Allan, who was the inspiration for the development of a strategic view of services in gastroenterology during his time as president of the British Society of Gastroenterology. Dr Michael Hellier took up this challenge when he was elected president, and we would also like to acknowledge the encouragement and support he has given us.
11. Principal team members
This study was conducted during 2004–05. Principal team members were:
Dr Faiz Ali, specialist registrar in gastroenterology/research fellow, Centre for Health Information, Research and EvaLuation (CHIRAL), School of Medicine, University of Wales Swansea.
Dr Wai‐Yee Cheung, senior lecturer in health service research, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Professor David Cohen, professor of health economics, School of Care Sciences, University of Glamorgan.
Mrs Gaynor Demery, personal assistant to Professor John Williams, School of Medicine, University of Wales Swansea.
Dr Adrian Edwards, reader in primary care, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Mrs Margot Greer, library and information services manager, National Public Health Service.
Dr Mike Hellier, president, British Society of Gastroenterology.
Dr Hayley Hutchings, statistician, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Dr Barry Ip, research officer, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Mrs Mirella Longo, research fellow, School of Care Sciences, University of Glamorgan.
Dr Stephen Roberts, lecturer of epidemiology, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Professor Ian Russell, professor of public health and director of Institute of Medical and Social Care Research, University of Wales Bangor.
Dr Helen Snooks, senior lecturer in health and social care research, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Professor John Williams, Project leader, consultant gastroenterologist/professor of health services research, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
Mrs Judy Williams, clerical officer, Centre for Health Information, Research and Evaluation (CHIRAL), School of Medicine, University of Wales Swansea.
12. Contributors
Dr Giles Croft, research fellow, Royal College of Physicians iLab, School of Medicine, University of Wales Swansea.
Dr Ian Frayling, director, all Wales Genetics Laboratory Service and consultant in genetic pathology, Institute of Medical Genetics, University of Wales Cardiff.
Ms Norma McGough, Coeliac UK.
Dr Alistair McIntyre, consultant gastroenterologist, Wycombe General Hospital, High Wycombe.
Dr Roland Valori, consultant gastroenterologist, Gloucester.
Professor Anne Williams, professor of nursing, School of Health Science, University of Wales Swansea.
Members of the Patient and Carer Network, Royal College of Physicians, London.
Mr Richard Driscoll, National Association for Crohn's and Colitis.
Footnotes
Competing interests: This was an independent study undertaken by the School of Medicine of the University of Wales Swansea, in collaboration with colleagues at the University of Glamorgan and University of Wales Bangor. Professor John Williams, Dr M D Hellier, Dr Alistair McIntyre, and Dr Roland Valori are practising gastroenterologists and members of the British Society of Gastroenterology. Dr Hellier is currently president of the Society (2004–05). Dr M F Ali is a trainee in gastroenterology.
All rights reserved. No part of the publication may be reproduced, stored on retrieval system or transmitted in any form or by any means, electronic, mechanical photocopying, recording or otherwise, without the prior permission of the copyright, except for brief, acknowledged quotations embodied in critical articles or reviews. ©University of Wales Swansea.
Published by the Centre for Health Information, Research and Evaluation, School of Medicine, University of Wales Swansea, SA2 8PP. ISBN – 1‐85987‐017‐1
Team roles
Professor J G Williams led the project throughout, devised the original research proposal, wrote some sections and edited the report. Professor I T Russell and Professor D R Cohen provided invaluable advice and guidance on the development of research methodology, and wrote or edited some sections.
Dr H A Snooks was responsible for the day‐to‐day management of the project, provided methodological input and analyses, and wrote some sections. Dr A Edwards and Mrs M Greer gave advice on the systematic literature search. Dr H A Hutchings and Dr W Y Cheung provided methodological and statistical input. Dr S E Roberts, Dr M F Ali, and Dr B Ip provided methodological input and analyses, reviewed the literature and wrote various sections. Working with Professor D R Cohen, Ms M F Longo carried out economic analyses, reviewed the health economics literature and wrote the health economics sections.
Mrs G Demery and Mrs J G Williams provided clerical support. Mrs Anne Seagrove proof read the report. Mrs Kymberley Thorne assisted with the appraisal of the research literature.
References
- 1.Wanless D.Securing our future health: taking a long‐term view ‐ interim report. London: HM Treasury, 2001
- 2.Pronect Team advised by Wanless D.The review of health and social care in Wales. Welsh Assembly Government 2003
- 3.Wanless D.Securing good health for the whole population ‐ final report. London: HM Treasury, 2004
- 4.NICE Guidlines on nutrition support in adults. London: NICE, 2006
- 5.Royal College of Physicians Nutrition ‐ a doctor's responsibility. London: Royal College of Physicians, 2002
- 6.Royal College of Physicians Storing up problems. The medical case for a slimmer nation. London: Royal College of Physicians, 2005
- 7.Department of Gastroenterology Division of Medicine (http://www.med.miami.edu, accessed 18 January 2007). Miami: University of Miami, 2005
- 8.Körner E.First report to the secretary of state of the steering group on health services information. London: HMSO, 1987
- 9.Cleary R, Beard R, Coles J.et al Comparative hospital databases: value for management and quality. Qual Health Care 199433–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon J, Sanderson C, Elliott P.et al Assessment of the reproducibility of clinical coding in routinely collected hospital activity data: a study in two hospitals. J Public Health Med 19982063–69. [DOI] [PubMed] [Google Scholar]
- 11.Williams J G, Mann R Y. Hospital episode statistics: time for clinicians to get involved? Clin Med 2002234–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen‐Van‐Tam J N, Logan R. Digestive diseases. In: The health of adult Britain, 1841–1994. London: HMSO, 1996
- 13.Goldacre M J. Cause‐specific mortality: understanding uncertain tips of the disease iceberg. J Epidemiol Community Health 199347491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn M, Babb P, Brock A.et alCancer trends in England and Wales, 1950–99. London: The Stationary Office, 2001
- 15.Mohammed I, Cherkas L F, Riley S A.et al Genetic influences in gastro‐oesophageal reflux disease: a twin study. Gut 2003521085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmood Z, McNamara D. Gastro‐oesophageal reflux disease and ulcer disease. Aliment Pharmacol Ther 200318(Suppl 3)31–37. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson M, Lagergren J. The relation between body mass and gastro‐oesophageal reflux. Best Pract Res Clin Gastroenterol 2004181117–1123. [DOI] [PubMed] [Google Scholar]
- 18.Kang J Y. Systematic review: geographical and ethnic differences in gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 200420705–707. [DOI] [PubMed] [Google Scholar]
- 19.Mahadeva S, Raman M C, Ford A C.et al Gastro‐oesophageal reflux is more prevalent in Western dyspeptics: a prospective comparison of British and South‐East Asian patients with dyspepsia. Aliment Pharmacol Ther 2005211483–1490. [DOI] [PubMed] [Google Scholar]
- 20.Spechler S J. Epidemiology and natural history of gastro‐oesophageal reflux disease. Digestion 199251(Suppl 1)24–29. [DOI] [PubMed] [Google Scholar]
- 21.Delaney B C. Review article: prevalence and epidemiology of gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 200420(Suppl 8)2–4. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy T M, Jones R H. The prevalence of gastro‐oesophageal reflux symptoms in a UK population and the consultation behaviour of patients with these symptoms. Aliment Pharmacol Ther 2000141589–1594. [DOI] [PubMed] [Google Scholar]
- 23.Kulig M, Nocon M, Vieth M.et al Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGERD study. J Clin Epidemiol 200457580–589. [DOI] [PubMed] [Google Scholar]
- 24.Pisegna J, Holtmann G, Howden C W.et al Review article: oesophageal complications and consequences of persistent gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 200420(Suppl 9)47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford A C, Forman D, Reynolds P D.et al A retrospective case control study to determine the risk of Barrett's oesophagus according to ethnic origin. Am J Epidemiol 2005162454–460. [DOI] [PubMed] [Google Scholar]
- 26.Caygill C P, Watson A, Lao‐Sirieix P.et al Barrett's oesophagus and adenocarcinoma. World J Surg Oncol 2004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlesman J F, Hameeteman W, Tytgat G N. Barrett's oesophagus. Eur J Cancer Prev 19921992323–325. [DOI] [PubMed] [Google Scholar]
- 28.Solaymani‐Dodaran M, Coupland C, Logan R F. Risk of oesophageal cancer in Barrett's oesophagus and in gastro‐oesophageal reflux. Gut 200352A20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd J A, Johnston D A, Dillon J F. The changing spectrum of gastroesophageal reflux disease. Eur J Cancer Prev 200211215–219. [DOI] [PubMed] [Google Scholar]
- 30.Caygill C P, Reed P I, Johnston B J.et al A single centre's 20 years' experience of columnar‐lined (Barrett's) oesophagus diagnosis. Eur J Gastroenterol Hepatol 1999111355–1358. [DOI] [PubMed] [Google Scholar]
- 31.Delaney B C, Moayyedi P. Dyspepsia. Health care needs assessment. Oxford, 2005 ( http://hcna.radcliffe‐oxford.com/dysframe.htm, accessed 20 December 2006)
- 32.Neumann C S, Cooper B T. Ethnic differences in gastro‐oesophageal reflux disease. Eur J Gastroenterol Hepatol 199911735–739. [DOI] [PubMed] [Google Scholar]
- 33.Kang J Y, Ho K Y. Different prevalences of reflux oesophagitis and hiatus hernia among dyspeptic patients in England and Singapore. Eur J Gastroenterol Hepatol 199911845–850. [DOI] [PubMed] [Google Scholar]
- 34.Delaney B, Moayyedi P. Dyspepsia ‐ HCNA. Health care needs assessment. Oxford, 2005 ( http://hcna.radcliffe‐oxford.com/dysframe.htm, accessed 20 December 2006)
- 35.Corder A P, Jones R H, Sadler G H.et al Heartburn, oesophagitis and Barrett's oesophagus in self‐medicating patients in general practice. Br J Clin Pract 199650245–248. [PubMed] [Google Scholar]
- 36.Penston J G, Pounder R E. A survey of dyspepsia in Great Britain. Aliment Pharmacol Ther 19961083–89. [DOI] [PubMed] [Google Scholar]
- 37.Moayyedi P, Forman D, Braunholtz D.et al The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti‐inflammatory drugs. Leeds HELP Study Group. Am J Gastroenterol 2000951448–1455. [DOI] [PubMed] [Google Scholar]
- 38.Jones R H, Lydeard S, Hobbs F.et al Dyspepsia in England and Scotland. Gut 199031401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones R H, Lydeard S. Prevalence of symptoms of dyspepsia in the community. BMJ 198929830–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy T M, Jones R H, Hungin A P.et al Irritable bowel syndrome, gastro‐oesophageal reflux, and bronchial hyper‐responsiveness in the general population. Gut 199843770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir R D, Backett E M. Studies of the epidemiology of peptic ulcer in a rural community: prevalence and natural history of dyspepsia and peptic ulcer. Gut 1968975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodward M, Morrison C E, McColl K E. The prevalence of dyspepsia and use of antisecretory medication in North Glasgow: role of Helicobacter pylori vs. lifestyle factors. Aliment Pharmacol Ther 1999131505–1509. [DOI] [PubMed] [Google Scholar]
- 43.Jones R H, Lydeard S. Irritable bowel syndrome in the general population. BMJ 199230487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill D, Mayou R, Dawes M.et al Presentation, management and course of angina and suspected angina in primary care. J Psychosom Res 199946349–358. [DOI] [PubMed] [Google Scholar]
- 45.Davie A P, Caesar D, Caruana L.et al Outcome from a rapid‐assessment chest pain clinic. Q J Med 199891339–343. [DOI] [PubMed] [Google Scholar]
- 46.Potts S G, Bass C M. Psychosocial outcome and use of medical resources in patients with chest pain and normal or near‐normal coronary arteries: a long‐term follow‐up study. Q J Med 199386583–593. [PubMed] [Google Scholar]
- 47.Grainger S L, Klass H J, Rake M O.et al Prevalence of dyspepsia: the epidemiology of overlapping symptoms. Postgrad Med J 199470154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doll R, Avery Jones F, Buckatzsch M M. Occupational factors in the aetiology of gastric and dudenal ulcers, with an estimate of their incidence in the general population. London: HMSO, 1951 (MRC special report series, No, 276) [PubMed]
- 49.Ford A, Delaney B, Forman D.et al Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev 2003(4)CD003840. [DOI] [PubMed]
- 50.Harvey R F, Spence R W, Lane J A.et al Relationship between the birth cohort pattern of Helicobacter pylori infection and the epidemiology of duodenal ulcer. Q J Med 200295519–525. [DOI] [PubMed] [Google Scholar]
- 51.Johnsen R, Straume B, Forde O H. Peptic ulcer and non‐ulcer dyspepsia—a disease and a disorder. Scand J Primary Health Care 19886239–243. [DOI] [PubMed] [Google Scholar]
- 52.Bernersen B, Johnsen R, Straume B. Non‐ulcer dyspepsia and peptic ulcer: the distribution in a population and their relation to risk factors. Gut 199638822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talley N J, O'Keefe E, Zinsmeister A.et al Prevalence of gastrointestinal symptoms in the elderly: a population‐based study. Gastroenterology 1992102895–901. [DOI] [PubMed] [Google Scholar]
- 54.Kay L, Jorgensen T. Epidemiology of upper dyspepsia in a random population. Prevalence, incidence, natural history, and risk factors. Scand J Gastroenterol 1994292–6. [PubMed] [Google Scholar]
- 55.Holtmann G, Goebell H, Holtmann M.et al Dyspepsia in healthy blood donors. Pattern of symptoms and association with Helicobacter pylori. Dig Dis Sci 1994391090–1098. [DOI] [PubMed] [Google Scholar]
- 56.Schlemper R J, van der Werf S D, Vandenbroucke J P.et al Nonulcer dyspepsia in a Dutch working population and Helicobacter pylori. Ulcer history as an explanation of an apparent association. Arch Intern Med 199515582–87. [PubMed] [Google Scholar]
- 57.Locke G R, 3rd, Talley N J, Fett S L.et al Prevalence and clinical spectrum of gastroesophageal reflux: a population‐based study in Olmsted County, Minnesota. Gastroenterology 19971121448–1456. [DOI] [PubMed] [Google Scholar]
- 58.Nandurkar S, Talley N J, Xia H.et al Dyspepsia in the community is linked to smoking and aspirin use but not to Helicobacter pylori infection. Arch Intern Med 19981581427–1433. [DOI] [PubMed] [Google Scholar]
- 59.Zober A, Schilling D, Ott M G.et al Helicobacter pylori infection: prevalence and clinical relevance in a large company. J Occup Environ Med 199840586–594. [DOI] [PubMed] [Google Scholar]
- 60.Caballero‐Plasencia A M, Sofos‐Kontoyannis S, Valenzuela‐Barranco M.et al Irritable bowel syndrome in patients with dyspepsia: a community‐based study in southern Europe. Eur J Gastroenterol Hepatol 199911517–522. [DOI] [PubMed] [Google Scholar]
- 61.Haque M, Wyeth J W, Stace N H.et al Prevalence, severity and associated features of gastro‐oesophageal reflux and dyspepsia: a population‐based study. N Z Med J 2000113178–181. [PubMed] [Google Scholar]
- 62.Agreus L, Talley N J, Svardsudd K.et al Identifying dyspepsia and irritable bowel syndrome: the value of pain or discomfort, and bowel habit descriptors. Scand J Gastroenterol 200035142–151. [DOI] [PubMed] [Google Scholar]
- 63.Boekema P J, van Dam van Isselt E F, Bots M L.et al Functional bowel symptoms in a general Dutch population and associations with common stimulants. Neth J Med 20015923–30. [DOI] [PubMed] [Google Scholar]
- 64.Olafsdottir L B, Gudjonsson H, Thjodleifsson B. [Epidemiological study of functional bowel disorders in Iceland]. Laeknabladid 200591329–333. [PubMed] [Google Scholar]
- 65.Westbrook J I, Talley N J. Empiric clustering of dyspepsia into symptom subgroups: a population‐based study. Scand J Gastroenterol 200237917–923. [DOI] [PubMed] [Google Scholar]
- 66.Brown R C, Langman M J, Lambert P M. Hospital admissions for peptic ulcer during 1958–1972. BMJ 1976135–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Primatesta P, Goldacre M J, Seagroatt V. Changing patterns in the epidemiology and hospital care of peptic ulcer. Int J Epidemiol 1994231206–1217. [DOI] [PubMed] [Google Scholar]
- 68.Coggon D, Lambert P, Langman M J. 20 years of hospital admissions for peptic ulcer in England and Wales. Lancet 198111302–1304. [DOI] [PubMed] [Google Scholar]
- 69.Jibril J M, Redpath A, Macintyre I M. Changing pattern of admission and operation for duodenal ulcer in Scotland. Br J Surg 19948187–89. [DOI] [PubMed] [Google Scholar]
- 70.Kang J Y, Elders A, Majeed A.et al Recent trends in hospital admissions and mortality rates for peptic ulcer in Scotland 1982–2002. Aliment Pharmacol Ther 20062465–79. [DOI] [PubMed] [Google Scholar]
- 71.Kang J Y, Tinto A, Higham J.et al Peptic ulceration in general practice in England and Wales 1994–98: period prevalence and drug management. Aliment Pharmacol Ther 2002161067–1074. [DOI] [PubMed] [Google Scholar]
- 72.Watkins R M, Dennison A R, Collin J. What has happened to perforated peptic ulcer? Br J Surg 198471774–776. [DOI] [PubMed] [Google Scholar]
- 73.Walt R, Katschinski B, Logan R.et al Rising frequency of ulcer perforation in elderly people in the United Kingdom. Lancet 19861489–492. [DOI] [PubMed] [Google Scholar]
- 74.Bardhan K D, Williamson M, Royston C.et al Admission rates for peptic ulcer in the Trent region, UK, 1972–2000. changing pattern, a changing disease? Dig Liver Dis 200436577–588. [DOI] [PubMed] [Google Scholar]
- 75.Higham J, Kang J Y, Majeed A. Recent trends in admissions and mortality due to peptic ulcer in England: increasing frequency of haemorrhage among older subjects. Gut 200250460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Langman M J, Weil J, Wainwright P.et al Risks of bleeding peptic ulcer associated with individual non‐steroidal anti‐inflammatory drugs. Lancet 19943431075–1078. [DOI] [PubMed] [Google Scholar]
- 77.Henry D, Lim L L, Garcia Rodriguez L A.et al Variability in risk of gastrointestinal complications with individual non‐steroidal anti‐inflammatory drugs: results of a collaborative meta‐analysis. BMJ 19963121563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moayyedi P, Axon A T. Is there a rationale for eradication of Helicobacter pylori? Cost‐benefit: the case for, Br Med Bull 199854243–250. [DOI] [PubMed] [Google Scholar]
- 79.Forman D, Webb P, Parsonnet J. H pylori and gastric cancer. Lancet 1994343243–244. [PubMed] [Google Scholar]
- 80.Goodwin C S, Mendall M M, Northfield T C. Helicobacter pylori infection. Lancet 1997349265–269. [DOI] [PubMed] [Google Scholar]
- 81.Sonnenberg A. Temporal trends and geographical variations of peptic ulcer disease. Aliment Pharmacol Ther 19959(Suppl 2)3–12. [PubMed] [Google Scholar]
- 82.Logan R F, Walker M M. ABC of the upper gastrointestinal tract: epidemiology and diagnosis of Helicobacter pylori infection. BMJ 2001323920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vreeburg E M, Snel P, de Bruijne J W.et al Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol 199792236–243. [PubMed] [Google Scholar]
- 84.Schiller K F, Truelove S C, Williams D G. Haematemesis and melaena, with special reference to factors influencing the outcome. Br Med J 197027–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berry A R, Collin J, Frostick S P.et al Upper gastrointestinal haemorrhage in Oxford. A prospective study. J R Coll Surg Edinb 198429134–138. [PubMed] [Google Scholar]
- 86.Johnston S J, Jones P F, Kyle J.et al Epidemiology and course of gastrointestinal haemorrhage in North‐east Scotland. BMJ 19733655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madden M V, Griffith G H. Management of upper gastrointestinal bleeding in a district general hospital. J R Coll Physicians Lond 198520213–215. [PMC free article] [PubMed] [Google Scholar]
- 88.Katschinski B D, Logan R F, Davies J.et al Audit of mortality in upper gastrointestinal bleeding. Postgrad Med J 198965913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holman R A, Davis M, Gough K R.et al Value of a centralised approach in the management of haematemesis and melaena: experience in a district general hospital. Gut 199031504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masson J, Bramley P N, Herd K.et al Upper gastrointestinal bleeding in an open‐access dedicated unit. J R Coll Physicians Lond 199630436–442. [PMC free article] [PubMed] [Google Scholar]
- 91.Rockall T A, Logan R F, Devlin H E.et al Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995311222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blatchford O, Davidson L A, Murray W R.et al Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ 1997315510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herner B, Lauritzen G. Haematemesis and melaena from a limited reception area during a 5‐year period. Acta Med Scand 1965177483–492. [PubMed] [Google Scholar]
- 94.Henriksson A E, Svensson J O. Upper gastrointestinal bleeding. With special reference to blood transfusion. Eur J Surg 1991157193–196. [PubMed] [Google Scholar]
- 95.Mino Fugarolas G, Jaramillo Esteban J L, Galvez Calderon C.et al [An analysis of a general prospective series of 3270 upper digestive hemorrhages]. Rev Esp Enferm Dig 1992827–15. [PubMed] [Google Scholar]
- 96.Hallas J, Lauritsen J, Villadsen H D.et al Nonsteroidal anti‐inflammatory drugs and upper gastrointestinal bleeding, identifying high‐risk groups by excess risk estimates. Scand J Gastroenterol 199530438–444. [DOI] [PubMed] [Google Scholar]
- 97.Longstreth G F. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study. Am J Gastroenterol 199590177–178. [PubMed] [Google Scholar]
- 98.Ahmed M E, al‐Knaway B, al‐Wabel A.et al Acute upper gastrointestinal bleeding in southern Saudi Arabia. J Royal Coll Physicians Lond 19973162–64. [PMC free article] [PubMed] [Google Scholar]
- 99.Soplepmann J, Udd M, Peetsalu A.et al Acute upper gastrointestinal haemorrhage in Central Finland Province, Finland, and in Tartu County, Estonia. Ann Chir Gynaecol 199786222–228. [PubMed] [Google Scholar]
- 100.Paspatis G A, Matrella E, Kapsoritakis A.et al An epidemiological study of acute upper gastrointestinal bleeding in Crete, Greece. Eur J Gastroenterol Hepatol 2000121215–1220. [DOI] [PubMed] [Google Scholar]
- 101.Laporte J R, Ibanez L, Vidal X.et al Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf 200427411–420. [DOI] [PubMed] [Google Scholar]
- 102.Loftus E V., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 20041261504–1517. [DOI] [PubMed] [Google Scholar]
- 103.Gut Week Digestive health in the UK. Gut Week Website 200484–89.
- 104.Feeney M, Ciegg A, Winwood P.et al A case‐control study of measles vaccination and inflammatory bowel disease. The East Dorset Gastroenterology Group. Lancet 1997350764–766. [DOI] [PubMed] [Google Scholar]
- 105.Thompson N P, Montgomery S M, Pounder R E.et al Is measles vaccination a risk factor for inflammatory bowel disease? Lancet 19953451071–1074. [DOI] [PubMed] [Google Scholar]
- 106.MacDougall I P. The cancer risk in ulcerative colitis. Lancet 1964ii655–658. [DOI] [PubMed] [Google Scholar]
- 107.Rosenqvist H, Ohrling H, Lagercrantz R.et al Ulcerative colitis and carcinoma coli. Lancet 1950i906–908. [DOI] [PubMed] [Google Scholar]
- 108.Gillen C D, Walmsley R S, Prior P.et al Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 1994351590–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mellemkjaer L, Olsen J H, Frisch M.et al Cancer in patients with ulcerative colitis. Int J Cancer 199560330–333. [DOI] [PubMed] [Google Scholar]
- 110.Eaden J A, Mayberry J F, British Society for Gastroenterology, Association of Coloproctology for Great Britain and Ireland Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut 200251(Suppl 5)V10–V12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Edwards F C, Truelove S C. The course and prognosis of ulcerative colitis; part III complications. Gut 196451–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grip O, Svensson P J, Lindgren S. Inflammatory bowel disease promotes venous thrombosis earlier in life. Scand J Gastroenterol 200035619–623. [DOI] [PubMed] [Google Scholar]
- 113.Miehsler W, Reinisch W, Valic E.et al Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut 200453542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Compston J E, Judd D, Crawley E O.et al Osteoporosis in patients with inflammatory bowel disease. Gut 198728410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scott E M, Gaywood I, Scott B B. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut 200046(Suppl 1)i1–i8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scott E M, Scott B B. A strategy for osteoporosis in gastroenterology. Eur J Gastroenterol Hepatol 199810689–696. [PubMed] [Google Scholar]
- 117.Shah A, Mayberry J F, Williams G.et al Epidemiological survey of coeliac disease and inflammatory bowel disease in first‐degree relatives of coeliac patients. Q J Med 199074283–288. [PubMed] [Google Scholar]
- 118.Cottone M, Marrone C, Casa A.et al Familial occurrence of inflammatory bowel disease in celiac disease. Inflamm Bowel Dis 20039321–323. [DOI] [PubMed] [Google Scholar]
- 119.Shepherd H A, Selby W S, Chapman R W.et al Ulcerative colitis and persistent liver dysfunction. Q J Med 198352503–513. [PubMed] [Google Scholar]
- 120.Cullen S, Chapman R. Primary sclerosing cholangitis. Autoimmun Rev 20032305–312. [DOI] [PubMed] [Google Scholar]
- 121.Thomas G A, Millar‐Jones D, Rhodes J.et al Incidence of Crohn's disease in Cardiff over 60 years: 1986–1990 an update. Eur J Gastroenterol Hepatol 19957401–405. [PubMed] [Google Scholar]
- 122.Yapp T R, Stenson R, Thomas G A.et al Crohn's disease incidence in Cardiff from 1930: an update for 1991–1995. Eur J Gastroenterol Hepatol 200012907–911. [DOI] [PubMed] [Google Scholar]
- 123.Kyle J. Crohn's disease in the northeastern and northern Isles of Scotland: an epidemiological review. Gastroenterology 1992103392–399. [DOI] [PubMed] [Google Scholar]
- 124.Fellows I W, Freeman J G, Holmes G K. Crohn's disease in the city of Derby, 1951–85. Gut 1990311262–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee F I, Costello F T. Crohn's disease in Blackpool ‐ incidence and prevalence 1968–1980. Gut 198526274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jayanthi V, Probert C S, Pinder D.et al Epidemiology of Crohn's disease in Indian migrants and the indigenous population in Leicestershire. Q J Med 199282125–138. [PubMed] [Google Scholar]
- 127.Logan R F. Inflammatory bowel disease incidence: up, down or unchanged? Gut 199842309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bjornsson S, Johannsson J H, Oddsson E. Inflammatory bowel disease in Iceland, 1980. A retrospective nationwide epidemiologic study. Scand J Gastroenterol 19983371–77. [DOI] [PubMed] [Google Scholar]
- 129.Molinie F, Gower‐Rousseau C, Yzet T.et al Opposite evolution in incidence of Crohn's disease and ulcerative colitis in Northern France (1988–1999). Gut 200453843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lakatos L, Mester G, Erdelyi Z.et al Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977–2001. World J Gastroenterol 200410404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Evans JG, Acheson ED. An epidemiological study of ulcerative colitis and regional enteritis in the Oxford area. Gut 19656311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones H W, Grogono J, Hoare A M. Surveillance in ulcerative colitis: burdens and benefit. Gut 198829325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Srivastava E D, Mayberry J F, Morris T J.et al Incidence of ulcerative colitis in Cardiff over 20 years: 1968–87. Gut 199233256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Devlin H B, Datta D, Dellipiani A W. The incidence and prevalence of inflammatory bowel disease in North Tees Health District. World J Surg 19804183–193. [DOI] [PubMed] [Google Scholar]
- 135.Sinclair T S, Brunt P W, Mowat N A. Nonspecific proctocolitis in northeastern Scotland: a community study. Gastroenterology 1983851–11. [PubMed] [Google Scholar]
- 136.Rubin G P, Hungin A P, Kelly P J.et al Inflammatory bowel disease: epidemiology and management in an English general practice population. Aliment Pharmacol Ther 2000141553–1559. [DOI] [PubMed] [Google Scholar]
- 137.Miller D S, Keighley A C, Langman M J. Changing patterns in epidemiology of Crohn's disease. Lancet 19742691–693. [DOI] [PubMed] [Google Scholar]
- 138.Smith I S, Young S, Gillespie G.et al Epidemiological aspects of Crohn's disease in Clydesdale 1961–1970. Gut 19751662–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tresadern J C, Gear M W, Nicol A. An epidemiological study of regional enteritis in the Gloucester area. Br J Surg 197360366–368. [DOI] [PubMed] [Google Scholar]
- 140.Humphreys W G, Brown J S, Parks T G. Crohn's disease in Northern Ireland—a retrospective study of 440 cases. Ulster Med J 19905930–35. [PMC free article] [PubMed] [Google Scholar]
- 141.Stone M A, Mayberry J F, Baker R. Prevalence and management of inflammatory bowel disease: a cross‐sectional study from central England. Eur J Gastroenterol Hepatol 200315275–280. [DOI] [PubMed] [Google Scholar]
- 142.Barton J R, Gillon S, Ferguson A. Incidence of inflammatory bowel disease in Scottish children between 1968 and 1983; marginal fall in ulcerative colitis, three‐fold rise in Crohn's disease. Gut 198930618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Armitage E, Drummond H, Ghosh S.et al Incidence of juvenile‐onset Crohn's disease in Scotland. Lancet 19993531496–1497. [DOI] [PubMed] [Google Scholar]
- 144.Watson A J, Johnston A T, Barker P M.et al The presentation and management of juvenile‐onset chronic inflammatory bowel disease in Northeastern Scotland. J Pediatr Surg 20023783–86. [DOI] [PubMed] [Google Scholar]
- 145.Cosgrove M, Al Atia R F, Jenkins H R. The epidemiology of paediatric inflammatory bowel disease. Arch Dis Child 199674460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahmed M, Davies I H, Hood K.et al Incidence of paediatric inflammatory bowel disease in South Wales. Arch Dis Child 200691344–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ehlin A G, Montgomery S M, Ekbom A.et al Prevalence of gastrointestinal diseases in two British national birth cohorts. Gut 2003521117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shivananda S, Lennard‐Jones J, Logan R.et al Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC‐IBD). Gut 199639690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jones J, Boorman J, Cann P.et al British Society of Gastroenterology guidelines for the management of irritable bowel syndrome. Gut 200047(Suppl 2)ii1–NaN19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harvey R F, Salih S Y, Read A E. Organic and functional disorders in 2000 gastroenterology outpatients. Lancet 19831632–634. [DOI] [PubMed] [Google Scholar]
- 151.Ferguson A, Sircus W, Eastwood M A. Frequency of “functional” gastrointestinal disorders. Lancet 19772613–614. [DOI] [PubMed] [Google Scholar]
- 152.Fielding J E. A year in out‐patients with the irritable bowel syndrome. Ir J Med Sci 1977146162–166. [DOI] [PubMed] [Google Scholar]
- 153.Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med 200411644–9S. [DOI] [PubMed] [Google Scholar]
- 154.Kennedy T M, Jones R H. Epidemiology of cholecystectomy and irritable bowel syndrome in a UK population. Br J Surg 2000871658–1663. [DOI] [PubMed] [Google Scholar]
- 155.Heaton K W, O'Donnell L J, Braddon F E.et al Symptoms of irritable bowel syndrome in a British urban community: consulters and nonconsulters. Gastroenterology 19921021962–1967. [DOI] [PubMed] [Google Scholar]
- 156.Thompson W G, Heaton K W, Smyth G T.et al Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 20004678–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thompson W G, Heaton K W. Functional bowel disorders in apparently healthy people. Gastroenterology 198079283–288. [PubMed] [Google Scholar]
- 158.Wilson S, Roberts L, Roalfe A.et al Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract 200454495–502. [PMC free article] [PubMed] [Google Scholar]
- 159.Drossman D A, Sandler R S, McKee D C.et al Bowel patterns among subjects not seeking health care. Use of a questionnaire to identify a population with bowel dysfunction. Gastroenterology 198283529–534. [PubMed] [Google Scholar]
- 160.Sandler R S, Drossman D A, Nathan H P.et al Symptom complaints and health care seeking behavior in subjects with bowel dysfunction. Gastroenterology 198487314–318. [PubMed] [Google Scholar]
- 161.Gaburri M, Bassotti G, Bacci G.et al Functional gut disorders and health care seeking behavior in an Italian non‐patient population. Recenti Prog Med 198980241–244. [PubMed] [Google Scholar]
- 162.Schlemper R J, van der Werf S D, Vandenbroucke J P.et al Peptic ulcer, non‐ulcer dyspepsia and irritable bowel syndrome in The Netherlands and Japan. Scand J Gastroenterol 1993200(Suppl)33–41. [DOI] [PubMed] [Google Scholar]
- 163.Drossman D A, Li Z, Andruzzi E.et al U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993381569–1580. [DOI] [PubMed] [Google Scholar]
- 164.Saito Y A, Locke G R, Talley N J.et al A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol 2000952816–2824. [DOI] [PubMed] [Google Scholar]
- 165.Agreus L, Svardsudd K, Nyren O.et al Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology 1995109671–680. [DOI] [PubMed] [Google Scholar]
- 166.Kay L, Jorgensen T, Jensen K H. The epidemiology of irritable bowel syndrome in a random population: prevalence, incidence, natural history and risk factors. J Intern Med 199423623–30. [DOI] [PubMed] [Google Scholar]
- 167.Boyce P M, Koloski N A, Talley N J. Irritable bowel syndrome according to varying diagnostic criteria: are the new Rome II criteria unnecessarily restrictive for research and practice? Am J Gastroenterol 2000953176–3183. [DOI] [PubMed] [Google Scholar]
- 168.Mearin F, Badia X, Balboa A.et al Irritable bowel syndrome prevalence varies enormously depending on the employed diagnostic criteria: comparison of Rome II versus previous criteria in a general population. Scand J Gastroenterol 2001361155–1161. [DOI] [PubMed] [Google Scholar]
- 169.Dapoigny M, Bellanger J, Bonaz B.et al Irritable bowel syndrome in France: a common, debilitating and costly disorder. Eur J Gastroenterol Hepatol 200416995–1001. [DOI] [PubMed] [Google Scholar]
- 170.Thompson W G, Irvine E J, Pare P.et al Functional gastrointestinal disorders in Canada: first population‐based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci 200247225–235. [DOI] [PubMed] [Google Scholar]
- 171.Barbezat G, Poulton R, Milne B.et al Prevalence and correlates of irritable bowel symptoms in a New Zealand birth cohort. N Z Med J 2002115U220. [PubMed] [Google Scholar]
- 172.Saito Y A, Talley N J, J Melton L.et al The effect of new diagnostic criteria for irritable bowel syndrome on community prevalence estimates. Neurogastroenterol Motil 200315687–694. [DOI] [PubMed] [Google Scholar]
- 173.Hogberg L, Falth‐Magnusson K, Grodzinsky E.et al Familial prevalence of coeliac disease: a twenty‐year follow‐up study. Scand J Gastroenterol 20033861–65. [DOI] [PubMed] [Google Scholar]
- 174.Sanders D S, Patel D, Stephenson T J.et al A primary care cross‐sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol 200315407–413. [DOI] [PubMed] [Google Scholar]
- 175.Scott B B, Losowsky M S. Coeliac disease: a cause of various associated diseases. Lancet 1975ii956–957. [DOI] [PubMed] [Google Scholar]
- 176.Swinson C M, Slavin G, Coles E C.et al Coeliac disease and malignancy. Lancet 19831111–115. [DOI] [PubMed] [Google Scholar]
- 177.Lawson A, West J, Aithal G P.et al Autoimmune cholestatic liver disease in people with coeliac disease: a population‐based study of their association. Aliment Pharmacol Ther 200521401–405. [DOI] [PubMed] [Google Scholar]
- 178.West J, Logan R F, Card T R.et al Fracture risk in people with celiac disease: a population‐based cohort study. Gastroenterology 2003125429–436. [DOI] [PubMed] [Google Scholar]
- 179.Logan R F, Ferguson A, Finlayson N D.et al Primary biliary cirrhosis and coeliac disease: an association? Lancet 19781230–233. [DOI] [PubMed] [Google Scholar]
- 180.Cooper B T, Holmes G K, Cooke W T. Coeliac disease and immunological disorders. BMJ 1978I537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sorensen H T, Thulstrup A M, Blomqvist P.et al Risk of primary biliary liver cirrhosis in patients with coeliac disease: Danish and Swedish cohort data. Gut 199944736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.West J, Logan R F, Smith C J.et al Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ 2004329716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holmes G K, Stokes P L, Sorahan T M.et al Coeliac disease, gluten‐free diet, and malignancy. Gut 197617612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Howdle P D, Jalal P K, Holmes G K.et al Primary small‐bowel malignancy in the UK and its association with coeliac disease. Q J Med 200396345–353. [DOI] [PubMed] [Google Scholar]
- 185.Kingham J G, Ramanaden D, Dawson A. Metachronous small‐bowel adenocarcinoma in coeliac disease: gluten‐free diet is not protective. Scand J Gastroenterol 199833218–222. [DOI] [PubMed] [Google Scholar]
- 186.West J, Logan R F, Hill P.et al Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut 200352960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schweizer J J, von Blomberg B M, Bueno‐de Mesquita H B.et al Coeliac disease in The Netherlands. Scand J Gastroenterol 200439359–364. [DOI] [PubMed] [Google Scholar]
- 188.Hovell C J, Collett J A, Vautier G.et al High prevalence of coeliac disease in a population‐based study from Western Australia: a case for screening? Med J Aust 2001175247–250. [DOI] [PubMed] [Google Scholar]
- 189.Lagerqvist C, Ivarsson A, Juto P.et al Screening for adult coeliac disease ‐ which serological marker(s) to use? J Intern Med 2001250241–248. [DOI] [PubMed] [Google Scholar]
- 190.Csizmadia C G, Mearin M L, von Blomberg B M.et al An iceberg of childhood coeliac disease in the Netherlands. Lancet 1999353813–814. [DOI] [PubMed] [Google Scholar]
- 191.Pratesi R, Gandolfi L, Garcia S G.et al Prevalence of coeliac disease: unexplained age‐related variation in the same population. Scand J Gastroenterol 200338747–750. [DOI] [PubMed] [Google Scholar]
- 192.Gomez J C, Selvaggio G S, Viola M.et al Prevalence of celiac disease in Argentina: screening of an adult population in the La Plata area. Am J Gastroenterol 2001962700–2704. [DOI] [PubMed] [Google Scholar]
- 193.Fasano A, Berti I, Gerarduzzi T.et al Prevalence of celiac disease in at‐risk and not‐at‐risk groups in the United States: a large multicenter study. Arch Intern Med 2003163286–292. [DOI] [PubMed] [Google Scholar]
- 194.Kolho K L, Farkkila M A, Savilahti E. Undiagnosed coeliac disease is common in Finnish adults. Scand J Gastroenterol 1998331280–1283. [DOI] [PubMed] [Google Scholar]
- 195.Johnston S D, Watson R G, McMillan S A.et al Prevalence of coeliac disease in Northern Ireland. Lancet 19973501370. [DOI] [PubMed] [Google Scholar]
- 196.Maki M, Mustalahti K, Kokkonen J.et al Prevalence of Celiac disease among children in Finland. N Engl J Med 20033482517–2524. [DOI] [PubMed] [Google Scholar]
- 197.Mustalahti K, Reunanen A, Heuer M.et alPrevalence of coeliac disease in four European countries. Belfast, Ireland: 11th International Symposium on Coeliac Disease, 200460
- 198.Simpson J, Spiller R. Colonic diverticular disease. Clin Evid 20039478–487. [PubMed] [Google Scholar]
- 199.Key T J, Davey G K, Appleby P N. Health benefits of a vegetarian diet. Proc Nutr Soc 199958271–275. [DOI] [PubMed] [Google Scholar]
- 200.Nair P, Mayberry J F. Vegetarianism, dietary fibre and gastro‐intestinal disease. Dig Dis Sci 199412177–185. [DOI] [PubMed] [Google Scholar]
- 201.Campbell K, Steele R J. Non‐steroidal anti‐inflammatory drugs and complicated diverticular disease: a case‐control study. Br J Surg 199178190–191. [DOI] [PubMed] [Google Scholar]
- 202.Day T K. Intestinal perforation associated with osmotic slow release indomethacin capsules. Br Med J (Clin Res Ed) 19832871671–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Morris C R, Harvey I M, Stebbings W S.et al Epidemiology of perforated colonic diverticular disease. Postgrad Med J 200278654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sheers R, Williams W R. NSAIDs and gut damage. Lancet 198921154. [DOI] [PubMed] [Google Scholar]
- 205.Canter J W, Shorb P E., Jr Acute perforation of colonic diverticula associated with prolonged adrenocorticosteroid therapy. Am J Surg 197112146–51. [DOI] [PubMed] [Google Scholar]
- 206.Hart A R, Kennedy H J, Stebbings W S.et al How frequently do large bowel diverticula perforate? An incidence and cross‐sectional study. Eur J Gastroenterol Hepatol 200012661–665. [DOI] [PubMed] [Google Scholar]
- 207.Kyle J, Adesola A O, Tinckler A F. Incidence of diverticulitis. Scand J Gastroenterol 1967277–80. [DOI] [PubMed] [Google Scholar]
- 208.Kang J Y, Dhar A, Pollok R.et al Diverticular disease of the colon: ethnic differences in frequency. Aliment Pharmacol Ther 200419765–769. [DOI] [PubMed] [Google Scholar]
- 209.Parks T G. Natural history of diverticular disease of the colon. Clin Gastroenterol 1975453–69. [PubMed] [Google Scholar]
- 210.Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther 200318(Suppl 3)71–74. [DOI] [PubMed] [Google Scholar]
- 211.Kyle J, Davidson A I. The changing pattern of hospital admissions for divertical disease of the colon. Br J Surg 197562537–541. [DOI] [PubMed] [Google Scholar]
- 212.Kang J Y, Hoare J D, Tinto A.et al Diverticular disease of the colon ‐ on the rise: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther 2003171189–1195. [DOI] [PubMed] [Google Scholar]
- 213.Campbell W B, Lee E J, Van de Sijpe K.et al A 25‐year study of emergency surgical admissions. Ann R Coll Surg Engl 200284273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.BASL, BSG, AUGIS, UK Liver Surgeons Group, Pancreatic Society UK Specialised services for hepatology and hepato‐pancreato‐biliary (HPB) surgery. London: BASL, BSG, AUGIS, UK Liver Surgeons Group and Pancreatic Society UK, 2004
- 215.Information and Statistics Division Cancer statistics for Scotland. Scotland: NHS in Scotland. 2004 ( http://www.isdscotland.org/isd/183.html, accessed 20 December 2006)
- 216.Tao N, Sussman S, Nieto J.et al Demographic characteristics of hospitalized patients with alcoholic liver disease and pancreatitis in los angeles county. Alcohol Clin Exp Res 2003271798–1804. [DOI] [PubMed] [Google Scholar]
- 217.Saunders J B, Walters J R, Davies A P.et al A 20‐year prospective study of cirrhosis. BMJ 1981282263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goodall J A, Bryan C. The low incidence of alcoholic cirrhosis in the islands of Lewis and Harris. Scott Med J 198833229–230. [DOI] [PubMed] [Google Scholar]
- 219.Scottish Health Statistics Scottish Health Statistics 2004. Available at http://www.cabinetoffice.gov.uk/strategy/work_areas/alcohol_misuse/, accessed 18 January 2007
- 220.Chick J. Evidence suggesting increasing health damage in Scotland related to alcohol. Health Bull (Edinb) 199755134–139. [PubMed] [Google Scholar]
- 221.Prime Minister's Strategy Unit Alcohol harm reduction strategy for England. London: Cabinet Office, 2004
- 222.Schmidt D N. Apparent risk factors for chronic and acute pancreatitis in Stockholm county. Spirits but not wine and beer. Int J Pancreatol 1991845–50. [DOI] [PubMed] [Google Scholar]
- 223.Collantes R, Ong J P, Younossi Z M. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med 200471657–664. [DOI] [PubMed] [Google Scholar]
- 224.Caldwell S H, Oelsner D H, Lezzoni J C.et al Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 199929664–669. [DOI] [PubMed] [Google Scholar]
- 225.Joy D, Thava V R, Scott B B. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 200315539–543. [DOI] [PubMed] [Google Scholar]
- 226.British Liver Trust 2002 ( http://www.britishlivertrust.org.uk, accessed 20 December 2006)
- 227.Burroughs A, McNamara D. Liver disease in Europe. Aliment Pharmacol Ther 200318(Suppl 3)54–59. [DOI] [PubMed] [Google Scholar]
- 228.Department of Health Hepatitis C strategy for England. London: Department of Health, 2002
- 229.Health Protection Agency, SCIE National Public Health Service for Wales, CDSC Northern Ireland, CRDHB, UASSG Shooting up: infections among injecting drug users in the United Kingdom 2003. London: Health Protection Agency, 2004
- 230.World Health Organisation Weekly epidemiological record. Geneva: World Health Organisation, 1999
- 231.Judd A, Hickman M, Jones S.et al Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. BMJ 200533024–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Department of Health/General Health Protection Hepatitis C action plan for England. London: Department of Health, 2004
- 233.Steinke D T, Weston T L, Morris A D.et al The epidemiology of liver disease in Tayside database: a population‐based record‐linkage study. J Biomed Informatics 200335186–193. [DOI] [PubMed] [Google Scholar]
- 234.Henry J A, Moloney C, Rivas C.et al Increase in alcohol related deaths: is hepatitis C a factor? J Clin Pathol 200255704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Deuffic S, Poynard T, Valleron A J. Correlation between hepatitis C virus prevalence and hepatocellular carcinoma mortality in Europe. J Viral Hepat 19996411–413. [DOI] [PubMed] [Google Scholar]
- 236.Metcalf J W, Howel D, James O F.et al Primary biliary cirrhosis: epidemiology helping the clinician. BMJ 19963121181–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Myszor M, James O F. The epidemiology of primary biliary cirrhosis in north‐east England: an increasingly common disease? Q J Med 199075377–385. [PubMed] [Google Scholar]
- 238.James O F, Bhopal R, Howel D.et al Primary biliary cirrhosis once rare, now common in the United Kingdom? Hepatology 199930390–394. [DOI] [PubMed] [Google Scholar]
- 239.Triger D R. Primary biliary cirrhosis: An epidemiological study. BMJ 1980281772–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hislop W S, Hopwood D, Bouchier I A. Primary biliary cirrhosis in elderly females. Age Ageing 198211153–159. [DOI] [PubMed] [Google Scholar]
- 241.Hamlyn A N, Macklon A F, James O. Primary biliary cirrhosis: a geographical clustering and symptomatic onset seasonality. Gut 198324940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Goudie B M, MacFarlane G, Boyle P. Epidemiology of antimitochondrial antibody seropositivity and primary biliary cirrhosis in west of Scotland. Gut 198728A1346 [Google Scholar]
- 243.Metcalf J V, Bhopal R S, Gray J.et al Incidence and prevalence of primary biliary cirrhosis in the city of Newcastle upon Tyne, England. Int J Epidemiol 199726830–836. [DOI] [PubMed] [Google Scholar]
- 244.Kingham J G, Parker D R. The association between primary biliary cirrhosis and coeliac disease: a study of relative prevalences. Gut 199842120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Danielsson A, Boqvist L, Uddenfeldt P. Epidemiology of primary biliary cirrhosis in a defined rural population in the northern part of Sweden. Hepatology 199011458–464. [DOI] [PubMed] [Google Scholar]
- 246.Eriksson S, Lindgren S. The prevalence and clinical spectrum of primary biliary cirrhosis in a defined population. Scand J Gastroenterol 198419971–976. [PubMed] [Google Scholar]
- 247.Lofgren J, Jarnerot G, Danielsson D R.et al Incidence and prevalence of primary biliary cirrhosis in a defined population in Sweden. Scand J Gastroenterol 198520647–650. [DOI] [PubMed] [Google Scholar]
- 248.Triger D R, Berg P, Rodes J. Epidemiology of primary biliary cirrhosis. Liver 19844195–200. [DOI] [PubMed] [Google Scholar]
- 249.Witt‐Sullivan H, Heathcote J, Cauch K.et al The demography of primary biliary cirrhosis in Ontario, Canada. Hepatology 19901295–105. [DOI] [PubMed] [Google Scholar]
- 250.Caballero Plasencia A M, Lopez Callejas C, Valenzuela Barranco M.et al [Epidemiology of primary biliary cirrhosis in the South area of Granada]. Med Clin (Barc) 199196481–485. [PubMed] [Google Scholar]
- 251.Watson R G, Angus P W, Dewar M.et al Low prevalence of primary biliary cirrhosis in Victoria, Australia. Melbourne Liver Group. Gut 199536927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Boberg K M, Aadland E, Jahnsen J.et al Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol 19983399–103. [DOI] [PubMed] [Google Scholar]
- 253.Remmel T, Remmel H, Uibo R.et al Primary biliary cirrhosis in Estonia. With special reference to incidence, prevalence, clinical features, and outcome. Scand J Gastroenterol 199530367–371. [DOI] [PubMed] [Google Scholar]
- 254.Bambha K, Kim W R, Talwalkar J.et al Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 20031251364–1369. [DOI] [PubMed] [Google Scholar]
- 255.Hurlburt K J, McMahon B J, Deubner H.et al Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol 2002972402–2407. [DOI] [PubMed] [Google Scholar]
- 256.Sood S, Gow P J, Christie J M.et al Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology 2004127470–475. [DOI] [PubMed] [Google Scholar]
- 257.Chapman R W. The management of primary sclerosing cholangitis. Curr Gastroenterol Rep 200359–17. [DOI] [PubMed] [Google Scholar]
- 258.Vera A, Moledina S, Gunson B.et al Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet 20023601943–1944. [DOI] [PubMed] [Google Scholar]
- 259.Levy C, Lindor K D. Treatment options for primary biliary cirrhosis and primary sclerosing cholangitis. Curr Treat Options Gastroenterol 2003693–103. [DOI] [PubMed] [Google Scholar]
- 260.Bergquist A, Ekbom A, Olsson R.et al Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 200236321–327. [DOI] [PubMed] [Google Scholar]
- 261.Broome U, Lofberg R, Veress B.et al Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology 1995221404–1408. [DOI] [PubMed] [Google Scholar]
- 262.Kingham J G. Risk of colon cancer in patients with ulcerative colitis is potentiated by the presence of primary sclerosing cholangitis. Hepatology 199725254. [DOI] [PubMed] [Google Scholar]
- 263.Hardy R G. ABC of colorectal cancer. Molecular basis for risk factors. BMJ 2000321886–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kingham J G, Kochar N, Gravenor M B. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology 20041261929–1930. [DOI] [PubMed] [Google Scholar]
- 265.Berdal J E, Ebbesen J, Rydning A. [Incidence and prevalence of autoimmune liver diseases]. Tidsskr Nor Laegeforen 19981184517–4519. [PubMed] [Google Scholar]
- 266.Aerts R, Penninckx F. The burden of gallstone disease in Europe. Aliment Pharmacol Ther 200318(Suppl 3)49–53. [DOI] [PubMed] [Google Scholar]
- 267.Heaton K W, Braddon F E, Mountford R A.et al Symptomatic and silent gall stones in the community. Gut 199132316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Holland C, Heaton K W. Increasing frequency of gall bladder operations in the Bristol clinical area. Br Med J 19723672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Plant J C, Percy I, Bates T.et al Incidence of gallbladder disease in Canada, England, and France. Lancet 19732249–251. [DOI] [PubMed] [Google Scholar]
- 270.Bateson M C. Gallstones and cholecystectomy in modern Britain. Postgrad Med J 200076700–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kang J Y, Ellis C, Majeed A.et al Gallstones‐an increasing problem: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther 200317561–569. [DOI] [PubMed] [Google Scholar]
- 272.Glambek I, Kvaale G, Arnesjo B.et al Prevalence of gallstones in a Norwegian population. Scand J Gastroenterol 1987221089–1094. [DOI] [PubMed] [Google Scholar]
- 273.Berndt H, Nurnberg D, Pannwitz H. Prevalence of cholelithiasis. Results of an epidemiologic study using sonography in East Germany. Z Gastroenterol 198927662–666. [PubMed] [Google Scholar]
- 274.Muhrbeck O, Ahlberg J. Prevalence of gallstone disease in a Swedish population. Scand J Gastroenterol 1995301125–1128. [DOI] [PubMed] [Google Scholar]
- 275.Caroli‐Bosc F X, Deveau C, Harris A.et al Prevalence of cholelithiasis: results of an epidemiologic investigation in Vidauban, southeast France. General Practitioner's Group of Vidauban. Dig Dis Sci 1999441322–1329. [DOI] [PubMed] [Google Scholar]
- 276.Attili A F, Carulli N, Roda E.et al Epidemiology of gallstone disease in Italy: prevalence data of the Multicenter Italian Study on Cholelithiasis (M.I.COL.). Am J Epidemiol 1995141158–165. [DOI] [PubMed] [Google Scholar]
- 277.Acalovschi M. Epidemiology of gallstone disease. In: Acalovschi M, Paumgartner G, eds. Hepatobiliary diseases: cholestasis and gallstones ‐ Falk Workshop London: Kluwer Academic Publishers, 2001117–130.
- 278.Barbara L, Sama C, Morselli Labate A M.et al A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology 19877913–917. [DOI] [PubMed] [Google Scholar]
- 279.Martinez de Pancorbo C, Carballo F, Horcajo P.et al Prevalence and associated factors for gallstone disease: results of a population survey in Spain. J Clin Epidemiol 1997501347–1355. [DOI] [PubMed] [Google Scholar]
- 280.GREPCO Prevalence of gallstone disease in an Italian adult female population. Rome Group for the Epidemiology and Prevention of Cholelithiasis (GREPCO). Am J Epidemiol 1984119796–805. [PubMed] [Google Scholar]
- 281.Jorgensen T. Prevalence of gallstones in a Danish population. Am J Epidemiol 1987126912–921. [DOI] [PubMed] [Google Scholar]
- 282.Kratzer W, Kron M, Hay B.et al Prevalence of cholecystolithiasis in South Germany—an ultrasound study of 2,498 persons of a rural population. Z Gastroenterol 1999371157–1162. [PubMed] [Google Scholar]
- 283.Connor S, Raraty M G, Howes N.et al Surgery in the treatment of acute pancreatitis—minimal access pancreatic necrosectomy. Scand J Surg 200594135–142. [DOI] [PubMed] [Google Scholar]
- 284.Trapnell J E, Duncan E H. Patterns of incidence in acute pancreatitis. BMJ 19752179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Corfield A P, Cooper M J, Williamson R C. Acute pancreatitis: a lethal disease of increasing incidence. Gut 198526724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bourke J B, Giggs J A, Ebdon D S. Variations in the incidence and the spatial distribution of patients with primary acute pancreatitis in Nottingham. Gut 197920366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Thomson S R, Hendry W S, McFarlane G A.et al Epidemiology and outcome of acute pancreatitis. Br J Surg 198774398–401. [DOI] [PubMed] [Google Scholar]
- 288.Toh S K, Phillips S, Johnson C D. A prospective audit against national standards of the presentation and management of acute pancreatitis in the South of England. Gut 200046239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.De Beaux A C, Palmer K R, Carter D C. Factors influencing morbidity and mortality in acute pancreatitis; an analysis of 279 cases. Gut 199537121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Norton S A, Cheruvu C V, Collins J.et al An assessment of clinical guidelines for the management of acute pancreatitis. Ann R Coll Surg Engl 200183399–405. [PMC free article] [PubMed] [Google Scholar]
- 291.Mero M. Changing aetiology of acute pancreatitis. Ann Chir Gynaecol 198271126–129. [PubMed] [Google Scholar]
- 292.Svensson J O, Norback B, Bokey E L.et al Changing pattern in aetiology of pancreatitis in an urban Swedish area. Br J Surg 197966159–161. [DOI] [PubMed] [Google Scholar]
- 293.Halvorsen F A, Ritland S. Acute pancreatitis in Buskerud county, Norway. Incidence and etiology. Scand J Gastroenterol 199631411–414. [DOI] [PubMed] [Google Scholar]
- 294.Benchimol D, Firtion O, Bereder J M.et al Acute pancreatitis treated in a surgery ward. Apropos of 57 cases. J Chir (Paris) 1996133208–213. [PubMed] [Google Scholar]
- 295.Minguez M, Garcia A, Boix V. [Acute pancreatitis. A prospective epidemiological study in the province of Alicante. A Hospital Group for Study of Digestive Diseases in Alicante]. Rev Esp Enferm Dig 199587869–873. [PubMed] [Google Scholar]
- 296.Gullo L, Migliori M, Olah A.et al Acute pancreatitis in five European countries: etiology and mortality. Pancreas 200224223–227. [DOI] [PubMed] [Google Scholar]
- 297.Milheiro A, Medeiros A, Castro e Sousa F. Acute pancreatitis. An analysis of 91 consecutive cases (1988–1991) with a brief review of the literature. Acta Med Port 19958269–277. [PubMed] [Google Scholar]
- 298.Gislason H, Horn A, Hoem D.et al Acute pancreatitis in Bergen, Norway. A study on incidence, etiology and severity. Scand J Surg 20049329–33. [DOI] [PubMed] [Google Scholar]
- 299.Lindkvist B, Appelros S, Manjer J.et al Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol Hepatol 20042831–837. [DOI] [PubMed] [Google Scholar]
- 300.Maes B, Hastier P, Buckley M J.et al Extensive aetiological investigations in acute pancreatitis: results of a 1‐year prospective study. Eur J Gastroenterol Hepatol 199911891–896. [PubMed] [Google Scholar]
- 301.Birgisson H, Moller P H, Birgisson S.et al Acute pancreatitis: a prospective study of its incidence, aetiology, severity, and mortality in Iceland. Eur J Surg 2002168278–282. [DOI] [PubMed] [Google Scholar]
- 302.Flint R, Windsor J, Bonham M. Trends in the management of severe acute pancreatitis: interventions and outcome. ANZ J Surg 200474335–342. [DOI] [PubMed] [Google Scholar]
- 303.Goldacre M J, Roberts S E. Hospital admission for acute pancreatitis in an English population, 1963–98: database study of incidence and mortality. BMJ 20043281466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Tinto A, Lloyd D A, Kang J Y.et al Acute and chronic pancreatitis‐diseases on the rise: a study of hospital admissions in England 1989/90–1999/2000. Aliment Pharmacol Ther 2002162097–2105. [DOI] [PubMed] [Google Scholar]
- 305.Wilson C, Imrie C W. Changing patterns of incidence and mortality from acute pancreatitis in Scotland, 1961–1985. Br J Surg 199077731–734. [DOI] [PubMed] [Google Scholar]
- 306.McKay C J, Evans S, Sinclair M.et al High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg 1999861302–1305. [DOI] [PubMed] [Google Scholar]
- 307.Appelros S, Borgstrom A. Incidence, aetiology and mortality rate of acute pancreatitis over 10 years in a defined urban population in Sweden. Br J Surg 199986465–470. [DOI] [PubMed] [Google Scholar]
- 308.Floyd A, Pedersen L, Nielsen G L.et al Secular trends in incidence and 30‐day case fatality of acute pancreatitis in North Jutland County, Denmark: a register‐based study from 1981–2000. Scand J Gastroenterol 2002371461–1465. [DOI] [PubMed] [Google Scholar]
- 309.Lankisch P G, Assmus C, Maisonneuve P.et al Epidemiology of pancreatic diseases in Luneburg county. A study in a defined German population. Pancreatology 20022469–477. [DOI] [PubMed] [Google Scholar]
- 310.Eland I A, Sturkenboom M J, Wilson J H.et al Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand J Gastroenterol 2000351110–1116. [DOI] [PubMed] [Google Scholar]
- 311.Tran D D, Van Schilfgaarde R. Prevalence and mortality from acute pancreatitis in the Netherlands during 1971–1990. Digestion 199455342–343. [Google Scholar]
- 312.Jaakkola M, Nordback I. Pancreatitis in Finland between 1970 and 1989. Gut 1993341255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lankisch P G, Assmus D, Pflichtohofer D. The burden of pancreatic disease in a well defined population. Gastroenterology 1998116A324 [Google Scholar]
- 314.Dite P, Stary K, Novotny I.et al Incidence of chronic pancreatitis in the Czech Republic. Eur J Gastroenterol Hepatol 200113749–750. [DOI] [PubMed] [Google Scholar]
- 315.Mitchell C. Chronic pancreatitis. Gastroenterology 200331122–125. [Google Scholar]
- 316.Garg P K, Tandon R K. Survey on chronic pancreatitis in the Asia‐Pacific region. J Gastroenterol Hepatol 200419998–1004. [DOI] [PubMed] [Google Scholar]
- 317.Otsuki M. Chronic pancreatitis in Japan: epidemiology, prognosis, diagnostic criteria, and future problems. J Gastroenterol Hepatol 200338315–326. [DOI] [PubMed] [Google Scholar]
- 318.Lin Y, Tamakoshi A, Matsuno S.et al Nationwide epidemiological survey of chronic pancreatitis in Japan. J Gastroenterol 200035136–141. [DOI] [PubMed] [Google Scholar]
- 319.McNamara D. Pancreatic diseases. Aliment Pharmacol Ther 200318(Suppl 3)60–65. [DOI] [PubMed] [Google Scholar]
- 320.Johnson C D, Hosking S. National statistics for diet, alcohol consumption, and chronic pancreatitis in England and Wales, 1960–88. Gut 1991321401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.National Statistics Living in Britain: results from the 2000 General Household Survey. London: HMSO, 2001
- 322.Levy P, Barthet M, Mollard B R.et al Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterol Clin Biol 200630838–844. [DOI] [PubMed] [Google Scholar]
- 323.Dzieniszewski J, Jarosz M, Ciok J. Chronic pancreatitis in Warsaw. Mater Med Pol 199022202–204. [PubMed] [Google Scholar]
- 324.Keighley M R. Gastrointestinal cancers in Europe. Aliment Pharmacol Ther 200318(Suppl 3)7–30. [DOI] [PubMed] [Google Scholar]
- 325.Slaney G, Brooke B N. Cancer in ulcerative colitis. Lancet 19592694–698. [DOI] [PubMed] [Google Scholar]
- 326.Ekbom A, Helmick C, Zack M.et al Ulcerative colitis and colorectal cancer. A population‐based study. N Engl J Med 19903231228–1233. [DOI] [PubMed] [Google Scholar]
- 327.Softley A, Clamp S E, Watkinson G.et al The natural history of inflammatory bowel disease: has there been a change in the last 20 years? Scand J Gastroenterol 1988144(Suppl)20–23. [PubMed] [Google Scholar]
- 328.Bray F, Sankila R, Ferlay J.et al Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 20023899–166. [DOI] [PubMed] [Google Scholar]
- 329.Lagergren J, Bergstrom R, Lindgren A.et al Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999340825–831. [DOI] [PubMed] [Google Scholar]
- 330.Malka D, Hammel P, Maire F.et al Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 200251849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kang J Y, Hoare J, Majeed A.et al Decline in admission rates for acute appendicitis in England. Br J Surg 2003901586–1592. [DOI] [PubMed] [Google Scholar]
- 332.Stoll B A. Association between breast and colorectal cancers. Br J Surg 1998851468–1472. [DOI] [PubMed] [Google Scholar]
- 333.Lieverse R J, Jansen J B, Masclee A A.et al Gastrointestinal disturbances with obesity. Scand J Gastroenterol Suppl 199320053–58. [DOI] [PubMed] [Google Scholar]
- 334.Key T J, Allen N E, Spencer E A.et al The effect of diet on risk of cancer. Lancet 2002360861–868. [DOI] [PubMed] [Google Scholar]
- 335.Walther C, Zilling T, Perfekt R.et al Increasing prevalence of adenocarcinoma of the oesophagus and gastro‐oesophageal junction: a study of the Swedish population between 1970 and 1997. Eur J Surg 2001167748–757. [DOI] [PubMed] [Google Scholar]
- 336.Vaughan T L, Kristal A R, Blount P L.et al Nonsteroidal anti‐inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev 200211745–752. [PubMed] [Google Scholar]
- 337.Engel L S, Chow W H, Vaughan T L.et al Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 2003951404–1413. [DOI] [PubMed] [Google Scholar]
- 338.Rozen P. Cancer of the gastrointestinal tract: early detection or early prevention? Eur J Cancer Prev 20041371–75. [DOI] [PubMed] [Google Scholar]
- 339.Caldwell S H, Crespo D M, Kang H S.et al Obesity and hepatocellular carcinoma. Gastroenterology 2004127(Suppl 1)S97–103. [DOI] [PubMed] [Google Scholar]
- 340.El‐Serag H B. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004127(Suppl 1)S27–S34. [DOI] [PubMed] [Google Scholar]
- 341.Bunout D. Nutritional and metabolic effects of alcoholism: their relationship with alcoholic liver disease. Nutrition 199915583–589. [DOI] [PubMed] [Google Scholar]
- 342.Day C P. Who gets alcoholic liver disease: nature or nurture? J R Coll Physicians Lond 200034557–562. [PMC free article] [PubMed] [Google Scholar]
- 343.Naveau S, Giraud V, Borotto E.et al Excess weight risk factor for alcoholic liver disease. Hepatology 199725108–111. [DOI] [PubMed] [Google Scholar]
- 344.Luyckx F H, Lefebvre P J, Scheen A J. Non‐alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab 20002698–106. [PubMed] [Google Scholar]
- 345.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol 200421048–1058. [DOI] [PubMed] [Google Scholar]
- 346.Friis‐Liby I, Aldenborg F, Jerlstad P.et al High prevalence of metabolic complications in patients with non‐alcoholic fatty liver disease. Scand J Gastroenterol 200439864–869. [DOI] [PubMed] [Google Scholar]
- 347.Shalauta M D, Saad R. Barrett's esophagus. Am Fam Physician 2004692113–2118. [PubMed] [Google Scholar]
- 348.Caygill C P, Johnston D A, Lopez M.et al Lifestyle factors and Barrett's esophagus. Am J Gastroenterol 2002971328–1331. [DOI] [PubMed] [Google Scholar]
- 349.Wilson L J, Ma W, Hirschowitz B I. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol 1999942840–2844. [DOI] [PubMed] [Google Scholar]
- 350.Kodera Y, Ito S, Yamamura Y.et al Obesity and outcome of distal gastrectomy with D2 lymphadenectomy for carcinoma. Hepatogastroenterology 2004511225–1228. [PubMed] [Google Scholar]
- 351.Flancbaum L, Choban P S. Surgical implications of obesity. Annu Rev Med 199849215–234. [DOI] [PubMed] [Google Scholar]
- 352.Funnell I C, Bornman P C, Weakley S P.et al Obesity: an important prognostic factor in acute pancreatitis. Br J Surg 199380484–486. [DOI] [PubMed] [Google Scholar]
- 353.Martinez J, Sanchez‐Paya J, Palazon J M.et al Is obesity a risk factor in acute pancreatitis? A meta‐analysis. Pancreatology 2004442–48. [DOI] [PubMed] [Google Scholar]
- 354.Singer M V. Effect of ethanol and alcoholic beverages on the gastrointestinal tract in humans. Rom J Gastroenterol 200211197–204. [PubMed] [Google Scholar]
- 355.Chick J. Alcohol problems in the general hospital. Br Med Bull 199450200–210. [DOI] [PubMed] [Google Scholar]
- 356.Findlay A. Alcohol misuse in Scotland—is there a growing health problem? Health Bull (Edinb) 199149273–283. [PubMed] [Google Scholar]
- 357.Waddell T S, Hislop W S. Analysis of alcohol‐related admissions in gastroenterology, cardiology and respiratory medicine. Scott Med J 200348114–116. [DOI] [PubMed] [Google Scholar]
- 358.Cumberland P J, Sethi D, Roderick P J.et al The infectious intestinal disease study of England: a prospective evaluation of symptoms and health care use after an acute episode. Epidemiol Infect 2003130453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lunniss P J, Gladman M A, Hetzer F H.et al Risk factors in acquired faecal incontinence. J R Soc Med 200497111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Norton C, Chelvanayagam S, Wilson‐Barnett J.et al Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology 20031251320–1329. [DOI] [PubMed] [Google Scholar]
- 361.Norton C, Chelvanayagam S.Bowel continence nursing. Beaconsfield: Beaconsfield Publishers, 2004
- 362.Hinds J P, Eidelman B H, Wald A. Prevalence of bowel dysfunction in multiple sclerosis. Gastroenterology 1990981538–1542. [DOI] [PubMed] [Google Scholar]
- 363.Chia Y W, Fowler C J, Kamm M A.et al Prevalence of bowel dysfunction in patients with multiple sclerosis and bladder dysfunction. J Neurol 1995242105–108. [DOI] [PubMed] [Google Scholar]
- 364.Wiesel P, Norton C, Glickman S.et al Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol 2001131–18. [DOI] [PubMed] [Google Scholar]
- 365.Edwards L L, Quigley E M, Pfeiffer R F. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 199242726–732. [DOI] [PubMed] [Google Scholar]
- 366.Malone P S, Wheeler R A, Williams J E. Continence in patients with spina bifida: long term results. Arch Dis Child 199470107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Nakayama H, Jorgensen H S, Pedersen P M.et al Prevalence and risk factors of incontinence after stroke: the Copenhagen Stroke Study. Stroke 19972858–62. [DOI] [PubMed] [Google Scholar]
- 368.Glickman S, Kamm M A. Bowel dysfunction in spinal‐cord‐injury patients. Lancet 19963471651–1653. [DOI] [PubMed] [Google Scholar]
- 369.Krogh K, Nielsen J, Djurhuus J C.et al Colorectal function in patients with spinal cord lesions. Dis Colon Rectum 1997401233–1239. [DOI] [PubMed] [Google Scholar]
- 370.Menter R, Weitzenkamp D, Cooper D.et al Bowel management outcomes in individuals with long‐term spinal cord injuries. Spinal Cord 199735608–612. [DOI] [PubMed] [Google Scholar]
- 371.Coggrave M, Wiesel P, Norton C.et al Bowel management for adults with neurological disease or injury (Cochrane review). The Cochrane Library, Issue 2. Chichester, UK: John Wiley & Sons, 2003
- 372.Poulton B, Thomas S. The nursing cost of constipation. Primary Health Care 1999917–20. [Google Scholar]
- 373.McKiernan P J, Baker A J, Kelly D A. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet 200035525–29. [DOI] [PubMed] [Google Scholar]
- 374.Jones B.Intestinal failure, short bowel syndrome and HPN. 2004 ( http://www.gastrospr.co.uk/officefiles/HPNSprs14dec04.ppt, accessed 20 December 2006)
- 375.Cook I J, Pavli P, Riley J W.et al Gastrointestinal investigation of iron deficiency anaemia. BMJ (Clin Res Ed) 19962921380–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Logan E C, Yates J M, Stewart R M.et al Investigation and management of iron deficiency anaemia in general practice: a cluster randomised controlled trial of a simple management prompt. Postgrad Med J 200278533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Yates J M, Logan E C, Stewart R M. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J 200480405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Hin H, Bird G, Fisher P.et al Coeliac disease in primary care: case finding study. BMJ 1999318164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Office of National Statistics Mortality statistics, 2000. Cause. Series DH2 no. 27. London: HMSO, 2001
- 380.Office of Population Censuses and Surveys Mortality statistics, 1990. Cause. Series DH2 no. 17. London: HMSO, 1992
- 381.Garcia Rodriguez L A, Ruigomez A, Hasselgren G.et al Comparison of mortality from peptic ulcer bleed between patients with or without peptic ulcer antecedents. Epidemiology 19989452–456. [DOI] [PubMed] [Google Scholar]
- 382.Irvin T T. Mortality and perforated peptic ulcer: a case for risk stratification in elderly patients. Br J Surg 198976215–218. [DOI] [PubMed] [Google Scholar]
- 383.Canoy D S, Hart A R, Todd C J. Epidemiology of duodenal ulcer perforation: a study on hospital admissions in Norfolk, United Kingdom. Dig Liver Dis 200234322–327. [DOI] [PubMed] [Google Scholar]
- 384.Jones A F. Haematemesis and melaena with special reference to bleeding peptic ulcer. Br Med J 1947ii441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Needham C D, McConachie J A. Haematemesis and melaena. Br Med J 19502133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Coghill N F, Wilcox R G. Factors in the prognosis of bleeding chronic gastric and duodenal ulcers. Q J Med 196029576–596. [PubMed] [Google Scholar]
- 387.Hoare A M. Comparative study between endoscopy and radiology in acute upper gastrointestinal haemorrhage. Br Med J 1975127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Allan R, Dykes P. A study of factors influencing mortality rates from gastrointestinal haemorrhage. QJ Med 197645533–550. [PubMed] [Google Scholar]
- 389.Mayberry J F, Penny W J, Counsell B R.et al Mortality in acute upper gastrointestinal haemorrhage: a six‐year survey from the University Hospital of Wales. Postgrad Med J 198157627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Brown S G, Salmon P R, Brown P.et al Upper gastrointestinal haemorrhage. J R Coll Physicians Lond 198115265–268. [PMC free article] [PubMed] [Google Scholar]
- 391.Clason A E, Macleod D A, Elton R A. Clinical factors in the prediction of further haemorrhage or mortality in acute upper gastrointestinal haemorrhage. Br J Surg 198673985–987. [DOI] [PubMed] [Google Scholar]
- 392.Sanderson J D, Taylor R F, Pugh S.et al Specialized gastrointestinal units for the management of upper gastrointestinal haemorrhage. Postgrad Med J 199066654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Daneshmend T K, Hawkey C J, Langman M J.et al Omeprazole versus placebo for acute upper gastrointestinal bleeding: randomised double blind controlled trial. BMJ 1992304143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Clements D, Aslan S, Foster D.et al Acute upper gastrointestinal haemorrhage in a district general hospital: audit of an agreed management policy. J R Coll Physicians Lond 19912527–30. [PMC free article] [PubMed] [Google Scholar]
- 395.Kapur K C, Green J T, Turner R G.et al Auditing mortality from upper gastrointestinal haemorrhage: impact of a high dependency unit. J R Coll Physicians Lond 199832246–250. [PMC free article] [PubMed] [Google Scholar]
- 396.Sanders D S, Perry M J, Jones S G.et al Effectiveness of an upper‐gastrointestinal haemorrhage unit: a prospective analysis of 900 consecutive cases using the Rockall score as a method of risk standardisation. Eur J Gastroenterol Hepatol 200416487–494. [DOI] [PubMed] [Google Scholar]
- 397.Lim C H, Vani D, Shah S G.et al The outcome of suspected upper gastrointestinal bleeding with 24‐hour access to upper gastrointestinal endoscopy: a prospective cohort study. Endoscopy 200638581–585. [DOI] [PubMed] [Google Scholar]
- 398.Rockall T A, Logan R F, Devlin H B.et al Variation in outcome after acute upper gastrointestinal haemorrhage. The National Audit of Acute Upper Gastrointestinal Haemorrhage. Lancet 1995346346–350. [DOI] [PubMed] [Google Scholar]
- 399.Logan R F, Finlayson N D. Death in acute upper gastrointestinal bleeding. Can endoscopy reduce mortality? Lancet 197611173–1175. [DOI] [PubMed] [Google Scholar]
- 400.Ch'ng C L, Kingham J G. Scoring systems and risk assessment for upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol 2001131137–1139. [DOI] [PubMed] [Google Scholar]
- 401.Probert C S, Jayanthi V, Wicks A C.et al Mortality from Crohn's disease in Leicestershire, 1972–1989: an epidemiological community based study. Gut 1992331226–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Probert C S, Jayanthi V, Wicks A C.et al Mortality in patients with ulcerative colitis in Leicestershire, 1972–1989. An epidemiological study. Dig Dis Sci 199338538–541. [DOI] [PubMed] [Google Scholar]
- 403.Farrokhyar F, Swarbrick E T, Grace R H.et al Low mortality in ulcerative colitis and Crohn's disease in three regional centers in England. Am J Gastroenterol 200196501–507. [DOI] [PubMed] [Google Scholar]
- 404.Mayberry J F, Newcombe R G, Rhodes J. Mortality in Crohn's disease. Q J Med 19804963–68. [PubMed] [Google Scholar]
- 405.Card T, Hubbard R, Logan R F. Mortality in inflammatory bowel disease: a population‐based cohort study. Gastroenterology 20031251583–1590. [DOI] [PubMed] [Google Scholar]
- 406.Logan R F, Rifkind E A, Turner I D.et al Mortality in celiac disease. Gastroenterology 198997265–271. [DOI] [PubMed] [Google Scholar]
- 407.Corrao G, Corazza G R, Bagnardi V.et al Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet 2001358356–361. [DOI] [PubMed] [Google Scholar]
- 408.Peters U, Askling J, Gridley G.et al Causes of death in patients with celiac disease in a population‐based Swedish cohort. Arch Intern Med 20031631566–1572. [DOI] [PubMed] [Google Scholar]
- 409.Cottone M, Termini A, Oliva L.et al Mortality and causes of death in celiac disease in a Mediterranean area. Dig Dis Sci 1999442538–2541. [DOI] [PubMed] [Google Scholar]
- 410.Nielsen O H, Jacobsen O, Pedersen E R.et al Non‐tropical sprue. Malignant diseases and mortality rate. Scand J Gastroenterol 19852013–18. [DOI] [PubMed] [Google Scholar]
- 411.Papagrigoriadis S, Debrah S, Koreli A.et al Impact of diverticular disease on hospital costs and activity. Colorectal Disease 2004681–84. [DOI] [PubMed] [Google Scholar]
- 412.Elliott T B, Yego S, Irvin T T. Five‐year audit of the acute complications of diverticular disease. Br J Surg 199784535–539. [PubMed] [Google Scholar]
- 413.Tudor R G, Farmakis N, Keighley M R. National audit of complicated diverticular disease: analysis of index cases. Br J Surg 199481730–732. [DOI] [PubMed] [Google Scholar]
- 414.Finlay I G, Carter D C. A comparison of emergency resection and staged management in perforated diverticular disease. Dis Colon Rectum 198730929–933. [DOI] [PubMed] [Google Scholar]
- 415.Leon D A, McCambridge J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: an analysis of routine data. Lancet 200636752–56. [DOI] [PubMed] [Google Scholar]
- 416.Vass A. Rates of liver cirrhosis rise in England, fall in Europe. BMJ 20013231388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Fisher N C, Hanson J N, Phillips A.et al Mortality from liver disease in the West Midlands, 1993–2000: observational study. BMJ 2002325312–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Degos F. Hepatitis C and alcohol. J Hepatol 199931(Suppl 1)113–118. [DOI] [PubMed] [Google Scholar]
- 419.Roberts S E, Goldacre M J, DY Trends in mortality after hospital admission for liver cirrhosis in an English population from 1968 to 1999. Gut 2005541615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Department of Health Annual Report of the Chief Medical Officer, 2001. London: Department of Health, 2001
- 421.Cucchiaro G, Watters C R, Rossitch J C.et al Deaths from gallstones. Incidence and associated clinical factors. Ann Surg 1989209149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Heatley M K, Crane J. Acute pancreatitis as a cause of sudden or unexpected death in Northern Ireland. Ulster Med J 19895851–55. [PMC free article] [PubMed] [Google Scholar]
- 423.Giggs J A, Bourke J B, Katschinski B. The epidemiology of primary acute pancreatitis in Greater Nottingham: 1969–1983. Soc Sci Med 19882679–89. [DOI] [PubMed] [Google Scholar]
- 424.Mann D V, Hershman M J, Hittinger R.et al Multicentre audit of death from acute pancreatitis. Br J Surg 199481890–893. [DOI] [PubMed] [Google Scholar]
- 425.Dube M G, Lobo D N, Rowlands B J.et al Audit of acute pancreatitis management: a tale of two hospitals. J R Coll Surg Edinb 200146292–296. [PubMed] [Google Scholar]
- 426.Tunnemann J, Easterbrook J R, Firth J.et al Management of acute pancreatitis: a comparative audit of clinical practice against the recommendations of the British Society of Gastrenterology. Br J Surg 200087362–373. [DOI] [PubMed] [Google Scholar]
- 427.Lankisch P G, Burchard‐Reckert S, Petersen M.et al Morbidity and mortality in 602 patients with acute pancreatitis seen between the years 1980–1994. Z Gastroenterol 199634371–377. [PubMed] [Google Scholar]
- 428.Cavallini G, Frulloni L, Bassi C.et al Prospective multicentre survey on acute pancreatitis in Italy (prolnf AISP): results on 1005 patients. Dig Dis Sci 200436205–211. [DOI] [PubMed] [Google Scholar]
- 429.Carter C R, McKay C J, Imrie C W. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg 2000232175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Wilson C, McArdle C S, Carter D C.et al Surgical treatment of acute necrotizing pancreatitis. Br J Surg 1988751119–1123. [DOI] [PubMed] [Google Scholar]
- 431.Beattie G C, Mason J, Swan D.et al Outcome of necrosectomy in acute pancreatitis: the case for continued vigilance. Scand J Gastroenterol 2002371449–1453. [DOI] [PubMed] [Google Scholar]
- 432.Schneider H, Boyle N, McCluckie A.et al Acute severe pancreatitis and multiple organ failure: total parenteral nutrition is still required in a proportion of patients. Br J Surg 200087362–373. [DOI] [PubMed] [Google Scholar]
- 433.Appelros S, Lindgren S, Borgstrom A. Short and long term outcome of severe acute pancreatitis. Eur J Surg 2001167281–286. [DOI] [PubMed] [Google Scholar]
- 434.Blum T, Maisonneuve P, Lowenfels A B.et al Fatal outcome in acute pancreatitis: its occurence and early prediction. Pancreatology 20011237–241. [DOI] [PubMed] [Google Scholar]
- 435.Lowenfels A B, Maisonneuve P, Cavallini G.et al Prognosis of chronic pancreatitis: an international multicenter study. International Pancreatitis Study Group. Lowenfels AB, Maisonneuve P, Cavallini. Am J Gastroenterol 1994891467–1471. [PubMed] [Google Scholar]
- 436.Lowenfels A B, Maisonneuve P, Cavallini G.et al Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 19933281433–1437. [DOI] [PubMed] [Google Scholar]
- 437.Ekbom A, McLaughlin J K, Karlsson B M.et al Pancreatitis and pancreatic cancer: a population‐based study. J Natl Cancer Inst 199486625–627. [DOI] [PubMed] [Google Scholar]
- 438.Miyake H, Harada H, Ochi K.et al Prognosis and prognostic factors in chronic pancreatitis. Dig Dis Sci 198934449–455. [DOI] [PubMed] [Google Scholar]
- 439.Talamini G, Falconi M, Bassi C.et al Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol 1999941253–1260. [DOI] [PubMed] [Google Scholar]
- 440.Karlson B M, Ekbom A, Josefsson S.et al The risk of pancreatic cancer following pancreatitis: an association due to confounding? Gastroenterology 1997113587–592. [DOI] [PubMed] [Google Scholar]
- 441.ONS Cancer survival, England and Wales, 1991–2001. Available at http://www.statistics.gov.uk/statbase/product.asp?vlnk = 10821/, accessed 18 January 2007
- 442.Metcalf J V, Smith J, Jones R.et al Incidence and causes of rectal bleeding in general practice as detected by colonoscopy. Br J Gen Pract 199646161–164. [PMC free article] [PubMed] [Google Scholar]
- 443.Hamilton W, Round A, Sharp D.et al Clinical features of colorectal cancer before diagnosis: a population‐based case‐control study. Br J Cancer 200593399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Crosland A, Jones R. Rectal bleeding: prevalence and consultation behaviour. BMJ 1995311486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Ciccolallo L, Capocaccia R, Coleman M P.et al Survival differences between European and US patients with colorectal cancer: role of stage at diagnosis and surgery. Gut 200554268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Taylor‐Robinson S D, Toledano M B, Arora S.et al Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut 200148816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Borgaonkar M R, Irvine E J. Quality of life measurement in gastrointestinal and liver disorders. Gut 200047444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Fitzpatrick R, Fletcher A, Gore S. Quality of life measures in health care. 1: Applications and issues in assessment, BMJ 19923051074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Koloski N A, Talley N J, Boyce P M. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol 20009567–71. [DOI] [PubMed] [Google Scholar]
- 450.Halder S L, Locke III G R, Talley N J.et al Impact of functional gastrointestinal disorders on health‐related quality of life: a population‐based care‐control study. Aliment Pharmacol Ther 200419233–242. [DOI] [PubMed] [Google Scholar]
- 451.Whincup P H, Mendall M A, Perry I J.et al Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart 199675568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Armitage E L, Aldhous M L, Anderson N.et al Incidence of juvenile‐onset Crohn's disease in Scotland: association with northern latitude and affluence. Gastroenterology 20041271051–1057. [DOI] [PubMed] [Google Scholar]
- 453.Department of Health Hospital episode statistics. London: Department of Health, 2004, ( http://www.dh.gov.uk/PublicationsAndStatistics/Statistics/HospitalEpisodeStatistics/fs/en, accessed 20 December 2006)
- 454.National Cancer Intelligence Centre Office for National Statistics Cancer statistics for England. ( http://www.dh.gov.uk/PolicyAndGuidance/HealthAndSocialCareTopics/Cancer/fs/en, accessed 20 December 2006)
- 455.Welsh Cancer Intelligence and Surveillance Unit Cancer incidence in Wales, 1992–2002. ( http://www.wales.nhs.uk/sites3/home.cfm?OrgID = 242, accessed 20 December 2006)
- 456.Northern Ireland Cancer Registry Cancer statistics for Northern Ireland. ( http://www.qub.ac.uk/research‐centres/nicr/, accessed 20 December 2006)
- 457.Talley N J, Piper D. Major life event stress and dyspepsia of unknown cause: a case control study. Gut 198627127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Baron J H, Sonnenberg A. Alimentary diseases in the poor and middle class in London 1773–1815, and in New York poor 1797–1818. Aliment Pharmacol Ther 2002161709–1714. [DOI] [PubMed] [Google Scholar]
- 459.Veldhuyzen van Zanten S. Do socio‐economic status, marital status and occupation influence the prevalence of Helicobacter pylori infection? Aliment Pharmacol Ther 19959(Suppl 2)41–44. [PubMed] [Google Scholar]
- 460.Webb P M, Knight T, Greaves S.et al Relation between infection with Helicobacter pylori and living conditions in childhood: evidence for person to person transmission in early life. BMJ 1994308750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Malcolm C A, MacKay W G, Shepherd A.et al Helicobacter pylori in children is strongly associated with poverty. Scott Med J 200449136–138. [DOI] [PubMed] [Google Scholar]
- 462.Sonnenberg A. Factors which influence the incidence and course of peptic ulcer. Scand J Gastroenterol 1988155(Suppl)119–140. [DOI] [PubMed] [Google Scholar]
- 463.Suadicani P, Hein H O, Gyntelberg F. Genetic and life‐style determinants of peptic ulcer. A study of 3387 men aged 54 to 74 years: the Copenhagen Male Study, Scand J Gastroenterol 19993412–17. [DOI] [PubMed] [Google Scholar]
- 464.Caygill C P, Hill M J, Knowles R L.et al Occupational and socioeconomic factors associated with peptic ulcer and with cancers following consequent gastric surgery. Ann Occup Hyg 19903419–27. [DOI] [PubMed] [Google Scholar]
- 465.Thompson N P, Montgomery S M, Wadsworth M E.et al Early determinants of inflammatory bowel disease: use of two national longitudinal birth cohorts. Eur J Gastroenterol Hepatol 20001225–30. [DOI] [PubMed] [Google Scholar]
- 466.Mendall M A, Kumar D. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). Eur J Gastroenterol Hepatol 19981059–62. [DOI] [PubMed] [Google Scholar]
- 467.Howell S, Talley N J, Quine S.et al The irritable bowel syndrome has origins in the childhood socioeconomic environment. Am J Gastroenterol 2004991572–1578. [DOI] [PubMed] [Google Scholar]
- 468.Kennedy T M, Jones R H. The epidemiology of hysterectomy and irritable bowel syndrome in a UK population. Int J Clin Pract 200054647–650. [PubMed] [Google Scholar]
- 469.Johnsen R, Jacobsen B K, Forde O H. Associations between symptoms of irritable colon and psychological and social conditions and lifestyle. Br Med J (Clin Res Ed) 19862921633–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Ford M J, Miller P M, Eastwood J.et al Life events, psychiatric illness and the irritable bowel syndrome. Gut 198728160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Nanda R, James R, Smith H.et al Food intolerance and the irritable bowel syndrome. Gut 1989301099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Talley N J, Phillips S F, Bruce B.et al Relation among personality and symptoms in nonulcer dyspepsia and the irritable bowel syndrome. Gastroenterology 199099327–333. [DOI] [PubMed] [Google Scholar]
- 473.Price B.Body image: nursing concepts and care. London: Prentice Hall, 1990
- 474.Harrison L, Gardiner E. Do the rich really die young? Alcohol‐related mortality and social class in Great Britain. Addiction 1999941871–1880. [DOI] [PubMed] [Google Scholar]
- 475.Marang‐van de Mheen P J, Davey Smith D, Hart C L.et al Socioeconomic diferentials in mortality among men within Great Britain: time trends and contributory causes. J Epidemiol Community Health 199852214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Hutchinson S J, Goldberg D J, King M.et al Hepatitis C virus among childbearing women in Scotland: prevalence, deprivation, and diagnosis. Gut 200453593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Stuver S O, Boschi‐Pinto C, Trichopoulos D. Infection with hepatitis B and C viruses, social class and cancer. IARC Sci Publ 1997319–324. [PubMed]
- 478.Brown J, Harding S, Bethune A.et al Longitudinal study of socio‐economic differences in the incidence of stomach, colorectal and pancreatic cancers. Popul Trends 199835–41. [PubMed]
- 479.Gerhardsson M, Norell S E, Kiviranta H.et al Sedentary jobs and colon cancer. Am J Epidemiol 1986123775–780. [DOI] [PubMed] [Google Scholar]
- 480.Adams J, White M, Barker G.et al Are there socio‐economic inequalities in age of resection of colorectal cancer in people with HNPCC? Fam Cancer 20032(3–4)169–173. [DOI] [PubMed] [Google Scholar]
- 481.Whynes D K, Frew E J, Manghan C M.et al Colorectal cancer, screening and survival: the influence of socio‐economic deprivation. Public Health 2003117389–395. [DOI] [PubMed] [Google Scholar]
- 482.McCaffery K, Wardle J, Nadel M.et al Socioeconomic variation in participation in colorectal cancer screening. J Med Screen 20029104–108. [DOI] [PubMed] [Google Scholar]
- 483.Lewison G.Gastroenterology in the UK: the burden of disease. London: The Wellcome Trust, 1997
- 484.Unit of Health‐Care Epidemiology Unit of Health‐Care Epidemiology. Oxford, University of Oxford, 2004( http://www.uhce.ox.ac.uk, accessed 20 December 2006)
- 485.McCormick A, Fleming D, Charlton J.Morbidity statistics from general practice. Fourth national study 1991–1992. London: Office of Population Censuses and Surveys, 1995
- 486.Royal College of General Practitioners, Office of Population Censuses and Surveys, Department of Health and Social Security Morbidity statistics from general practice. Third national study 1981–1982. London: HMSO, 1986, (Series MB5 No 1. )
- 487.Rigby M J, Severs M P, Swayne J.et al Time to outlaw the episode. Br J Healthcare Computing 19941126–28. [Google Scholar]
- 488.Clarke A, McKee M. The consultant episode: an unhelpful measure. BMJ 19923051307–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.BSG & RCP GI services for patients in the new millennium: a national framework responsive to change (unpublished concensus document). BSG & RCP 2003
- 490.Royal College of General Practitioners Consultant physicians working with patients, the duties, responsibilities and practice of physicians in general medicine and the specialties. 3rd ed. London: RGCP, 20051–335.
- 491.Williams J G, Russell I, Durai D.et al What are the clinical outcome and cost‐effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi‐Institution Nurse Endoscopy Trial (MINuET). Health Technol Assess 200610 [DOI] [PubMed] [Google Scholar]
- 492.Douglass A B, Powell A, Bramble M G. The nurse endoscopist contribution to service delivery. Gastrointestinal Nursing 2004221–24. [Google Scholar]
- 493.Russo M W, Wei J T, Thiny M T.et al Digestive and liver diseases statistics, 2004. Gastroenterology 20041261448–1453. [DOI] [PubMed] [Google Scholar]
- 494.Driscoll R. The painful truth about colitis and Crohn's disease. NACC News 2004
- 495.Driscoll R, Kane S. Member survey. NACC News 1992
- 496.Walters S. NACC Audit of IBD. Chichester: Aeneas Press, 2000
- 497.Leigh S, Goss S.Counselling project report. 2001. ( http://www.nacc.org.uk/research, accessed 26 December 2006)
- 498.Department of Health NHS reference costs, 2001. London: Department of Health, 2002
- 499.Office of Health Economics Compendium of health statistics (2002–03). Abingdon: Radcliffe Publishing Ltd, 2004
- 500.Froehlich F, Burnand B, Pache I.et al Overuse of upper gastrointestinal endoscopy in a country with open‐access endoscopy: a prospective study in primary care. Gastrointest Endosc 19974513–19. [PubMed] [Google Scholar]
- 501.Gralnek I M. Outpatient management of “low‐risk” nonvariceal upper GI hemorrhage. Are we ready to put evidence into practice? Gastrointest Endosc 200255131–134. [DOI] [PubMed] [Google Scholar]
- 502.Silcock J G, Bramble M G. Open access gastroscopy: second survey of current practice in the United Kingdom. Gut 199740192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Watt G. The inverse care law today. Lancet 2002360252–254. [DOI] [PubMed] [Google Scholar]
- 504.Dunnill M G, Pounder R E. Medical outpatients: changes that can benefit patients. Clin Med 2004445–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Association of Coloproctology of GB and Ireland Guidelines for the management of colorectal cancer. London: Association of Coloproctology of GB and Ireland, 2001
- 506.Duff S E, Wood C, McCredie V.et al Waiting times for treatment of rectal cancer in North West England. J R Soc Med 200497117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Flashman K, O‐Leary D P, Senapati A.et al The department of health's two week standard for bowel cancer: is it working? Gut 200453387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Hellier M. Delivery of GI services in the new millennium. BSG News 200210 [Google Scholar]
- 509.Navarro F, Hanauer S B. Treatment of Inflammatory Bowel Disease: Safety and Tolerability Issues. Am J Gastroenterol 200398(Suppl 12)S18–S23. [DOI] [PubMed] [Google Scholar]
- 510.Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut 200148272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Dick A, Keady S, Mohamed F.et al Use of unlicensed and off‐label medications in paediatric gastroenterology with a review of the commonly used formularies in the UK. Aliment Pharm Ther 200317571–575. [DOI] [PubMed] [Google Scholar]
- 512.Chan F K, Graham D Y. Review article: prevention of non‐steroidal anti‐inflammatory drug gastrointestinal complications—review and recommendations based on risk assessment. Aliment Pharmacol Ther 2004191051–1061. [DOI] [PubMed] [Google Scholar]
- 513.Sheen C L, Colin‐Jones D G. Review article: over‐the‐counter drugs and the gastrointestinal tract. Aliment Pharmacol Ther 2001151263–1270. [DOI] [PubMed] [Google Scholar]
- 514.Bloor K, Maynard A. Is there scope for improving the cost‐effective prescribing of nonsteroidal anti‐inflammatory drugs? Pharmacoeconomics 19969484–496. [DOI] [PubMed] [Google Scholar]
- 515.Feagan B G. Maintenance therapy for inflammatory bowel disease. Am J Gastroenterol 200398(Suppl 12)S6–17. [DOI] [PubMed] [Google Scholar]
- 516.Belsey J D. Non‐steroidal anti‐inflammatory induced upper gastrointestinal event rates in patients awaiting joint replacement in the United Kingdom. An epidemiologically‐based burden of disease model. Curr Med Res Opin 200319306–312. [DOI] [PubMed] [Google Scholar]
- 517.Cook D J, Guyatt G H, Salena B J.et al Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta‐analysis. Gastroenterology 1992102139–148. [DOI] [PubMed] [Google Scholar]
- 518.Heuschkel R, Afzal N, Wuerth A.et al Complementary medicine use in children and young adults with inflammatory bowel disease. Am J Gastroenterol 200297382–388. [DOI] [PubMed] [Google Scholar]
- 519.Galloway J M, Gibson J, Dalrymple J. Endoscopy in primary care—a survey of current practice. Br J Gen Pract 200252536–538. [PMC free article] [PubMed] [Google Scholar]
- 520.Abbas S, Shaw S, Campbell D.et al Outpatient upper gastrointestinal endoscopy: large, prospective study of the morbidity and mortality rate at a single endoscopy unit in England. Dig Endosc 200416113–116. [Google Scholar]
- 521.Quine M A, Bell G D, McCloy R F.et al Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut 199536462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Van Kouwen M C, Drenth J P, Verhoeven H M.et al Upper gastrointestinal endoscopy in patients aged 85 years or more. Results of a feasibility study in a district general hospital. Arch Gerontol Geriatr 20033745–50. [DOI] [PubMed] [Google Scholar]
- 523.NCEPOD Scoping our practice. The 2004 report of the national confidential enquiry into patient outcome and death. London: NCEPOD, 2004
- 524.Page M J, Poritz L S, Kunselman S J.et al Factors affecting surgical risk in elderly patients with inflammatory bowel disease. J Gastrointest Surg 20026606–613. [DOI] [PubMed] [Google Scholar]
- 525.Moorthy K, Shaul T, Foley R J. Factors that predict conversion in patients undergoing laparoscopic surgery for Crohn's disease. Am J Surg 200418747–51. [DOI] [PubMed] [Google Scholar]
- 526.Ripamonti C, De Conno F, Ventafridda V.et al Management of bowel obstruction in advanced and terminal cancer patients. Ann Oncol 1993415–21. [DOI] [PubMed] [Google Scholar]
- 527.Yim H B, Jacobson B C, Saltzman J R.et al Clinical outcome of the use of enteral stents for palliation of patients with malignant upper GI obstruction. Gastrointest Endosc 200153329–332. [DOI] [PubMed] [Google Scholar]
- 528.Mukherjee S, Sloper P, Turnbull A. An insight into the experiences of parents with inflammatory bowel disease. J Adv Nurs 200237355–363. [DOI] [PubMed] [Google Scholar]
- 529.Institute of Food Research 2004 ( http://www.ifr.ac.uk/, accessed 26 December 2006)
- 530.Hawkey G M, Hawkey C J. Effect of information leaflets on knowledge in patients with gastrointestinal diseases. Gut 1989301641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.NICE Healthcare services for bowel (colorectal) cancer. London: NICE, 2004
- 532.Thompson K, Melby V, Parahoo K.et al Information provided to patients undergoing gastroscopy procedures. J Clin Nurs 200312899–911. [DOI] [PubMed] [Google Scholar]
- 533.Stone M A, Mayberry J F, Baker R. Prevalence and management of inflammatory bowel disease: a cross‐sectional study from central England. Eur J Gastroenterol Hepatol 2003151275–1280. [DOI] [PubMed] [Google Scholar]
- 534.Sewitch M J, Abrahamowicz M, Bitton A.et al Psychosocial correlates of patient‐physician discordance in inflammatory bowel disease. Am J Gastroenterol 2002972174–2183. [DOI] [PubMed] [Google Scholar]
- 535.Sewitch M J, Abrahamowicz M, Barkun A.et al Patient nonadherence to medication in inflammatory bowel disease. Am J Gastroenterol 2003981535–1544. [DOI] [PubMed] [Google Scholar]
- 536.Greiner K A, Engelman K K, Hall M A.et al Barriers to colorectal cancer screening in rural primary care. Prev Med 200438269–275. [DOI] [PubMed] [Google Scholar]
- 537.Mansfield J C, Tanner A R, Bramble M G. Information for patients about inflammatory bowel disease. J R Coll Physicians Lond 199731184–187. [PMC free article] [PubMed] [Google Scholar]
- 538.Berg D F, Bahadursingh A M, Kaminski D.et al Acute surgical emergencies in inflammatory bowel disease. Am J Surg 200218445–51. [DOI] [PubMed] [Google Scholar]
- 539.Farmer M, Petras R E, Hunt L E.et al The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol 2000953184–3188. [DOI] [PubMed] [Google Scholar]
- 540.Spray C, Debelle G D, Murphy M S. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr 200190400–405. [PubMed] [Google Scholar]
- 541.Jenkins H. Update on paediatric inflammatory bowel disease. Curr Paediatr 200111260–263. [Google Scholar]
- 542.Schmulson M W, Chang L. Diagnostic approach to the patient with irritable bowel syndrome. Am J Med 1999107(5A)20S–26S. [DOI] [PubMed] [Google Scholar]
- 543.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology 2001120652–668. [DOI] [PubMed] [Google Scholar]
- 544.de Dombal F. Acute abdominal pain in the elderly. J Clin Gastroenterol 199419331–335. [DOI] [PubMed] [Google Scholar]
- 545.Ofman J J, Dorn G H, Fennerty M B.et al The clinical and economic impact of competing management strategies for gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 200216261–273. [DOI] [PubMed] [Google Scholar]
- 546.Talley N J, Colin‐Jones D G, Koch K.et al Functional dyspepsia: a classification with guidelines for diagnosis and management. Gastroenterol Int 19914145–160. [Google Scholar]
- 547.Hansen J M, Bytzer P, Schaffalitzky De Muckadell O. Management of dyspeptic patients in primary care—value of the unaided clinical diagnosis and of dyspepsia subgrouping. Scand J Gastroenterol 199833799–805. [DOI] [PubMed] [Google Scholar]
- 548.Brignoli R, Watkins P, Halter F. The Omega Project—a comparison of two diagnostic strategies for risk‐ and cost‐oriented management of dyspepsia. Eur J Gastroenterol Hepatol 19979337–343. [DOI] [PubMed] [Google Scholar]
- 549.Summerton N, Paes R. The clinical assessment of patients with large bowel symptoms by general practitioners. Eur J Gen Pract 2000643–47. [Google Scholar]
- 550.Diamanti A, Gambarara M, Ferretti F.et al Is severe liver disease an indication for early transplantation in patients with ultra‐short bowel disease? Transplant Proc 200234876–877. [DOI] [PubMed] [Google Scholar]
- 551.Drossman D A, Whitehead W E, Toner B B.et al What determines severity among patients with painful functional bowel disorders? Am J Gastroenterol 200095974–980. [DOI] [PubMed] [Google Scholar]
- 552.Sands B E, Arsenault J E, Rosen M J. Risk of early surgery for Crohn's disease: implications for early treatment strategies. Am J Gastroenterol 2003982712–2718. [DOI] [PubMed] [Google Scholar]
- 553.Angelelli G, Scardapane A, Memeo M.et al Acute bowel ischemia: CT findings. Eur J Radiol 20045037–47. [DOI] [PubMed] [Google Scholar]
- 554.Limpert P, Longo W E, Kelemen P R.et al Colon and rectal cancer in the elderly. High incidence of asymptomatic disease, less surgical emergencies, and a favorable short‐term outcome. Crit Rev Oncol Hematol 200348159–163. [DOI] [PubMed] [Google Scholar]
- 555.Martin I G, Young S, Sue‐Ling H.et al Delays in the diagnosis of oesophagogastric cancer: a consecutive case series. BMJ 1997314467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Hamilton W, Sharp D. Diagnosis of lung cancer in primary care: a structured review. Fam Pract 200421605–611. [DOI] [PubMed] [Google Scholar]
- 557.NHS Centre for Reviews and Dissemination Management of upper gastro‐intestinal cancers. 2000;6 [Google Scholar]
- 558.Tunaci A. Postoperative imaging of gastrointestinal tract cancers. Eur J Radiol 200242224–230. [DOI] [PubMed] [Google Scholar]
- 559.NHS Centre for Reviews and Dissemination The management of colorectal cancer. 1997;3 [Google Scholar]
- 560.NHS Centre for Reviews and Dissemination The management of colorectal cancers. 2004;8 [Google Scholar]
- 561.Gatta G, Capocaccia R, Sant M.et al Understanding variations in survival for colorectal cancer in Europe: a eurocare high resolution study. Gut 200047533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Shah R J, Fenoglio‐Preiser C, Bleau B L.et al Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea. Am J Gastroenterol 2001961091–1095. [DOI] [PubMed] [Google Scholar]
- 563.Erickson R A, Glick M E. Why have controlled trials failed to demonstrate a benefit of esophagogastroduodenoscopy in acute upper gastrointestinal bleeding? A probability model analysis. Dig Dis Sci 198631760–768. [DOI] [PubMed] [Google Scholar]
- 564.Parry S D, Welfare M R, Cobden I.et al Push enteroscopy in a UK district general hospital: experience of 51 cases over 2 years. Eur J Gastroenterol Hepatol 200214305–309. [DOI] [PubMed] [Google Scholar]
- 565.Yasui W, Yokozaki H, Shimamoto F.et al Molecular‐pathological diagnosis of gastrointestinal tissues and its contribution to cancer histopathology. Pathol Int 199949763–774. [DOI] [PubMed] [Google Scholar]
- 566.Lang M, Niskanen M, Miettinen P.et al Outcome and resource ultilisation in gastroenterological surgery. Br J Surg 2001881006–1014. [DOI] [PubMed] [Google Scholar]
- 567.Schofield P. Medical negligence in coloproctology. Colorectal Dis 1999160–63. [DOI] [PubMed] [Google Scholar]
- 568.Jeffery G, Hickey B, Hider P. Follow‐up strategies for patients treated for non‐metastatic colorectal cancer. Cochrane Database Syst Rev 2002(1)CD002200. [DOI] [PubMed]
- 569.Limburg P J, Ahlquist D A. Second primary colorectal cancer: the consequence of management failure at several potential levels. Ann Intern Med 2002136335–337. [DOI] [PubMed] [Google Scholar]
- 570.Allum W H, Griffin S M, Watson A, Colin‐Jones D, Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland; British Society of Gastroenterology; British Association of Surgical Oncology Guidelines for the management of oesophageal and gastric cancer. Gut 200250(Suppl V)v1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Ahmed N, Ahmedzai S, Vora V.et al Supportive care for patients with gastrointestinal cancer. Cochrane Database Syst Rev 2004(3)CD003445. [DOI] [PMC free article] [PubMed]
- 572.Bardou M, Barkun A N, Ghosn J.et al Effect of chronic intake of NSAIDs and cyclooxygenase 2‐selective inhibitors on esophageal cancer incidence. Clin Gastroenterol Hepatol 20042880–887. [DOI] [PubMed] [Google Scholar]
- 573.Smith A M, Maxwell‐Armstrong C A, Welch N T.et al Surveillance for Barrett's oesophagus in the UK. Br J Surg 199986276–280. [DOI] [PubMed] [Google Scholar]
- 574.BSG Dyspepsia management guidelines. BSG News 2002
- 575.Spiegel B M, Vakil N B, Ofman J J. Dyspepsia management in primary care: a decision analysis of competing strategies. Gastroenterology 20021221270–1285. [DOI] [PubMed] [Google Scholar]
- 576.Bodger K, Eastwood P G, Manning S I.et al Dyspepsia workload in urban general practice and implications of the British Society of Gastroenterology Dyspepsia guidelines. Aliment Pharmacol Ther 200014413–420. [DOI] [PubMed] [Google Scholar]
- 577.NICE Dyspepsia: managing adult patient in primary care. London: NICE, 2003
- 578.Parry J M, Foy R C, Woodman C B. How might general practitioner knowledge of patient Helicobacter pylori status change the management of dyspepsia in primary care? J Public Health Med 199820133–136. [DOI] [PubMed] [Google Scholar]
- 579.Delaney B C, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 20043 [DOI] [PubMed] [Google Scholar]
- 580.Banait G, Sibbald B, Thompson D, Summerton C, Hann M, Talbot S; Salford and Trafford Ulcer Research Network Modifying dyspepsia management in primary care: a cluster randomised controlled trial of educational outreach compared with passive guideline dissemination. Br J Gen Pract 20035394–100. [PMC free article] [PubMed] [Google Scholar]
- 581.McNamara D A, Buckley M, O'Morain C A. Nonulcer dyspepsia: current concepts and management. Gastroenterol Clin North Am 200029807–818. [DOI] [PubMed] [Google Scholar]
- 582.Delaney B C. Role of Helicobacter pylori in gastrointestinal disease: implications for primary care of a revolution in management of dyspepsia. Br J Gen Pract 199545489–494. [PMC free article] [PubMed] [Google Scholar]
- 583.Tremaine W J, Sandborn W J, Loftus E V.et al A prospective cohort study of practice guidelines in inflammatory bowel disease. Am J Gastroenterol 2001962401–2406. [DOI] [PubMed] [Google Scholar]
- 584.Carter M J, Lobo A J, Travis S P, IBD Section BSoG Guidelines for the management of inflammatory bowel disease in adults. Gut 200453(Suppl V)v1–v16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Moum B. Medical treatment: does it influence the natural course of inflammatory bowel disease? Eur J Intern Med 200011197–203. [DOI] [PubMed] [Google Scholar]
- 586.Wright J, Manning A P, Bolus J.et al Do all patients in primary care who may benefit from eradication of Helicobacter pylori have access to effective care? Public Health 2001115282–285. [DOI] [PubMed] [Google Scholar]
- 587.Milne R, Logan R P, Harwood D.et al Helicobacter pylori and upper gastrointestinal disease: a survey of gastroenterologists in the United Kingdom. Gut 199537314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Verma S, Giaffer M H. Helicobacter pylori eradication ameliorates symptoms and improves quality of life in patients on long‐term acid suppression. A large prospective study in primary care. Dig Dis Sci 2002471567–1574. [DOI] [PubMed] [Google Scholar]
- 589.Whitaker M J, Brun J, Carelli F. Controversy and consensus in the management of upper gastrointestinal disease in primary care. The International Gastro Primary Care Group. Int J Clin Pract 199751239–243. [PubMed] [Google Scholar]
- 590.Paton S. Cost‐effective treatment of gastro‐oesophageal reflux disease—a comparision of two therapies commonly used in general practice. Br J Med Economics 1995885–95. [Google Scholar]
- 591.Conio M, Demarquay J F, De Luca L.et al Endoscopic treatment of pancreatico‐biliary malignancies. Crit Rev Oncol Hematol 200137127–135. [DOI] [PubMed] [Google Scholar]
- 592.Drossman D A, Li Z, Toner B B.et al Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci 199540986–995. [DOI] [PubMed] [Google Scholar]
- 593.Raine R, Carter S, Sensky T.et al General practitioners' perceptions of chronic fatigue syndrome and beliefs about its management, compared with irritable bowel syndrome: qualitative study. BMJ 20043281354–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Ryder S D. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 200352(Suppl 3)iii1–iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Feuer D. Management of intestinal obstruction. CME Bull Palliat Med 1999135–40. [Google Scholar]
- 596.Bodger K, Daly M J, Heatley R V. Prescribing patterns for dyspepsia in primary care: a prospective study of selected general practitioners. Aliment Pharmacol Ther 199610889–895. [DOI] [PubMed] [Google Scholar]
- 597.MacKenzie S, Norrie J, Vella M.et al Randomized clinical trial comparing consultant‐led or open access investigation for large bowel symptoms. Br J Surg 200390941–947. [DOI] [PubMed] [Google Scholar]
- 598.Rockall T A, Logan R F, Devlin H B.et al Selection of patients for early discharge or outpatient care after acute upper gastrointestinal haemorrhage. Lancet 19963471138–1140. [DOI] [PubMed] [Google Scholar]
- 599.Fisher D A, Jeffreys A, Grambow S C.et al Mortality and follow‐up colonoscopy after colorectal cancer. Am J Gastroenterol 200398901–906. [DOI] [PubMed] [Google Scholar]
- 600.Wexner S D, Eisen G M, Simmang C. Principles of privileging and credentialing for endoscopy and colonoscopy. Surg Endosc 200216367–369. [DOI] [PubMed] [Google Scholar]
- 601.Manes G, Balzano A, Marone P.et al Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open‐access endoscopy system: a prospective observational study based on the Maastricht guidelines. Aliment Pharmacol Ther 200216105–110. [DOI] [PubMed] [Google Scholar]
- 602.Axon A T, Bell G D, Jones R H.et al Guidelines on appropriate indications for upper gastrointestinal endoscopy. Working Party of the Joint Committee of the Royal College of Physicians of London, Royal College of Surgeons of England, Royal College of Anaesthetists, Association of Surgeons, the British Society of Gastroenterology, and the Thoracic Society of Great Britain. BMJ 1995310853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Association of Coloproctology of GB and Ireland Design for coloproctology. 3rd ed. London: Association of Coloproctology of GB and Ireland, 1999
- 604.Probert C S, Jayanthi V, Mayberry J F. British gastroenterologists' care profile for patients with inflammatory bowel disease: the need for a patients' charter. J R Soc Med 199386271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Department of Health Renal services information implementation strategy. London: Department of Health, 2004
- 606.Kubba A K, Whyman M R. Upper gastro‐intestinal disease in Scotland: a survey of practice amongst Scottish gastroenterologists. J R Coll Surg Edinb 199641302–306. [PubMed] [Google Scholar]
- 607.Garvican L. Planning for a possible national colorectal cancer screening programme. J Med Screen 19985187–194. [DOI] [PubMed] [Google Scholar]
- 608.Rozen P, Winawer S J, Waye J D. Prospects for the worldwide control of colorectal cancer through screening. Gastrointest Endosc 200255755–759. [DOI] [PubMed] [Google Scholar]
- 609.Mandel J S, Bond J H, Church T R.et al Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 19933281365–1371. [DOI] [PubMed] [Google Scholar]
- 610.UK Colorectal Cancer Screening Pilot Group Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ 2004329133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Mpofu C, Watson A, Rhodes J. Strategies for detecting colon cancer and‐or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev 2004(2)CD000279. [DOI] [PubMed]
- 612.Gross C P, Canto M I, Hixson J.et al Management of Barrett's esophagus: a national study of practice patterns and their cost implications. Am J Gastroenterol 1999943440–3447. [DOI] [PubMed] [Google Scholar]
- 613.Lin O S, Mannava S, Hwang K L.et al Reasons for current practices in managing Barrett's esophagus. Dis Esophagus 20021539–45. [DOI] [PubMed] [Google Scholar]
- 614.Mason J, Axon A T, Forman D.et al The cost‐effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther 200216559–568. [DOI] [PubMed] [Google Scholar]
- 615.Gerson L B, Groeneveld P W, Triadafilopoulos G. Cost‐effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 20042868–879. [DOI] [PubMed] [Google Scholar]
- 616.Duncan A, Morris A J, Cameron A.et al Laxative induced diarrhoea—a neglected diagnosis. J R Soc Med 199285203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Ramsey S D, Mandelson M T, Berry K.et al Cancer‐attributable costs of diagnosis and care for persons with screen‐detected versus symptom‐detected colorectal cancer. Gastroenterology 20031251645–1650. [DOI] [PubMed] [Google Scholar]
- 618.Renehan A G, O'Dwyer S T, Whynes D K. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ 200432881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Pignone M, Saha S, Hoerger T.et al Cost‐effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 200213796–104. [DOI] [PubMed] [Google Scholar]
- 620.Nietert P J, Silverstein M D, Mokhashi M S.et al Cost‐effectiveness of screening a population with chronic gastroesophageal reflux. Gastrointest Endosc 200357311–318. [DOI] [PubMed] [Google Scholar]
- 621.Loeve F, Brown M L, Boer R.et al Endoscopic colorectal cancer screening: a cost‐saving analysis. J Natl Cancer Inst 200092557–563. [DOI] [PubMed] [Google Scholar]
- 622.Atkin W S. Flexible sigmoidoscopy as a mass screening tool. Eur J Gastroenterol Hepatol 199810219–223. [DOI] [PubMed] [Google Scholar]
- 623.Dubinsky M C, Johanson J F, Seidman E G.et al Suspected inflammatory bowel disease—the clinical and economic impact of competing diagnostic strategies. Am J Gastroenterol 2002972333–2342. [DOI] [PubMed] [Google Scholar]
- 624.Bejes C, Marvel M K. Attempting the improbable: offering colorectal cancer screening to all appropriate patients. Fam Pract Res J 19921283–90. [PubMed] [Google Scholar]
- 625.Helm J, Choi J, Sutphen R.et al Current and evolving strategies for colorectal cancer screening. Cancer Control 200310193–204. [DOI] [PubMed] [Google Scholar]
- 626.Ganz P A, Farmer M M, Belman M.et al Improving colorectal cancer screening rates in a managed care health plan: recruitment of provider organizations for a randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev 200312824–829. [PubMed] [Google Scholar]
- 627.Kronborg O. Screening guidelines for colorectal cancer. Scand J Gastroenterol 199227(Suppl 192)123–129. [DOI] [PubMed] [Google Scholar]
- 628.Hill J, Walsh S, Evans D G. Screening of patients at high risk of colorectal cancer. Colorectal Dis 20013308–311. [DOI] [PubMed] [Google Scholar]
- 629.Taylor T, Williamson S, Wardle J.et al Acceptability of flexible sigmoidoscopy screening in older adults in the United Kingdom. J Med Screen 2000738–45. [DOI] [PubMed] [Google Scholar]
- 630.Rae L C. Community screening for colorectal cancer in north‐eastern New South Wales, 1987–1996. Med J Aust 1998168382–385. [DOI] [PubMed] [Google Scholar]
- 631.Sonnenberg A. Cost effectiveness of competing strategies to prevent or treat GORD‐related dysphagia. Pharmacoeconomics 200017391–401. [DOI] [PubMed] [Google Scholar]
- 632.Macafee D A, Scholefield J H. Population based endoscopic screening for colorectal cancer. Gut 200352323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Doria‐Rose V P, Levin T R, Selby J V.et al The incidence of colorectal cancer following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology 2004127714–722. [DOI] [PubMed] [Google Scholar]
- 634.Hur C, Gazelle G S, Zalis M E.et al An analysis of the potential impact of computed tomographic colonography (virtual colonoscopy) on colonoscopy demand. Gastroenterology 20041271312–1321. [DOI] [PubMed] [Google Scholar]
- 635.Cotton P B, Durkalski V L, Pineau B C.et al Virtual colonoscopy: good results and not so good results—why the difference? JAMA 20042911713–1719.15082698 [Google Scholar]
- 636.Cairnes, Scholefield J H. Guidelines for col‐rectal cancer screening in high risk groups. Gut 200251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Department of Health Screening for bowel cancer (colorectal cancer) UK. London, 2005. ( http://www.cancerscreening.nhs.uk/colorectal/screening.html#programme, accessed 26 December 2006)
- 638.Dunlop M G, British Society for Gastroenterology, Association of Coloproctology for Great Britain and Ireland Guidance on large bowel surveillance for people with two first degree relatives with colorectal cancer or one first degree relative diagnosed with colorectal cancer under 45 years. Gut 200251(Suppl 5)v17–v20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Dunlop M G, British Society for Gastroenterology, Association of Coloproctology for Great Britain and Ireland Guidance on gastrointestinal surveillance for hereditary non‐polyposis colorectal cancer, familial adenomatous polypolis, juvenile polyposis, and Peutz‐Jeghers syndrome. Gut 200251(Suppl 5)v21–v27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Bradshaw N, Holloway S, Penman I.et al Colonoscopy surveillance of individuals at risk of familial colorectal cancer. Gut 2003521748–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Mowat A M. Coeliac disease‐a meeting point for genetics, immunology, and protein chemistry. Lancet 20033611290–1292. [DOI] [PubMed] [Google Scholar]
- 642.Newman B, Siminovitch K A. Recent advances in the genetics of inflammatory bowel disease. Curr Opin Gastroenterol 200521401–407. [PubMed] [Google Scholar]
- 643.Ahmad T, Marshall S, Jewell D. Genotype‐based phenotyping heralds a new taxonomy for inflammatory bowel disease. Curr Opin Gastroenterol 200319327–335. [DOI] [PubMed] [Google Scholar]
- 644.Brueckl W M, Heinze E, Milsmann C.et al Prognostic significance of microsatellite instability in curatively resected adenocarcinoma of the small intestine. Cancer Lett 2004203181–190. [DOI] [PubMed] [Google Scholar]
- 645.Ahmad T, Tamboli C P, Jewell D.et al Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology 20041261533–1549. [DOI] [PubMed] [Google Scholar]
- 646.Anwar S, Frayling I M, Scott N A.et al Systematic review of genetic influences on the prognosis of colorectal cancer. Br J Surg 2004911275–1291. [DOI] [PubMed] [Google Scholar]
- 647.Westra J L, Plukker J T, Buys C H.et al Genetic alterations in locally advanced stage II/III colon cancer: a search for prognostic markers. Clin Colorectal Cancer 20044252–259. [DOI] [PubMed] [Google Scholar]
- 648.Charara M, Edmonston T B, Burkholder S.et al Microsatellite status and cell cycle associated markers in rectal cancer patients undergoing a combined regimen of 5‐FU and CPT‐11 chemotherapy and radiotherapy. Anticancer Res 2004243161–3167. [PubMed] [Google Scholar]
- 649.Clark A J, Barnetson R, Farrington S M.et al Prognosis in DNA mismatch repair deficient colorectal cancer: are all MSI tumours equivalent? Fam Cancer 2004385–91. [DOI] [PubMed] [Google Scholar]
- 650.Bataille F, Rummele P, Dietmaier W.et al Alterations in p53 predict response to preoperative high dose chemotherapy in patients with gastric cancer. Mol Pathol 200356286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Hulscher M, Wensing M, van der Weijden T.et al Interventions to implement prevention in primary care. The Cochrane Library 20043 [DOI] [PubMed] [Google Scholar]
- 652.Muller A D, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case‐control study of 32,702 veterans. Ann Intern Med 1995123904–910. [DOI] [PubMed] [Google Scholar]
- 653.Thomson M A, Booth I W. Treatment of traveller's diarrhoea: economic aspects. Pharmacoeconomics 19969382–391. [DOI] [PubMed] [Google Scholar]
- 654.O'Connor H, Sebastian S. The burden of Helicobacter pylori infection in Europe. Aliment Pharmacol Ther 200318(Suppl 3)38–44. [DOI] [PubMed] [Google Scholar]
- 655.Thornton J. Traditional or systematic reviews? Rev Gynaecol Pract 200223 [Google Scholar]
- 656.Wagenaar A C. Importance of systematic reviews and meta‐analyses for research and practice. Am J Prev Med 1999169–11. [DOI] [PubMed] [Google Scholar]
- 657.Dixon‐Woods M, Agarwal S, Jones D.et al Synthesising qualitative and quantitative evidence: a review of possible methods. J Health Serv Res Policy 20051045–53. [DOI] [PubMed] [Google Scholar]
- 658.Davies P. The relevance of systematic reviews to educational policy and practice. Oxford Review of Education 200026365–378. [Google Scholar]
- 659.Gough D, Elbourne D. Systematic research synthesis to inform policy, practice, and democratic debate. Social Policy & Society 20021225–236. [Google Scholar]
- 660.Centre for R e v i e w, Dissemination ( C R D )Undertaking systematic reviews of research on effectiveness. 2nd ed. York: Centre for Reviews and Dissemination (CRD), 2001, No 4.
- 661.Horvath A R, Pewsner D. Systematic reviews in laboratory medicine: principles, processes and practical considerations. Clin Chim Acta 200434223–39. [DOI] [PubMed] [Google Scholar]
- 662.Khan K, Popay J, Kleijnen J. Phase 2—development of a review protocol. York: Centre for Reviews and Dissemination, 2001, No 4.
- 663.Verhagen A P, de Vet H C, de Bie R A.et al The art of quality assessment of RCTs included in systematic reviews. J Clin Epidemiol 200154651–654. [DOI] [PubMed] [Google Scholar]
- 664.Appraisal of Guidelines Research and Evaluation Collaboration AGREE instrument. 2001 ( http://www.agreecollaboration.org/, accessed 26 December 2006)
- 665.Verhagen A P, de Vet H C, de Bie R A.et al The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998511235–1241. [DOI] [PubMed] [Google Scholar]
- 666.Jadad A.Randomised controlled trials. London: BMJ Publishing, 1998
- 667.AGREE Collaboration (Cluzeau F, Burgers J, Brouwers M, et al) Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Health Care 20031218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Pallant J.SPSS survival manual: Buckingham, UK: Open University Press 2001
- 669.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Measure 19602037–46. [Google Scholar]
- 670.Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs 20031277–84. [DOI] [PubMed] [Google Scholar]
- 671.NICE Guideline development methods. Information for national collaborating centres and guideline developers. London: NICE, 2004
- 672.Cook J R, Drummond M, Glick H.et al Assessing the appropriateness of combining economic data from multinational clinical trials. Stat Med 2003221955–1976. [DOI] [PubMed] [Google Scholar]
- 673.Department of Health National standards, local action. Health and social care standards and planning framework. London: Department of Health, 2004
- 674.Department of Health Improving chronic disease management. London: Department of Health, 2004
- 675.Ham C, York N, Sutch S.et al Hospital bed utilisation in the NHS, Kaiser Permanente, and the US Medicare programme: analysis of routine data. BMJ 20033271257–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Rubin G P, Hungin A P, Chinn D.et al Quality of life in patients with established inflammatory bowel disease: a UK general practice survey. Aliment Pharmacol Ther 200419529–535. [DOI] [PubMed] [Google Scholar]
- 677.Royal College of Physicians of London, Royal College of General Practitioners and NHS Alliance Clinicians, services and commissioning in chronic disease management in the NHS: the need for coordinated management programmes. London and Nottingham: Royal College of Physicians of London, Royal College of General Practitioners, and NHS Alliance, 2004, ( http://www.rcgp.org.uk/PDF/Corp_chronic_disease_nhs.pdf, accessed 26 December 2006)
- 678.Singh D.Making the shift: key success factors. A rapid review of best practice in shifting hospital care into the community. Birmingham: Health Services Management Centre, University of Birmingham and NHS Institute for Innovation and Improvement, 2006
- 679.Williams J G, Cheung W Y, Russell I T.et al Open access follow up for inflammatory bowel disease: pragmatic randomised trial and cost effectiveness study. BMJ 2000320544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Robinson A. Review article: inflammatory bowel disease—empowering the patient and improving outcome. Aliment Pharmacol Ther 200420(Suppl 4)84–87. [DOI] [PubMed] [Google Scholar]
- 681.Robinson A, Thompson D G, Wilkin D.et al Guided self‐management and patient‐directed follow‐up of ulcerative colitis: a randomised trial. Lancet 2001358976–981. [DOI] [PubMed] [Google Scholar]
- 682.Kennedy A P, Nelson E, Reeves D.et al A randomised controlled trial to assess the effectiveness and cost of a patient orientated self management approach to chronic inflammatory bowel disease. Gut 2004531639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Kennedy A P, Nelson E, Reeves D.et al A randomised controlled trial to assess the impact of a package comprising a patient‐orientated, evidence‐based self‐help guidebook and patient‐centred consultations on disease management and satisfaction in inflammatory bowel disease. Health Technol Assess 200371–113. [DOI] [PubMed] [Google Scholar]
- 684.Kennedy A, Robinson A, Rogers A. Incorporating patients' views and experiences of life with IBS in the development of an evidence based self‐help guidebook. Patient Educ Counsel 200350303–310. [DOI] [PubMed] [Google Scholar]
- 685.NACC Adolescence into adulthood in inflammatory bowel disease (IBD): the transition from paediatric to adult care. London: NACC, 2005
- 686.Evans J P, Steinhart A H, Cohen Z.et al Home total parenteral nutrition: an alternative to early surgery for complicated inflammatory bowel disease. J Gastrointest Surg 20037562–566. [DOI] [PubMed] [Google Scholar]
- 687.De Rooy E C, Toner B B, Maunder R G.et al Concerns of patients with inflammatory bowel disease: results from a clinical population. Am J Gastroenterol 2001961816–1821. [DOI] [PubMed] [Google Scholar]
- 688.O'Hanrahan T, Irving M H. The role of HPN in the management of intestinal failure—report of 400 cases. Clin Nutr 199211331–336. [DOI] [PubMed] [Google Scholar]
- 689.Shepperd S, Iliffe S. Hospital at home versus in‐patient hospital care. The Cochrane Library 20043 [Google Scholar]
- 690.NHS Modernisation Agency Practitioners with special interests—a step‐by‐step guide to setting up a GPwSI service. London: NHS Modernisation Agency, 2003
- 691.Department of Health and Royal College of General Practitioners Implementing a scheme for GPSI. London: Department of Health and Royal College of General Practitioners, 2002
- 692.Scott A, Vale L. Increased general practice workload due to a primary care led National Health Service: the need for evidence to support rhetoric. Br J Gen Pract 1998481085–1088. [PMC free article] [PubMed] [Google Scholar]
- 693.Kernick D P. Developing intermediate care provided by general practitioners with a special interest: the economic perspective. Br J Gen Pract 200353553–556. [PMC free article] [PubMed] [Google Scholar]
- 694.Scott A. Primary or secondary care? What can economics contribute to evaluation at the interface? J Public Health Med 19961819–26. [DOI] [PubMed] [Google Scholar]
- 695.Bass C, Hyde G, Bond A.et al A survey of frequent attenders at a gastroenterology clinic. J Psychosom Res 200150107–109. [DOI] [PubMed] [Google Scholar]
- 696.Levy R L, Von Korff M, Whitehead W E.et al Costs of care for irritable bowel syndrome patients in a health maintenance organization. Am J Gastroenterol 2001963122–3129. [DOI] [PubMed] [Google Scholar]
- 697.Haycox A, Butterworth M, Walley T.et al Development of an economic model for the management of upper gastrointestinal disease in primary care. Preliminary findings. Pharmacoeconomics 199814(Suppl 2)11–23. [DOI] [PubMed] [Google Scholar]
- 698.Primary Care Society for Gastroenterology Endoscopy in primary care. Primary Care Society for Gastroenterology, 2001. ( http://www.pcsg.org.uk/downloads/Endoscopy%20in%20primary%20care%202001.pdf, accessed 26 December 2006)
- 699.Nocon A, Leese B. The role of UK general practitioners with special clinical interests: implications for policy and service delivery. Br J Gen Pract 20045450–56. [PMC free article] [PubMed] [Google Scholar]
- 700.Littlewood J, Webb E, Beer N.et al A survey of postgraduate education programmes and research interests of GPs in community trusts in an inner city area. J R Soc Health 200012096–99. [DOI] [PubMed] [Google Scholar]
- 701.Jones R H, Bartholomew J. General practitioners with special clinical interests: a cross‐sectional survey. Br J Gen Pract 200252833–834. [PMC free article] [PubMed] [Google Scholar]
- 702.Gerada C, Harris L. Appraisal and revalidation of general practitioners with special interests (GPwSIs). Education for Primary Care 200314572–576. [Google Scholar]
- 703.Gerada C, Limber C. General practitioners with special interests:implications for clinical governance. Qual Primary Care 20031147–52. [Google Scholar]
- 704.Provenzale D, Ofman J, Gralnek I.et al Gastroenterologist specialist care and care provided by generalists—an evaluation of effectiveness and efficiency. Am J Gastroenterol 20039821–28. [DOI] [PubMed] [Google Scholar]
- 705.Aly E A, Milne R, Johnson C D. Non‐compliance with national guidelines in the management of acute pancreatitis in the United kingdom. Dig Surg 200219192–198. [DOI] [PubMed] [Google Scholar]
- 706.Bohra S, Byrne M F, Manning D.et al A prospective analysis of inpatient consultations to a gastroenterology service. Ir Med J 200396263–265. [PubMed] [Google Scholar]
- 707.Quirk D M, Barry M J, Aserkoff B.et al Physician specialty and variations in the cost of treating patients with acute upper gastrointestinal bleeding. Gastroenterology 19971131443–1448. [DOI] [PubMed] [Google Scholar]
- 708.Bassi A, Dodd S, Williamson P.et al Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut 2004531471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Moore R, Phillips C. Cost of NSAID adverse effects of the UK national health service. J Med Economics 1999245–55. [Google Scholar]
- 710.Meyer G S, Cheng E Y, Elting J. Differences between generalists and specialists in characteristics of patients receiving gastrointestinal procedures. J Gen Intern Med 200015188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Pathmakanthan S, Murray I, Smith K.et al Nurse endoscopists in United Kingdom health care: a survey of prevalence, skills and attitudes. J Adv Nurs 200136705–710. [DOI] [PubMed] [Google Scholar]
- 712.Smale S, Bjarnason I, Forgacs I.et al Upper gastrointestinal endoscopy performed by nurses: scope for the future? Gut 2003521090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.McKinlay A, MacKenzie J, Mowat N.Modernisation of the gastroenterology service in Scotland: towards European standards. Scottish Society of Gastroenterology 2003
- 714.Moshakis V, Ruban R, Wood G. Role of the nurse endoscopist in colorectal practice. Br J Surg 1996831399. [DOI] [PubMed] [Google Scholar]
- 715.Schoenfeld P, Lipscomb S, Crook J.et al Accuracy of polyp detection by gastroenterologists and nurse endoscopists during flexible sigmoidoscopy: a randomised trial. Gastroenterology 1999117312–318. [DOI] [PubMed] [Google Scholar]
- 716.Bachmann M, Peters T J, Harvey I M. Costs and concentration of cancer care: evidence for pancreatic, oesophageal and gastric cancers in National Health Service hospitals. J Health Serv Res Policy 2003875–82. [DOI] [PubMed] [Google Scholar]
- 717.Chen S C, Rex D K. Registered nurse‐administered propofol sedation for endoscopy. Aliment Pharmacol Ther 200419147–155. [DOI] [PubMed] [Google Scholar]
- 718.BSG Non‐medical endoscopists. A report of the working party of the British Society of Gastroenterology. London: BSG, 2005
- 719.Axon A T. Cancer surveillance in ulcerative colitis—a time for reappraisal. Gut 199435587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Lim C H, Dixon M F, Vail A.et al Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut 2003521127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Senate of Surgery of GB and Ireland Reconfiguration of surgical, accident and emergency and trauma services in the UK. Senate of Surgery of GB and Ireland, 2004. ( http://www.rcseng.ac.uk/rcseng/content/publications/docs/reconfiguration.html, accessed 26 December 2006)
- 722.Williams R.Provision of specialist liver services in England: a survey of their siting and extent. Foundation for Liver Research and the British Liver Trust. ( www.bsg.org.uk/pdf_word_docs/hepservices.doc, accessed 26 December 2006)
- 723.Bachmann M, Alderson D, Edwards D.et al Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg 200289914–922. [DOI] [PubMed] [Google Scholar]
- 724.Delaney B C, Wilson S, Roalfe A.et al Cost effectiveness of initial endoscopy for dyspepsia in patients over age 50 years: a randomised controlled trial in primary care. Lancet 20003561965–1969. [DOI] [PubMed] [Google Scholar]
- 725.Duxbury M S, Brodribb A J, Oppong F C.et al Management of colorectal cancer: variations in practice in one hospital. Eur J Surg Oncol 200329400–402. [DOI] [PubMed] [Google Scholar]
- 726.Senapati P S, Bhattarcharya D, Harinath G.et al A survey of the timing and approach to the surgical management of cholelithiasis in patients with acute biliary pancreatitis and acute cholecystitis in the UK. Ann R Coll Surg Engl 200385306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Davenport M, De Ville de Goyet J, Stringer M D.et al Seamless management of biliary atresia in England and Wales (1999–2002). Lancet 20043631354–1357. [DOI] [PubMed] [Google Scholar]
- 728.Majeed A W, Price C. Resource and manpower calculations for the provision of hepatobiliary surgical services in the UK. Ann R Coll Surg Engl 20048691–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Parks R W, Bettschart V, Frame S.et al Benefits of specialisation in the management of pancreatic cancer: results of a Scottish population‐based study. Br J Cancer 200491459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Bachmann M O, Alderson D, Peters T J.et al Influence of specialization on the management and outcome of patients with pancreatic cancer. Br J Surg 200390171–177. [DOI] [PubMed] [Google Scholar]
- 731.Neoptolemos J P, Russell R C, Bramhall S.et al Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. Br J Surg 1997841370–1376. [PubMed] [Google Scholar]
- 732.Andren‐Sandberg A, Neoptolemos J. Resection for pancreatic cancer in the new millennium. Pancreatology 20022431–439. [DOI] [PubMed] [Google Scholar]
- 733.Ferguson B, Posnett J, Sheldon T.Concentration and choice in the provision of hospital services: summary support. York: NHS Centre for Reviews and Dissemination, 1997
- 734.Grilli R, Minozzi S, Tinazzi A.et al Do specialists do it better? The impact of specialization on the processes and outcomes of care for cancer patients. Ann Oncol 19989365–374. [DOI] [PubMed] [Google Scholar]
- 735.Hutchins R R, Kojodjojo P, Ho R.et al Short and long‐term outcome of pancreatic surgery in a district general hospital. J R Coll Surg Edinb 200247548–551. [PubMed] [Google Scholar]
- 736.Sowden A.Relationship between volume and quality of health care: a review of the literature. York: NHS Centre for Reviews and Dissemination, 1995
- 737.Bodger K. Cost of illness of Crohn's disease. Pharmacoeconomics 200220639–652. [DOI] [PubMed] [Google Scholar]
- 738.Ward F M, Bodger K, Daly M J.et al Clinical economics review: medical management of inflammatory bowel disease. Aliment Pharmacol Ther 19991315–25. [DOI] [PubMed] [Google Scholar]
- 739.Wells N E, Hahn B A, Whorwell P J. Clinical economics review: irritable bowel syndrome. Aliment Pharmacol Ther 1997111019–1030. [DOI] [PubMed] [Google Scholar]
- 740.Akehurst R L, Brazier J E, Mathers N.et al Health‐related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics 200220455–462. [DOI] [PubMed] [Google Scholar]
- 741.Creed F, Ratcliffe J, Fernandez L.et al Health‐related quality of life and health care costs in severe, refractory irritable bowel syndrome. Ann Intern Med 2001134(Pt 2)860–868. [DOI] [PubMed] [Google Scholar]
- 742.Haycox A, Dubois D, Butterworth M. Customising an international disease management model to the needs of individual countries. Application to upper gastrointestinal disease. Pharmacoeconomics 199814(Suppl 2)39–56. [DOI] [PubMed] [Google Scholar]
- 743.Duggan A K. Modelling different approaches to the management of upper gastrointestinal disease. Pharmacoeconomics 199814(Suppl 2)25–37. [DOI] [PubMed] [Google Scholar]
- 744.Morant S V, McMahon A D, Cleland J G.et al Cardiovascular prophylaxis with aspirin: costs of supply and management of upper gastrointestinal and renal toxicity. Br J Clin Pharmacol 200357188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Sheridan W G, White A T, Havard T.et al Non‐specific abdominal pain: the resource implications. Ann R Coll Surg Engl 199274181–185. [PMC free article] [PubMed] [Google Scholar]
- 746.McLoughlin R, Sebastian S S, Qasim A.et al Coeliac disease in Europe. Aliment Pharmacol Ther 200318(Suppl 3)45–48. [DOI] [PubMed] [Google Scholar]
- 747.Moayyedi P, Mason J. Clinical and economic consequences of dyspepsia in the community. Gut 200250(Suppl 4)iv10–iv12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Logan R F, Delaney B. ABC of the upper gastrointestinal tract: implications of dyspepsia for the NHS. BMJ 2001323675–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Somasekar K, Shankar P J, Foster M E.et al Costs of waiting for gall bladder surgery. Postgrad Med J 200278668–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Valori R M, Brown C M, Strangeways P.et al Reducing community dyspepsia drug costs: a controlled trial. Gut 200149495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Delaney B C, Wilson S, Roalfe A.et al Randomised controlled trial of Helicobacter pylori testing and endoscopy for dyspepsia in primary care. BMJ 2001322898–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Bowles C J, Leicester R, Romaya C.et al A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 200453277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Association of Coloproctology of GB and Ireland Resources for coloproctology. London: Association of Coloproctology of GB and Ireland, 2001
- 754.South Wales Cancer Network Service development plan for adult cancer services. South Wales Cancer Network 2003
- 755.Burling D, Halligan S, Taylor S A.et al CT colonography practice in the UK: a national survey. Clin Radiol 20045939–43. [DOI] [PubMed] [Google Scholar]
- 756.Sawczenko A, Lynn R, Sandhu B K. Variations in initial assessment and management of inflammatory bowel disease across Great Britain and Ireland. Arch Dis Child 200388990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Burnett C A, Juszczak E, Sullivan P B. Nurse management of intractable functional constipation: a randomised controlled trial. Arch Dis Child 200489717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Maule W F. Screening for colorectal cancer by nurse endoscopists. N Engl J Med 1994330183–187. [DOI] [PubMed] [Google Scholar]
- 759.Melleney E M, Willoughby C P. Audit of a nurse endoscopist based one stop dyspepsia clinic. Postgrad Med J 200278161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Richards D M, Deeks J J, Sheldon T A.et al Home parenteral nutrition: a systematic review: Health Technol Assess 199711–59. [PubMed] [Google Scholar]
- 761.Puntis J W. The economics of home parenteral nutrition. Nutrition 199814809–812. [DOI] [PubMed] [Google Scholar]
- 762.British Society of Gastroenterology Information Working Party Specification of core requirements for clinical information systems in support of gastroenterology. London: BSG, 2001
- 763.Williams J G, Cheung W Y. Clinical information processes and information use in gastroenterology in the United Kingdom. Gastroenterol Today 20011182–86. [Google Scholar]
- 764.Mann R Y, Williams J G. Standards in medical record keeping. Clin Med 20033329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Croft G P, Williams J G. Breaking the cycle of poor data quality. Clin Med 2005547–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Lewsey J D, Leyland A H, Murray G D.et al Using routine data to complement and enhance the results of randomised controlled trials. Health Technol Assess 200041–55. [PubMed] [Google Scholar]
- 767.Williams J G, Cheung W Y, Cohen D.et al The value of routine data in health technology assaessment: can randomised trials rely on electronic data? Health Technol Assess 20037 [DOI] [PubMed] [Google Scholar]
- 768.Ellis B W. Management importance of common treatments: contribution of top 20 procedures to surgical workload and cost. BMJ 1991302882–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Beard S M, Holmes M, Majeed A.et alHepatic resection as a treatment for liver metastases in colorectal cancer. Nottingham: Trent Institute for Health Services Research, 1999
- 770.Jones R H. Clinical economics review: gastrointestinal disease in primary care. Aliment Pharmacol Ther 199610233–239. [DOI] [PubMed] [Google Scholar]
- 771.Read A M, Stone M A, Rathbone B J.et al Production and evaluation of guidelines for the management of inflammatory bowel disease: the Leicester experience. Postgrad Med J 199975147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Moody G A, Mann R, Gay S.et al The gastroenterology service: a survey of general practitioners' requirements. J R Soc Med 19938626–27. [PMC free article] [PubMed] [Google Scholar]
- 773.Hungin A P, Thomas P R, Bramble M G.et al What happens to patients following open access gastroscopy? An outcome study from general practice. Br J Gen Pract 199444519–521. [PMC free article] [PubMed] [Google Scholar]
- 774.Parry J M, Foy R C, Woodman C B J.et al A randomised contolled trial to assess the impact of a printed summary of research findings in general practice. Br J Gen Pract 199949634–638. [Google Scholar]
- 775.Smith G D, Steinke D T, Kinnear M.et al A comparison of irritable bowel syndrome patients managed in primary and secondary care: the Episode IBS Study. Br J Gen Pract 200454503–507. [PMC free article] [PubMed] [Google Scholar]
- 776.Guillou P J, Windsor A J, Nejim A. Clinical economics review: the health‐care economic implications of minimal access gastrointestinal surgery. Aliment Pharmacol Ther 199610707–713. [DOI] [PubMed] [Google Scholar]
- 777.Moayyedi P, Wardman M, Toner J.et al Establishing patient preferences for gastroenterology clinic reorganization using conjoint analysis. Eur J Gastroenterol Hepatol 200214429–433. [DOI] [PubMed] [Google Scholar]
- 778.Mathew J, Shankar P, Aldean I M. Audit on flexible sigmoidoscopy for rectal bleeding in a district general hospital: are we over‐loading the resources? Postgrad Med J 20048038–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Roderick P, Davies R, Raftery J.et al Cost‐effectiveness of population screening for Helicobacter pylori in preventing gastric cancer and peptic ulcer disease, using simulation. J Med Screen 200310148–156. [DOI] [PubMed] [Google Scholar]
- 780.Moayyedi P, Soo S, Deeks J.et al Pharmacological interventions for non‐ulcer dyspepsia. Cochrane Database Syst Rev 2004(3)CD002301. [DOI] [PubMed]
- 781.Paterson C, Ewings P, Brazier J.et al Treating dyspepsia with acupuncture and homeopathy: reflections on a pilot study by researchers, practitioners and participants. Complement Ther Med 20031178–84. [DOI] [PubMed] [Google Scholar]
- 782.Bate C M, Riley S A, Chapman R W.et al Evaluation of omeprazole as a cost‐effective diagnostic test for gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 19991359–66. [DOI] [PubMed] [Google Scholar]
- 783.Dominitz J A, Young J C, Boyko E J. Outcomes of infants born to mothers with inflammatory bowel disease: a population‐based cohort study. Am J Gastroenterol 200297641–648. [DOI] [PubMed] [Google Scholar]
- 784.De Lillo A R, Rose S. Functional bowel disorders in the geriatric patient: constipation, fecal impaction, and fecal incontinence. Am J Gastroenterol 200095901–905. [DOI] [PubMed] [Google Scholar]
- 785.Hislop W S, Heading R. Impact of alcohol related disease and inpatient workload of gastroenterologists in Scotland. Scott Med J 20044957–60. [DOI] [PubMed] [Google Scholar]
- 786.Plevris J, Schina M, Hayes P. The management of acute liver failure [review]. Aliment Pharmacol Ther 199812405–418. [DOI] [PubMed] [Google Scholar]
- 787.Morris C. Non‐ulcer dyspepsia. J Psychosom Res 199135129–140. [DOI] [PubMed] [Google Scholar]
- 788.American Gastroenterogical Association The burden of gastrointestinal diseases. New York, USA: American Gastroenterogical Association, 2001
- 789.Ghanchi F D, Rembacken B J. Inflammatory bowel disease and the eye. Surv Ophthalmol 200348663–676. [DOI] [PubMed] [Google Scholar]
- 790.Mamula P, Telega G W, Markowitz J E.et al Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol 2002972005–2010. [DOI] [PubMed] [Google Scholar]
- 791.Wong W M, Lai K C, Lam K F.et al Prevalence, clinical spectrum and health care utilisation of gastro‐oesophageal reflux disease in a Chinese population: a population‐based study. Aliment Pharmacol Ther 200318595–604. [DOI] [PubMed] [Google Scholar]
- 792.Pimentel M, Chow E J, Lin H C. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000953503–3506. [DOI] [PubMed] [Google Scholar]
- 793.Russel M G. Changes in the incidence of inflammatory bowel disease: what does it mean? Eur J Intern Med 200011191–196. [DOI] [PubMed] [Google Scholar]
- 794.Moum B, Ekbom A. Epidemiology of inflammatory bowel disease—methodological considerations. Dig Liver Dis 200234364–369. [DOI] [PubMed] [Google Scholar]
- 795.Farrokhyar F, Swarbrick E T, Irvine E J. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol 2001362–15. [DOI] [PubMed] [Google Scholar]
- 796.Lapane K L, Spooner J J, Pettitt D. The effect of nonsteroidal anti‐inflammatory drugs on the use of gastroprotective medication in people with arthritis. Am J Manag Care 20017402–408. [PubMed] [Google Scholar]
- 797.Chiang D T, Anozie A, Fleming W R.et al Comparative study on acute pancreatitis management. ANZ J Surg 200474218–221. [DOI] [PubMed] [Google Scholar]
- 798.Fass R, Fullerton S, Tung S.et al Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol 2000951195–1200. [DOI] [PubMed] [Google Scholar]
- 799.Parry S D, Stansfield R, Jelley D.et al Is irritable bowel syndrome more common in patients presenting with bacterial gastroenteritis? A community‐based, case‐control study. Am J Gastroenterol 200398327–331. [DOI] [PubMed] [Google Scholar]
- 800.Bernstein C N, Blanchard J F, Rawsthorne P.et al The prevalence of extraintestinal diseases in inflammatory bowel disease: a population‐based study. Am J Gastroenterol 2001961116–1122. [DOI] [PubMed] [Google Scholar]
- 801.Payne N, Saul C. What common disorders do those reporting limiting long‐term illness experience, and what is their survival and health service utilization experience? J Public Health Med 200022324–329. [DOI] [PubMed] [Google Scholar]
- 802.Ruigomez A, Wallander M A, Johansson S.et al One‐year follow‐up of newly diagnosed irritable bowel syndrome patients. Aliment Pharmacol Ther 1999131097–1102. [DOI] [PubMed] [Google Scholar]
- 803.ONS Cancer trends in England and Wales, 1950–1999. 2001 ( http://www.ons.org/, accessed 27 December 2006)
- 804.Blower A L, Brooks A, Fenn G C.et al Emergency admissions for upper gastrointestinal disease and their relation to NSAID use. Aliment Pharmacol Ther 199711283–291. [DOI] [PubMed] [Google Scholar]
- 805.Sawczenko A, Sandhu B K, Logan R F.et al Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet 20013571093–1094. [DOI] [PubMed] [Google Scholar]
- 806.Griffin M, McCulloch P, Davies S.et alAUGIS database report 2002. London: Association of Upper Gastrointestinal Surgeons of GB and Ireland, 2002
- 807.Cooper D L, Smith G E, O'Brien S J.et al What can analysis of calls to NHS direct tell us about the epidemiology of gastrointestinal infections in the community? J Infect 200346101–105. [DOI] [PubMed] [Google Scholar]
- 808.CSCG Board Meeting Response to NICE service guidance: upper gastro‐intestinal cancers. CSCG Board Meeting 2003
- 809.Jones R H. Likely impacts of recruitment site and methodology on characteristics of enrolled patient population: Irritable Bowel Syndrome Clinical Trial Design. Am J Med 199910785–90S. [DOI] [PubMed] [Google Scholar]
- 810.Department of H e a l t h, NICEImproving outcomes in colorectal cancers. London: Department of Health and NICE, 2004
- 811.Ashorn M. Gastrointestinal diseases in the paediatric age groups in Europe: epidemiology and impact on healthcare. Aliment Pharmacol Ther 200318(Suppl 3)80–83. [DOI] [PubMed] [Google Scholar]
- 812.Delvaux M. Digestive health in the elderly: faecal incontinence in adults. Aliment Pharmacol Ther 200318(Suppl 3)84–89. [DOI] [PubMed] [Google Scholar]
- 813.Delvaux M. Functional bowel disorders and irritable bowel syndrome in Europe. Aliment Pharmacol Ther 200318(Suppl 3)75–79. [DOI] [PubMed] [Google Scholar]
- 814.Munkholm P. The incidence and prevalence of colorectal cancer in inflammatory bowel disease [review]. Aliment Pharmacol Ther 200318(Suppl 2)1–5. [DOI] [PubMed] [Google Scholar]
- 815.Davis D L, Hoel D, Fox J.et al International trends in cancer mortality in France, West Germany, Italy, Japan, England and Wales, and the USA. Lancet 1990336474–481. [DOI] [PubMed] [Google Scholar]
- 816.Stanley K, Stjernsward J, Koroltchouk V. Cancers of the stomach, lung and breast: mortality trends and control strategies. World Health Stat Q 198841107–114. [PubMed] [Google Scholar]
- 817.Khan S A, Taylor‐Robinson S D, Toledano M B.et al Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 200237806–813. [DOI] [PubMed] [Google Scholar]
- 818.Cucino C, Sonnenberg A. Occupational mortality from inflammatory bowel disease in the United States 1991–1996. Am J Gastroenterol 2001961101–1105. [DOI] [PubMed] [Google Scholar]
- 819.Maroun J, Ng E, Berthelot J M.et al Lifetime costs of colon and rectal cancer management in Canada. Chronic Dis Can 20032491–101. [PubMed] [Google Scholar]
- 820.Fernandez E, La Vecchia C, Porta M.et al Trends in pancreatic cancer mortality in Europe, 1955–1989. Int J Cancer 199457786–792. [DOI] [PubMed] [Google Scholar]
- 821.La Vecchia C, Lucchini F, Franceschi S.et al Trends in mortality from primary liver cancer in Europe. Eur J Cancer 200036909–915. [DOI] [PubMed] [Google Scholar]
- 822.Maheswaran R, Strachan D P, Dodgeon B.et al A population‐based case‐control study for examining early life influences on geographical variation in adult mortality in England and Wales using stomach cancer and stroke as examples. Int J Epidemiol 200231375–382. [PubMed] [Google Scholar]
- 823.Payne J N, Coy J, Milner P C.et al Are deprivation indicators a proxy for morbidity? A comparison of the prevalence of arthritis, depression, dyspepsia, obesity and respiratory symptoms with unemployment rates and Jarman scores. J Public Health Med 199315161–170. [DOI] [PubMed] [Google Scholar]
- 824.Sharp L, Black R J, Muir C S.et al Will the Scottish cancer target for the year 2000 be met? The use of cancer registration and death records to predict future cancer incidence and mortality in Scotland. Br J Cancer 1996731115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Pye J K, Crumplin M K, Charles J.et al One‐year survey of carcinoma of the oesophagus and stomach in Wales. Br J Surg 200188278–285. [DOI] [PubMed] [Google Scholar]
- 826.Association of Upper Gastrointestinal Surgeons of GB and Ireland Provision of upper gastrointestinal surgical services. London: Association of Upper Gastrointestinal Surgeons of GB and Ireland, 1999
- 827.Tekkis P P, Poloniecki J D, Thompson M R.et al Operative mortality in colorectal cancer: prospective national study. BMJ 20033271196–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Payne J N, Coy J, Patterson S.et al Is use of hospital services a proxy for morbidity? A small area comparison of the prevalence of arthritis, depression, dyspepsia, obesity, and respiratory disease with inpatient admission rates for these disorders in England. J Epidemiol Community Health 19944874–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.McCulloch P, Ward J, Tekkis P P.et al Mortality and morbidity in gastro‐oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ 20033271192–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Talley N J, Holtmann G, Agreus L.et al Gastrointestinal symptoms and subjects cluster into distinct upper and lower groupings in the community: a four nations study. Am J Gastroenterol 2000951439–1447. [DOI] [PubMed] [Google Scholar]
- 831.Levenstein S, Li Z, Almer S.et al Cross‐cultural variation in disease‐related concerns among patients with inflammatory bowel disease. Am J Gastroenterol 2001961822–1830. [DOI] [PubMed] [Google Scholar]
- 832.Longobardi T, Jacobs P, Bernstein C N. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol 2003981064–1072. [DOI] [PubMed] [Google Scholar]
- 833.Longobardi T, Jacobs P, Wu L.et al Work losses related to inflammatory bowel disease in Canada: results from a National Population Health Survey. Am J Gastroenterol 200398844–849. [DOI] [PubMed] [Google Scholar]
- 834.Danese S, Sans M, Fiocchi C. Inflammatory autoimmune bowel disease: the role of environmental factors. Autoimmun Rev 20043394–400. [DOI] [PubMed] [Google Scholar]
- 835.McKinney P, Sharp L, Macfarlane G.et al Oesophageal and gastric cancer in Scotland 1960–1990. Br J Cancer 199571411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Dean B B, Crawley J A, Schmitt C M.et al The burden of illness of gastro‐oesophageal reflux disease: impact on work productivity. Aliment Pharmacol Ther 2003171309–1317. [DOI] [PubMed] [Google Scholar]
- 837.Bernstein C N, Kraut A, Blanchard J F.et al The relationship between inflammatory bowel disease and socioeconomic variables. Am J Gastroenterol 2001962117–2125. [DOI] [PubMed] [Google Scholar]
- 838.Vaughn C, Leff J, Sarner M. Relatives' expressed emotion and the course of inflammatory bowel disease. J Psychosom Res 199947461–469. [DOI] [PubMed] [Google Scholar]
- 839.Crane C, Martin M. Social learning, affective state and passive coping in irritable bowel syndrome and inflammatory bowel disease. Gen Hosp Psychiatry 20042650–58. [DOI] [PubMed] [Google Scholar]
- 840.Casati J, Toner B B. Psychosocial aspects of inflammatory bowel disease. Biomed Pharmacother 200054388–393. [DOI] [PubMed] [Google Scholar]
- 841.Sewitch M J, Abrahamowicz M, Bitton A.et al Psychological distress, social support, and disease activity in patients with inflammatory bowel disease. Am J Gastroenterol 2001961470–1479. [DOI] [PubMed] [Google Scholar]
- 842.Soo S, Moayyedi P, Deeks J.et al Psychological interventions for non‐ulcer dyspepsia (Cochrane review). Cochrane Database Syst Rev 2004(3)CD002301. [DOI] [PubMed]
- 843.Guthrie E. Psychodynamic‐interpersonal therapy for functional bowel disorders. International Congress Series 20021241121–125. [Google Scholar]
- 844.Kisely S. Multiple readmissions: an analysis of patients using routine contracting data. Clinician in Management 19998211–219. [Google Scholar]
- 845.Tojek T M, Lumley M A, Corlis M.et al Maternal correlates of health status in adolescents with inflammatory bowel disease. J Psychosom Res 200252173–179. [DOI] [PubMed] [Google Scholar]
- 846.Jahnsen J, Falch J A, Mowinckel P.et al Body composition in patients with inflammatory bowel disease: a population‐based study. Am J Gastroenterol 2003981556–1562. [DOI] [PubMed] [Google Scholar]
- 847.Hatch M L. Conceptualization and treatment of bowel obsessions: two case reports. Behav Res Ther 199735253–257. [DOI] [PubMed] [Google Scholar]
- 848.Guthrie E, Creed F, Fernandes L.et al Cluster analysis of symptoms and health seeking behaviour differentiates subgroups of patients with severe irritable bowel syndrome. Gut 2003521616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Drossman D A. Do psychosocial factors define symptom severity and patient status in irritable bowel syndrome? Am J Med 199910741–50S. [DOI] [PubMed] [Google Scholar]
- 850.Pachler J, Wille‐Jorgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev 2004(3)CD004323. [DOI] [PubMed]
- 851.Yacavone R F, Locke G R, 3rd, Provenzale D T.et al Quality of life measurement in gastroenterology: what is available? Am J Gastroenterol 200196285–297. [DOI] [PubMed] [Google Scholar]
- 852.Simren M, Axelsson J, Gillberg R.et al Quality of life in inflammatory bowel disease in remission: the impact of IBS‐like symptoms and associated psychological factors. Am J Gastroenterol 200297389–396. [DOI] [PubMed] [Google Scholar]
- 853.Hahn B A, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion 19996077–81. [DOI] [PubMed] [Google Scholar]
- 854.Blondel‐Kucharski, Chircop C, Marquis P.et al Health‐related quality of life in Crohn's disease: a prospective longitudinal study in 231 patients. Am J Gastroenterol 2001962915–2920. [DOI] [PubMed] [Google Scholar]
- 855.Gonsalkorale W M, Toner B B, Whorwell P J. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res 200456271–278. [DOI] [PubMed] [Google Scholar]
- 856.El‐Serag H B, Olden K, Bjorkman D. Health‐related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther 2002161171–1185. [DOI] [PubMed] [Google Scholar]
- 857.O'Keefe E A, Talley N J, Zinsmeister A R.et al Bowel disorders impair functional status and quality of life in the elderly: a population‐based study. J Gerontol A Biol Sci Med Sci 199550M184–M189. [DOI] [PubMed] [Google Scholar]
- 858.Gralnek I, Hays R, Kilbourne A.et al The impact of irritable bowel syndrome on health‐related quality of life. Gastroenterology 2000119654–660. [DOI] [PubMed] [Google Scholar]
- 859.Pfau P R, Cooper G S, Carlson M D.et al Success and shortcomings of a clinical care pathway in the management of acute nonvariceal upper gastrointestinal bleeding. Am J Gastroenterol 200499425–431. [DOI] [PubMed] [Google Scholar]
- 860.Slade P E, Davidson A R, Steel A.et al Reducing the endoscopic workload: does serological testing for Helicobacter pylori help? Eur J Gastroenterol Hepatol 199911857–862. [PubMed] [Google Scholar]
- 861.Lamy V, McNamara D. Gastroenterology and hepatology services in Europe. Aliment Pharmacol Ther 200318(Suppl 3)90–92. [DOI] [PubMed] [Google Scholar]
- 862.Rayner H C, Temple R M, Marshall T.et al A comparison of hospital readmission rates between two general physicians with different outpatient review practices. BMC Health Serv Res 2002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Westbrook J I, Rushworth R L, Rob M I.et al Diagnostic procedures and health outcomes. Upper gastrointestinal tract investigations in the elderly. Med J Aust 1993159242–245. [DOI] [PubMed] [Google Scholar]
- 864.Grassi A. Tracking regional endoscopy activity. Gastrointest Endosc 200255298–300. [DOI] [PubMed] [Google Scholar]
- 865.BSG Future requirements for colonoscopy in Britain. Report by the Endoscopy Section Committee of the British Society of Gastroenterology. Gut 198728772–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Parente F, Bargiggia S, Bianchi Porro B. Prospective audit of gastroscopy under the ‘three day rule': a regional initiative in Italy to reduce waiting time for suspected malignancy. Aliment Pharmacol Ther 2002161011–1014. [DOI] [PubMed] [Google Scholar]
- 867.Quine M A, Bell G D, McCloy R F.et al Prospective audit of perforation rates following upper gastrointestinal endoscopy in two regions of England. Br J Surg 199582530–533. [DOI] [PubMed] [Google Scholar]
- 868.Mulcahy H E, Hennessy E, Connor P.et al Changing patterns of sedation use for routine out‐patient diagnostic gastroscopy between 1989 and 1998. Aliment Pharmacol Ther 200115217–220. [DOI] [PubMed] [Google Scholar]
- 869.Rockey D C, Paulson E, Niedzwiecki D.et al Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005365305–311. [DOI] [PubMed] [Google Scholar]
- 870.Halligan S, Atkin W. Unbiased studies are needed before virtual colonoscopy can be dismissed. Lancet 2005365275–276. [DOI] [PubMed] [Google Scholar]
- 871.Meineche‐Schmidt V. Empiric treatment with high and standard dose of omeprazole in general practice: two‐week randomized placebo‐controlled trial and 12‐month follow‐up of health‐care consumption. Am J Gastroenterol 2004991050–1058. [DOI] [PubMed] [Google Scholar]
- 872.Milne R P, Logan R, Harwood D.et al Helicobacter pylori and upper gastrointestinal disease: a survey of gastroenterologists in the United Kingdom. Gut 199537314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Forman D, Stockton D, Moller H.et al Cancer prevalence in the UK: results from the EUROPREVAL study. Ann Oncol 200314648–654. [DOI] [PubMed] [Google Scholar]
- 874.Sant M, Capocaccia R, Coleman M P.et al Cancer survival increases in Europe, but international differences remain wide. Eur J Cancer 2001371659–1667. [DOI] [PubMed] [Google Scholar]
- 875.Askling J, Dickman P W, Karlen P.et al Colorectal cancer rates among first‐degree relatives of patients with inflammatory bowel disease: a population‐based cohort study. Lancet 2001357262–266. [DOI] [PubMed] [Google Scholar]
- 876.Lynch H T, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003348919–932. [DOI] [PubMed] [Google Scholar]
- 877.Rhodes J M, Campbell B J. Inflammation and colorectal cancer: IBD‐associated and sporadic cancer compared. Trends Mol Med 2002810–16. [DOI] [PubMed] [Google Scholar]
- 878.Lewis J D. Prevention and treatment of colorectal cancer: pay now or pay later. Ann Intern Med 2000133647–649. [DOI] [PubMed] [Google Scholar]
- 879.Sonnenberg A, Inadomi J M, Becker L A. Economic analysis of step‐wise treatment of gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 1999131003–1013. [DOI] [PubMed] [Google Scholar]
- 880.Polito J, II, Rees R, Childs B.et al Preliminary evidence for genetic anticipation in Crohn's disease. Lancet 1996347798–800. [DOI] [PubMed] [Google Scholar]
- 881.Morris‐Yates A, Talley N J, Boyce P M.et al Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol 1998931311–1317. [DOI] [PubMed] [Google Scholar]
- 882.Faybush E M, Blanchard J F, Rawsthorne P.et al Generational differences in the age at diagnosis with Ibd: genetic anticipation, bias, or temporal effects. Am J Gastroenterol 200297636–640. [DOI] [PubMed] [Google Scholar]
- 883.Greer S.Four way bet: how devolution has led to four different models for the NHS. London: The Constitution Unit, University College London, 2004
- 884.Lilford R, Mohammed M A, Spiegelhalter D.et al Use and misuse of process and outcome data in managing performance of acute medical care: avoiding institutional stigma. Lancet 20043631147–1154. [DOI] [PubMed] [Google Scholar]
- 885.Rockall T A, Logan R F, Devlin H B.et al Risk assessment after acute upper gastrointestinal haemorrhage. Gut 199638316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Johansen J F, Schmitt C M, Deas T M., Jret al Quality and outcomes assessment in Gastrointestinal Endoscopy. Gastrointest Endosc 200052827–830. [PubMed] [Google Scholar]
- 887.Dougall A, Russell A, Rubin G.et al Rethinking patient satisfaction: patient experiences of an open access flexible sigmoidoscopy service. Soc Sci Med 20005053–62. [DOI] [PubMed] [Google Scholar]
- 888.Feuer D, Broadley K. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. The Cochrane Library 20043 [DOI] [PubMed] [Google Scholar]
- 889.Fletcher D R. Peptic disease: Can we afford current management? ANZ J Surg 19976775–80. [DOI] [PubMed] [Google Scholar]
- 890.Bodenheimer T, Wagner E H, Grumbach K. Improving primary care for patients with chronic illness. JAMA 20022881775–1779. [DOI] [PubMed] [Google Scholar]
- 891.van der Eijk I, Stockbrugger R, Russel M. Influence of quality of care on quality of life in inflammatory bowel disease (IBD): literature review and studies planned. Eur J Intern Med 200011228–234. [DOI] [PubMed] [Google Scholar]
- 892.Gilmore I T, Burroughs A, Murray‐Lyon I M.et al Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut 199536437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Kurlberg G, Forssell H, Aly A. National registry of patients with short bowel syndrome. Transplant Proc 200436253–254. [DOI] [PubMed] [Google Scholar]
- 894.Palmer K, Morris A I. A snapshot of colonoscopy practice in England: stimulus for improvement. Gut 200453163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Macarthur D C, Nixon S J, Aitken R J. Avoidable deaths still occur after large bowel surgery. Scottish Audit of Surgical Mortality, Royal College of Surgeons of Edinburgh. Br J Surg 19988580–83. [DOI] [PubMed] [Google Scholar]
- 896.van der Eijk I, Verheggen F W, Russel M G.et al “Best practice” in inflammatory bowel disease: an international survey and audit. Eur J Intern Med 200415113–120. [DOI] [PubMed] [Google Scholar]
- 897.Eaden J A, Ward B A, Mayberry J F. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc 200051123–128. [DOI] [PubMed] [Google Scholar]
- 898.Scott C M, Verhoef M J, Hilsden R J. Inflammatory bowel disease patients' decisions to use complementary therapies: links to existing models of care. Complement Ther Med 20031122–27. [DOI] [PubMed] [Google Scholar]
- 899.Hinton J. Which patients with terminal cancer are admitted from home care? Palliat Med 19948197–210. [DOI] [PubMed] [Google Scholar]
- 900.Barrett J, Goh S, Todd C.et al A description of an intermediate care service using routinely collected data. J Nurs Manag 200210221–227. [DOI] [PubMed] [Google Scholar]
- 901.Gill A J, Martin I G. Survival from upper gastrointestinal cancer in New Zealand: the effect of distance from a major hospital, socio‐economic status, ethnicity, age and gender. ANZ J Surg 200272643–646. [DOI] [PubMed] [Google Scholar]
- 902.Whynes D K, Thornton P. Measuring concentration in primary care. Health Care Manag Sci 2000343–49. [DOI] [PubMed] [Google Scholar]
- 903.Gonsalkorale W M, Houghton L, Whorwell P. Hypnotherapy in irritable bowel syndrome: a large‐scale audit of a clinical service with examination of factors influencing responsiveness. Am J Gastroenterol 200297954–961. [DOI] [PubMed] [Google Scholar]
- 904.van Dam J, Cotton P, Johnson C D.et al AGA future trends report: CT colonography. Gastroenterology 2004127970–984. [DOI] [PubMed] [Google Scholar]
- 905.Podolsky D. The AGA and future trends in gastroenterology: CT colonography. Gastroenterology 2004127985–986. [DOI] [PubMed] [Google Scholar]
- 906.Baron T H, Kimery B D, Sorbi D.et al Strategies to address increased demand for colonoscopy: guidelines in an open endoscopy practice. Clin Gastroenterol Hepatol 20042178–182. [DOI] [PubMed] [Google Scholar]
- 907.Cheung W Y, Dove J, Russell I T.et al Shared care in gastroenterology: GPs' views of open access to out‐patient follow‐up for patients with inflammatory bowel disease. Fam Pract 20021953–56. [DOI] [PubMed] [Google Scholar]
- 908.Smith G D, Watson R, Roger D.et al Impact of a nurse‐led counselling service on quality of life in patients with inflammatory bowel disease. J Adv Nurs 200238152–160. [DOI] [PubMed] [Google Scholar]
- 909.Ilnyckyj A, Graff L A, Blanchard J F.et al Therapeutic value of a gastroenterology consultation in irritable bowel syndrome. Aliment Pharmacol Ther 200317871–880. [DOI] [PubMed] [Google Scholar]
- 910.Pasricha P J. The future of therapeutic endoscopy. Clin Gastroenterol Hepatol 20042286–289. [DOI] [PubMed] [Google Scholar]
- 911.Axon A. Open access endoscopy in Britain: a service in evolution. Gastrointest Endosc 199848653–658. [PubMed] [Google Scholar]
- 912.Department of Health Improving outcomes in upper gastro‐intestinal cancers. London: Department of Health, 2001
- 913.Talley N J, Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet 2002360555–564. [DOI] [PubMed] [Google Scholar]
- 914.Nord N. Gastrointestinal endoscopy at the dawn of the new millennium. Gastroenterol Endosc 199950721–725. [DOI] [PubMed] [Google Scholar]
- 915.Abuksis G, Mor M, Segal N.et al A patient education program is cost‐effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001961786–1790. [DOI] [PubMed] [Google Scholar]
- 916.Lewin van den Broek N T, Numans M E, Buskens E.et al A randomised controlled trial of four management strategies for dyspepsia: relationships between symptom subgroups and strategy outcome. Br J Gen Pract 200151619–624. [PMC free article] [PubMed] [Google Scholar]
- 917.Delaney B C, Moayyedi P. Dyspepsia—HCNA. Health Care Needs Assessment, Updated. Available at http://hcna.radcliffe‐oxford.com/dyspepsia.htm, accessed 18 January 2007
- 918.Heaney A, Collins J S, Watson R G. Open access gastroscopy—3 year experience of a new service. Ir J Med Sci 1998167136–137. [DOI] [PubMed] [Google Scholar]
- 919.Hansen J M, Bytzer P, Bondesen S.et al Efficacy and outcome of an open access endoscopy service. Dan Med Bull 199138288–290. [PubMed] [Google Scholar]
- 920.Paisley A M, Madhavan K K, Paterson‐Brown S.et al Role of the surgical trainee in upper gastrointestinal resectional surgery. Ann R Coll Surg Engl 19998140–45. [PMC free article] [PubMed] [Google Scholar]
- 921.Chin K, Newton J. Survey of training in minimal access surgery in the West Midlands region of the UK. Gynaecol Endosc 19965329–333. [Google Scholar]
- 922.Pardo A, Durandez R, Hernandez M.et al Impact of physician specialty on the cost of nonvariceal upper GI bleeding care. Am J Gastroenterol 2002971535–1542. [DOI] [PubMed] [Google Scholar]
- 923.Waye J D, Toouli J, Guelrud M.et al Who is permitted to do endoscopy? Gastrointest Endosc 200153267–269. [DOI] [PubMed] [Google Scholar]
- 924.Bini E J, Weinshel E H, Generoso R.et al Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology 2001341089–1095. [DOI] [PubMed] [Google Scholar]
- 925.Eaden J, Abrams K, McKay H.et al Inter‐observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol 2001194152–157. [DOI] [PubMed] [Google Scholar]
- 926.Barrison I, Bramble M, Wilkinson M.et alProvision of endoscopy related services in district general hospitals. BSG Working Party 2001
- 927.Lim A G, Martin R M, Montileone M.et al Helicobacter pylori serology and the management of young dyspeptics: a UK survey of gastroenterologists and general practitioners with an interest in gastroenterology. Aliment Pharmacol Ther 199711299–303. [DOI] [PubMed] [Google Scholar]
- 928.Cockel R, Colin Jones D G, Schiller K F. Gastrointestinal endoscopy services—a review of the 70s with predictions for the 80s. Health Trends 19821446–49. [PubMed] [Google Scholar]
- 929.Knight‐Davis D K, Fouweather M G, Srivastava E D.et al Cross cover for physicians: an additional burden. J R Coll Physicians Lond 199832130–132. [PMC free article] [PubMed] [Google Scholar]
- 930.Nightingale J, Hogg P. The gastrointestinal advanced practitioner: an emerging role for the modern radiology service. Radiography 20039151–160. [Google Scholar]
- 931.Farthing M J, Williams R, Swan C H.et al Nature and standards of gastrointestinal and liver services in the United Kingdom. Gut 1993341728–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932.Colin‐Thome D. The NHS plan: general practitioners with special interests. Br J Cardiol 20029359–360. [Google Scholar]
- 933.Birch K.Developing practitioners with special interest (PwSI) services: managing the risks. Healthcare Standards Unit, Keele, NHS and NatPaCT 200453553–556. [Google Scholar]
- 934.Pearson S D, Moreno R, Trnka Y. Informal consultations provided to general internists by the gastroenterology department of an HMO. J Gen Intern Med 199813435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Williams S, Ryan D, Price D.et al General practitioners with a special clinical interest: a model for improving respiratory disease management. Br J Gen Pract 200252838–843. [PMC free article] [PubMed] [Google Scholar]
- 936.Department of Health and Royal College of General Practitioners Guidelines for the appointment of GPSI. With the role of service development. London: Department of Health and Royal College of General Practitioners, 2002
- 937.Ryan D. Respiratory disease: why GPSIs are a breath of fresh air. Primary Care Report 2003516–17. [Google Scholar]
- 938.Gruffydd‐Jones K. The framework for general practitioners with a special interest in respiratory medicine. Primary Care Resp J 20031235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Kernick D P. The economic perspective of respiratory general practitioners with a special interest (GPwSIs): proceed with caution. Primary Care Resp J 200312108–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940.Richardson C. Benefit or burden? The rise of GPs with a special interest. Primary Care Report 2002438–39. [Google Scholar]
- 941.Barry J D, Edwards P, Lewis W G.et al Special interest radiology improves the perceived preoperative stage of gastric cancer. Clin Radiol 200257984–988. [DOI] [PubMed] [Google Scholar]
- 942.Fullerton S. Functional digestive disorders (FDD) in the year 2000—economic impact. Eur J Surg 1998(Suppl 582)62–64. [DOI] [PubMed]
- 943.Robinson A, Lee V, Kennedy A.et al A randomised controlled trial of self‐help interventions in patients with a primary care diagnosis of IBS. Gut 200555643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Norton C, Kamm M A. Specialist nurses in gastroenterology. J R Soc Med 200295331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945.Wade B E. Colostomy patients: psychological adjustment at 10 weeks and 1 year after surgery in districts which employed stoma‐care nurses and districts which did not. J Adv Nurs 1990151297–1304. [DOI] [PubMed] [Google Scholar]
- 946.Erwin‐Toth P, Spencer M. A survey of patient perception of quality of care. J ET Nurs 199118122–125. [DOI] [PubMed] [Google Scholar]