Abstract
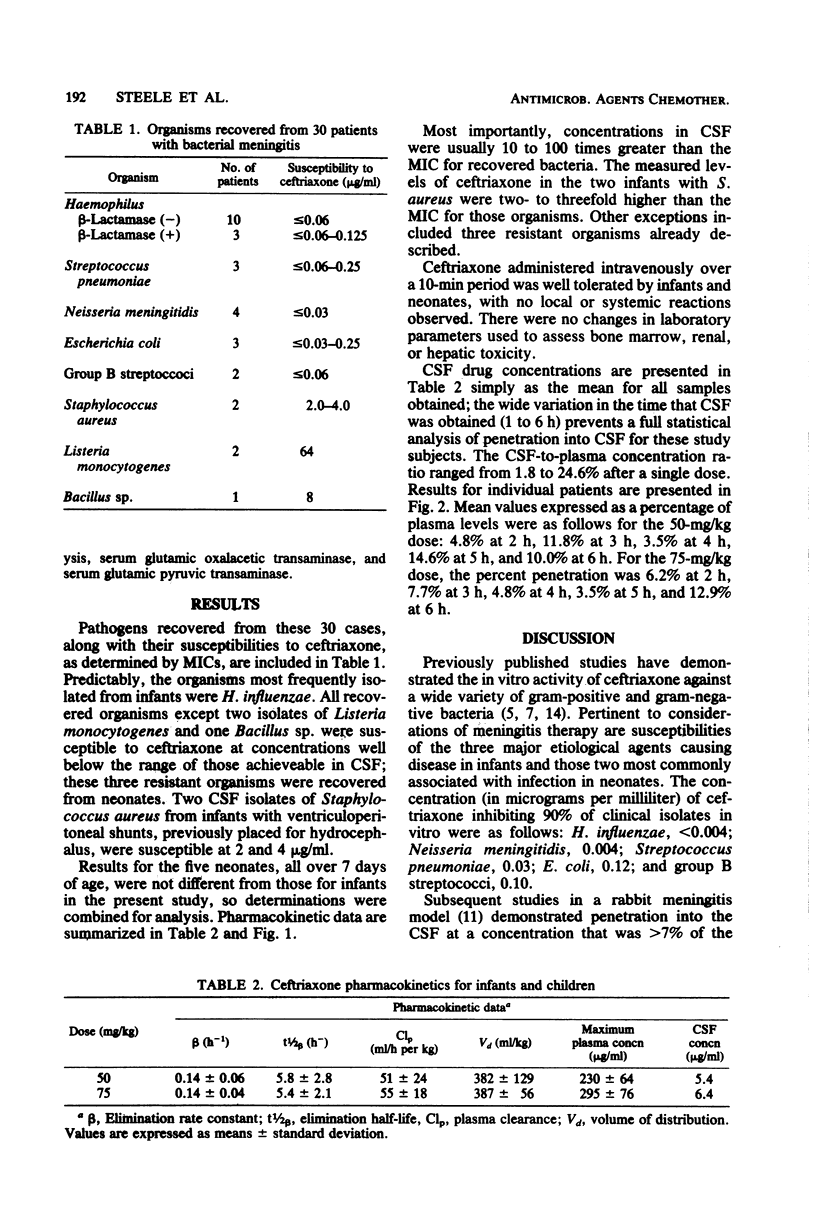
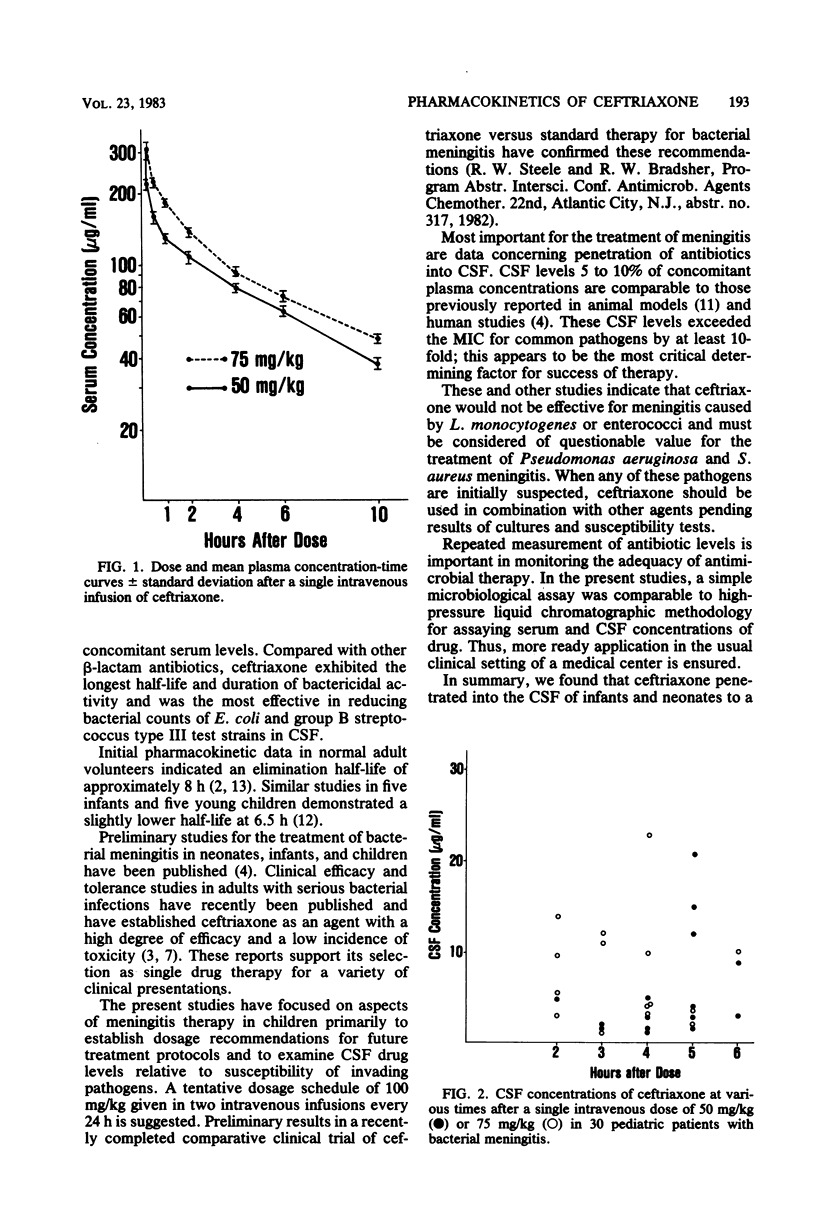
Pharmacokinetics of ceftriaxone after a single dose of 50 or 75 mg/kg were determined in 30 pediatric patients with bacterial meningitis. Data for doses of 50 and 75 mg/kg, respectively, were as follows (mean ± standard deviation): maximum plasma concentrations, 230 ± 64 and 295 ± 76 μg/ml; elimination rate constant, 0.14 ± 0.06 and 0.14 ± 0.04 h−1; harmonic elimination half-life, 5.8 ± 2.8 and 5.4 ± 2.1 h; plasma clearance, 51 ± 24 and 55 ± 18 ml/h per kg; volume of distribution, 382 ± 129 and 387 ± 56 ml/kg; mean concentration in cerebrospinal fluid 1 to 6 h after infusion, 5.4 and 6.4 μg/ml. A dosage schedule of 50 mg/kg every 12 h for bacterial meningitis caused by susceptible organisms is suggested for pediatric patients over 7 days of age.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskid G., Christenson J. G., Cleeland R., DeLorenzo W., Trown P. W. In vivo activity of ceftriaxone (Ro 13-9904), a new broad-spectrum semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981 Aug;20(2):159–167. doi: 10.1128/aac.20.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradsher R. W. Ceftriaxone (Ro 13-9904) therapy of serious infection. Antimicrob Agents Chemother. 1982 Jul;22(1):36–42. doi: 10.1128/aac.22.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoz M., Denis F., Félix H., Diop Mar I. Treatment of purulent meningitis with a new cephalosporin-Rocephin (Ro 13-9904). Clinical, bacteriological and pharmacological observations in 24 cases. Chemotherapy. 1981;27 (Suppl 1):57–61. doi: 10.1159/000238030. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Goetter W. E., Elliott A. M., Cobbs C. G. Ceftriaxone: in vitro studies and clinical evaluation. Antimicrob Agents Chemother. 1982 Jul;22(1):1–9. doi: 10.1128/aac.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriologic efficacy of moxalactam, cefotaxime, cefoperazone, and rocephin in experimental bacterial meningitis. J Infect Dis. 1981 Feb;143(2):156–163. doi: 10.1093/infdis/143.2.156. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Stoeckel K. Single-dose pharmacokinetics of ceftriaxone in infants and young children. Antimicrob Agents Chemother. 1982 Feb;21(2):248–253. doi: 10.1128/aac.21.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton S., Nelson J. D., McCracken G. H., Jr In vitro susceptibility of gram-negative bacilli from pediatric patients to moxalactam, cefotaxime, Ro 13-9904, and other cephalosporins. Antimicrob Agents Chemother. 1980 Sep;18(3):476–479. doi: 10.1128/aac.18.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]


