Abstract
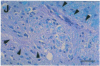
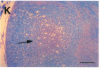
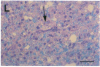
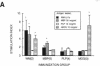
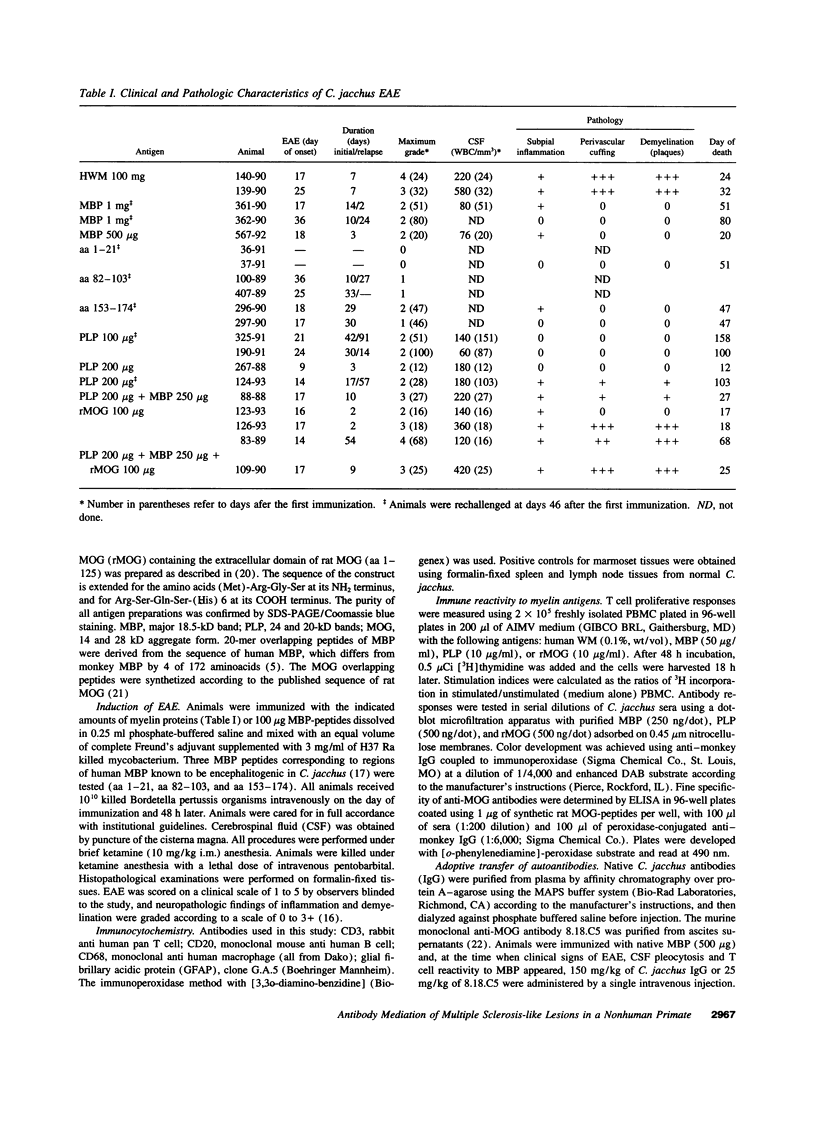
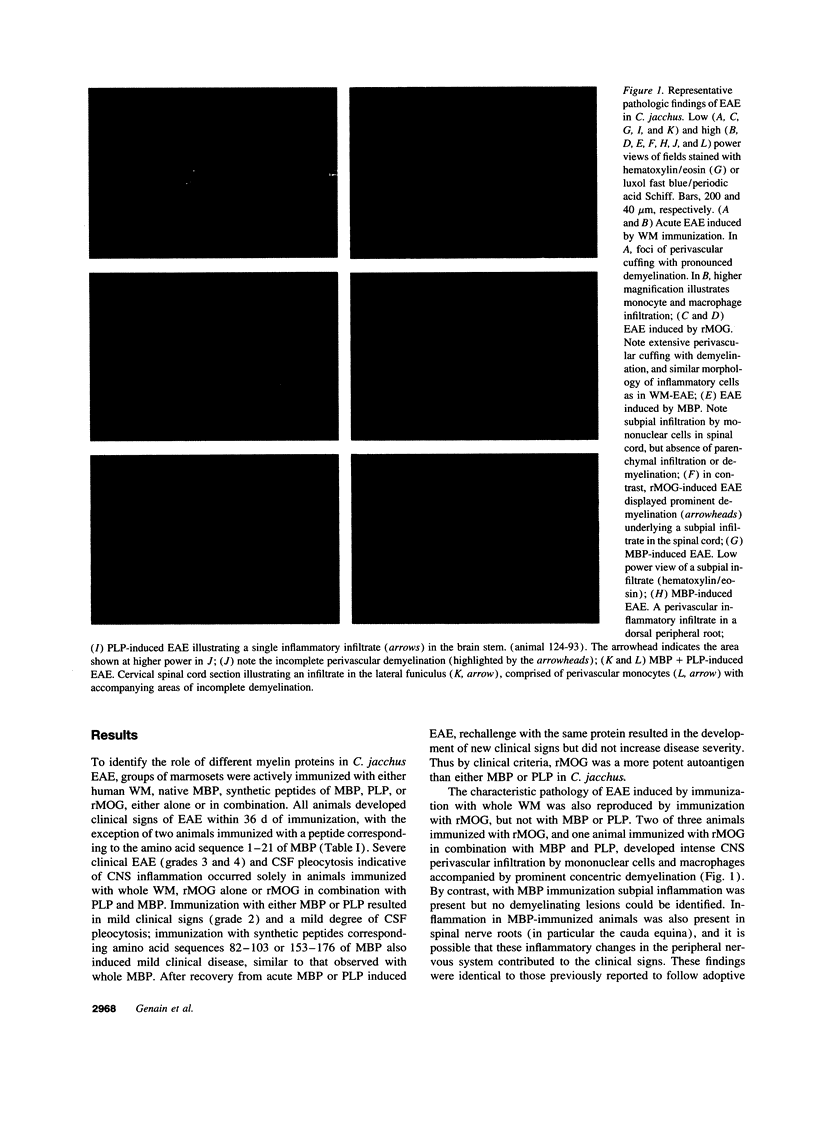
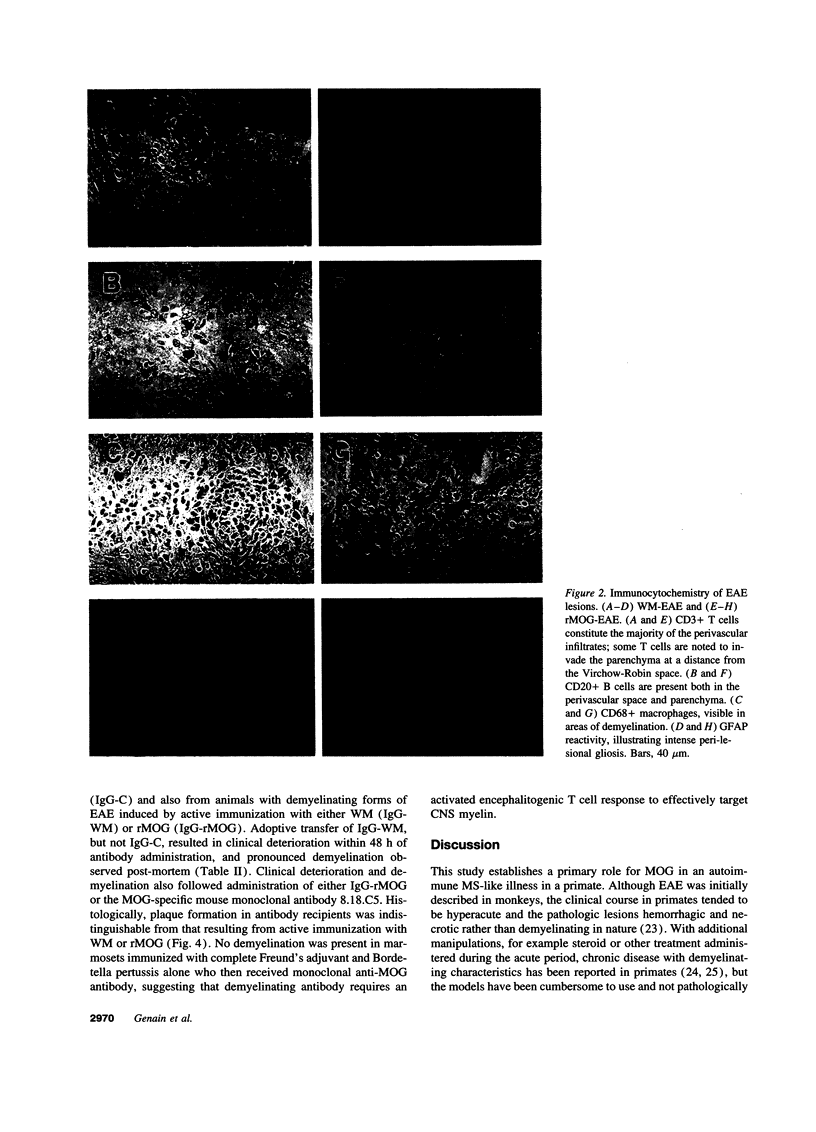
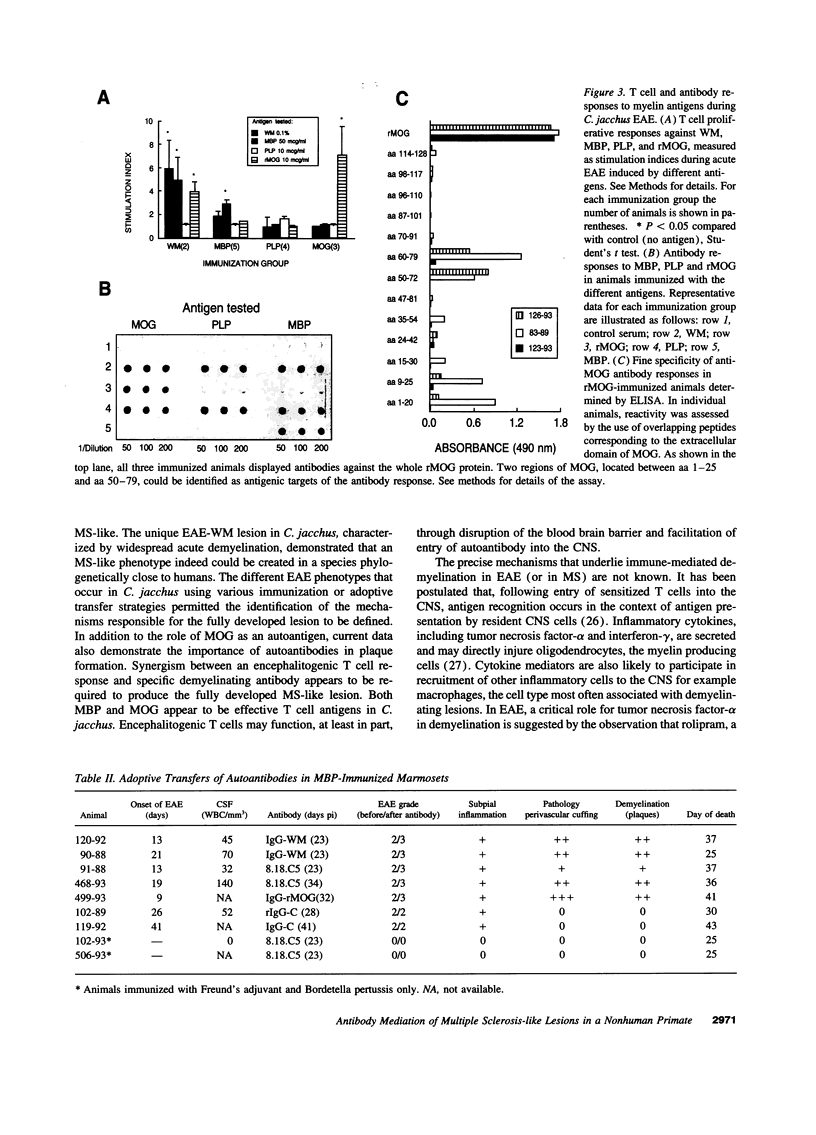
In the human disease multiple sclerosis (MS), the immune mechanisms responsible for selective destruction of central nervous system myelin are unknown. In the common marmoset Callithrix jacchus, a unique demyelinating form of experimental allergic encephalomyelitis resembling MS can be induced by immunization with whole myelin. Here we show that the MS-like lesion can be reproduced by immunization against the extracellular domain of a single myelin protein, myelin/oligodendrocyte glycoprotein (MOG). By contrast, immunization against the quantitatively major myelin proteins myelin basic protein or proteolipid protein results in inflammation but little or no demyelination. Furthermore, in the presence of encephalitogenic (e.g., disease-inducing) T cells, the fully demyelinated lesion is reconstructed by systemic administration of IgG purified from whole myelin-, or MOG-immunized animals, and equally by a monoclonal antibody against MOG, but not by control IgG. Encephalitogenic T cells may contribute to the MS-like lesion through disruption of the blood-brain barrier that permits access of demyelinating antibody into the nervous system. The identification of MOG as a major target antigen for autoimmune demyelination in a nonhuman primate should facilitate development of specific immunotherapies for human MS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretta M., Nicklas J. A., Sriram S., Albertini R. J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990 Feb 9;247(4943):718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Amor S., Groome N., Linington C., Morris M. M., Dornmair K., Gardinier M. V., Matthieu J. M., Baker D. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 1994 Nov 15;153(10):4349–4356. [PubMed] [Google Scholar]
- BORNSTEIN M. B., APPEL S. H. TISSUE CULTURE STUDIES OF DEMYELINATION. Ann N Y Acad Sci. 1965 Mar 31;122:280–286. doi: 10.1111/j.1749-6632.1965.tb20212.x. [DOI] [PubMed] [Google Scholar]
- Benveniste E. N. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992 Jul;263(1 Pt 1):C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Brunner C., Lassmann H., Waehneldt T. V., Matthieu J. M., Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2',3'-cyclic nucleotide 3'-phosphodiesterase in the CNS of adult rats. J Neurochem. 1989 Jan;52(1):296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Tuohy V. K., Raine C. S. Development of reactivity to new myelin antigens during chronic relapsing autoimmune demyelination. Cell Immunol. 1993 Feb;146(2):261–269. doi: 10.1006/cimm.1993.1025. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Gardinier M. V., Amiguet P., Linington C., Matthieu J. M. Myelin/oligodendrocyte glycoprotein is a unique member of the immunoglobulin superfamily. J Neurosci Res. 1992 Sep;33(1):177–187. doi: 10.1002/jnr.490330123. [DOI] [PubMed] [Google Scholar]
- Gausas J., Paterson P. Y., Day E. D., Dal Canto M. C. Intact B-cell activity is essential for complete expression of experimental allergic encephalomyelitis in Lewis rats. Cell Immunol. 1982 Sep 15;72(2):360–366. doi: 10.1016/0008-8749(82)90484-1. [DOI] [PubMed] [Google Scholar]
- Genain C. P., Lee-Parritz D., Nguyen M. H., Massacesi L., Joshi N., Ferrante R., Hoffman K., Moseley M., Letvin N. L., Hauser S. L. In healthy primates, circulating autoreactive T cells mediate autoimmune disease. J Clin Invest. 1994 Sep;94(3):1339–1345. doi: 10.1172/JCI117454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain C. P., Roberts T., Davis R. L., Nguyen M. H., Uccelli A., Faulds D., Li Y., Hedgpeth J., Hauser S. L. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Bornstein M. B. Multiple sclerosis: serum gamma globulin and demyelination in organ culture. Neurology. 1980 Jul;30(7 Pt 1):749–754. doi: 10.1212/wnl.30.7.749. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Chofflon M., Kurt-Jones E., Weiner H. L. Interleukin-1 corrects the defective autologous mixed lymphocyte response in multiple sclerosis. Clin Immunol Immunopathol. 1991 Jan;58(1):115–125. doi: 10.1016/0090-1229(91)90153-2. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N., Milo R., Lees M. B., Burger D., Bernard C. C., Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis. Peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993 Dec;92(6):2602–2608. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K., Berger T., Lassmann H., Hinze-Selch D., Zhang Y., Gehrmann J., Reske K., Wekerle H., Linington C. Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100 beta molecule, a calcium binding protein of astroglia. J Exp Med. 1994 Sep 1;180(3):817–829. doi: 10.1084/jem.180.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Linington C., Engelhardt B., Kapocs G., Lassman H. Induction of persistently demyelinated lesions in the rat following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibody. J Neuroimmunol. 1992 Oct;40(2-3):219–224. doi: 10.1016/0165-5728(92)90136-9. [DOI] [PubMed] [Google Scholar]
- Massacesi L., Genain C. P., Lee-Parritz D., Letvin N. L., Canfield D., Hauser S. L. Active and passively induced experimental autoimmune encephalomyelitis in common marmosets: a new model for multiple sclerosis. Ann Neurol. 1995 Apr;37(4):519–530. doi: 10.1002/ana.410370415. [DOI] [PubMed] [Google Scholar]
- Massacesi L., Joshi N., Lee-Parritz D., Rombos A., Letvin N. L., Hauser S. L. Experimental allergic encephalomyelitis in cynomolgus monkeys. Quantitation of T cell responses in peripheral blood. J Clin Invest. 1992 Aug;90(2):399–404. doi: 10.1172/JCI115874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron R. M., Fallis R. J., McFarlin D. E. Alterations in T cell antigen specificity and class II restriction during the course of chronic relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1990 Sep-Oct;29(1-3):73–79. doi: 10.1016/0165-5728(90)90149-h. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Traugott U., Farooq M., Norton W. T., Raine C. S. Experimental autoimmune encephalomyelitis. Augmentation of demyelination by different myelin lipids. Lab Invest. 1984 Oct;51(4):416–424. [PubMed] [Google Scholar]
- Oksenberg J. R., Begovich A. B., Erlich H. A., Steinman L. Genetic factors in multiple sclerosis. JAMA. 1993 Nov 17;270(19):2362–2369. [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch H. S., Hooper C. J., Johnson K. P. CSF antibody to myelin basic protein. Measurement in patients with multiple sclerosis and subacute sclerosing panencephalitis. Arch Neurol. 1980 Apr;37(4):206–209. doi: 10.1001/archneur.1980.00500530044005. [DOI] [PubMed] [Google Scholar]
- Pelfrey C. M., Trotter J. L., Tranquill L. R., McFarland H. F. Identification of a second T cell epitope of human proteolipid protein (residues 89-106) recognized by proliferative and cytolytic CD4+ T cells from multiple sclerosis patients. J Neuroimmunol. 1994 Sep;53(2):153–161. doi: 10.1016/0165-5728(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Pham-Dinh D., Allinquant B., Ruberg M., Della Gaspera B., Nussbaum J. L., Dautigny A. Characterization and expression of the cDNA coding for the human myelin/oligodendrocyte glycoprotein. J Neurochem. 1994 Dec;63(6):2353–2356. doi: 10.1046/j.1471-4159.1994.63062353.x. [DOI] [PubMed] [Google Scholar]
- Piddlesden S. J., Lassmann H., Zimprich F., Morgan B. P., Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993 Aug;143(2):555–564. [PMC free article] [PubMed] [Google Scholar]
- Piddlesden S., Lassmann H., Laffafian I., Morgan B. P., Linington C. Antibody-mediated demyelination in experimental allergic encephalomyelitis is independent of complement membrane attack complex formation. Clin Exp Immunol. 1991 Feb;83(2):245–250. doi: 10.1111/j.1365-2249.1991.tb05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. A., Röyttä M., Yu R. K., Raine C. S., Bornstein M. B. Antisera to different glycolipids induce myelin alterations in mouse spinal cord tissue cultures. Brain Res. 1985 Jul 22;339(1):9–18. doi: 10.1016/0006-8993(85)90616-x. [DOI] [PubMed] [Google Scholar]
- Schluesener H. J., Sobel R. A., Linington C., Weiner H. L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987 Dec 15;139(12):4016–4021. [PubMed] [Google Scholar]
- Scolding N. J., Morgan B. P., Houston W. A., Linington C., Campbell A. K., Compston D. A. Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature. 1989 Jun 22;339(6226):620–622. doi: 10.1038/339620a0. [DOI] [PubMed] [Google Scholar]
- Seboun E., Robinson M. A., Doolittle T. H., Ciulla T. A., Kindt T. J., Hauser S. L. A susceptibility locus for multiple sclerosis is linked to the T cell receptor beta chain complex. Cell. 1989 Jun 30;57(7):1095–1100. doi: 10.1016/0092-8674(89)90046-9. [DOI] [PubMed] [Google Scholar]
- Sergott R. C., Brown M. J., Lisak R. P., Miller S. L. Antibody to myelin-associated glycoprotein produces central nervous system demyelination. Neurology. 1988 Mar;38(3):422–426. doi: 10.1212/wnl.38.3.422. [DOI] [PubMed] [Google Scholar]
- Shaw C. M., Alvord E. C., Jr, Hruby S. Chronic remitting-relapsing experimental allergic encephalomyelitis induced in monkeys with homologous myelin basic protein. Ann Neurol. 1988 Dec;24(6):738–748. doi: 10.1002/ana.410240608. [DOI] [PubMed] [Google Scholar]
- Sommer N., Löschmann P. A., Northoff G. H., Weller M., Steinbrecher A., Steinbach J. P., Lichtenfels R., Meyermann R., Riethmüller A., Fontana A. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med. 1995 Mar;1(3):244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Tourtellotte W. W., Potvin A. R., Fleming J. O., Murthy K. N., Levy J., Syndulko K., Potvin J. H. Multiple sclerosis: measurement and validation of central nervous system IgG synthesis rate. Neurology. 1980 Mar;30(3):240–244. doi: 10.1212/wnl.30.3.240. [DOI] [PubMed] [Google Scholar]
- Tuohy V. K., Lu Z., Sobel R. A., Laursen R. A., Lees M. B. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol. 1989 Mar 1;142(5):1523–1527. [PubMed] [Google Scholar]
- Tuohy V. K. Peptide determinants of myelin proteolipid protein (PLP) in autoimmune demyelinating disease: a review. Neurochem Res. 1994 Aug;19(8):935–944. doi: 10.1007/BF00968703. [DOI] [PubMed] [Google Scholar]
- Vine A. K. Severe periphlebitis, peripheral retinal ischemia, and preretinal neovascularization in patients with multiple sclerosis. Am J Ophthalmol. 1992 Jan 15;113(1):28–32. doi: 10.1016/s0002-9394(14)75748-4. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- Zhang J., Markovic-Plese S., Lacet B., Raus J., Weiner H. L., Hafler D. A. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994 Mar 1;179(3):973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]