Abstract
Aim
To compare clonal T cell receptor γ (TCRγ) gene rearrangements in frozen and formalin‐fixed paraffin wax‐embedded (FFPE) tissue, using capillary electrophoresis for use in diagnostics, as T cell lymphomas may be difficult to diagnose by conventional methods.
Methods
The DNA for PCR was extracted from frozen and FFPE tissue, cell lines and blood. PCR primers Vγ1‐8, Vγ9, Vγ10 or Vγ11 (5′ end labelled) combined with a mixture of JγP1/JγP/JγP2/Jγ2 (unlabelled) were used. Monoclonal cases were sequenced and clonality, reproducibility, sensitivity and specificity analyses were carried out.
Results
In all cases the molecular test was found to be in agreement with the histological diagnosis. Discrepancies were found between frozen and FFPE tissue in 18 of 56 (32%) tests. The method was highly reproducible. The sensitivity was found to be 0.5% for cell lines and 1% for patient specimens and the specificity 100%. The junctional region between the Vγ and Jγ segments was specific for each patient.
Conclusions
Capillary electrophoresis of PCR products from frozen and FFPE tissue is suitable for detecting clonal TCRγ gene rearrangements. It is important, however, to correlate the results with conventional morphological and immunohistochemical studies.
The diagnosis of lymphoproliferative lesions may be difficult with conventional histopathological methods. Consequently, it is of interest to develop and implement research routines—for example, molecular techniques for clinical use—to identify and characterise these lesions. Moreover, it is of importance to validate the accessible tumour cells in frozen and formalin‐fixed and paraffin wax‐embedded (FFPE) tissue in that FFPE tissue is often the only diagnostic tissue available. The present study focuses on clonal rearrangements in the T cell receptor γ chain (TCRγ) gene.
In general, it is agreed that most malignancies are clonal in origin and represent a clonal growth from a single transformed cell—for example, a clonal TCRγ rearrangement is a specific characteristic of each malignant T cell.
T cells possess a surface antigen receptor that appears in both αβ and γδ configurations in post‐thymic T cells. The γδ configuration constitutes the minor fraction.1,2 The TCRγ locus has 14 variable gene segments (Vγ), five joining segments divided into two groups, Jγ1 (JγP1, JγP and Jγ1) and Jγ2 (JγP2 and Jγ2), and two constant segments (Cγ1 and Cγ2). The Vγ gene segments are separated into four subgroups on the basis of their sequence homology. Group I (Vγ1–8) consists of nine segments, five of which are functional and four are pseudogenes. The groups II, III and IV each consist of a single segment Vγ9, Vγ10 and Vγ11. The remaining genes, VγA and VγB (pseudogenes), are located upstream of Vγ9 and Vγ11, respectively.3,4
During early T cell differentiation, the germline variable (Vγ) and joining segments (Jγ) of the TCRγ gene complexes rearrange and each lymphocyte thereby acquires a particular combination of Vγ/Jγ segments. The joining of the Vγ and Jγ segments results from small variable‐sized deletions at the ends of the rearranged segments and the insertion of short stretches of random sequences—that is, hypervariable N regions that exist between the Vγ and Jγ segments before they join—and thus increasing the junctional diversity.5,6,7 The TCRγ gene may have only one or both alleles rearranged.
The junctional sequences of rearranged TCRγ genes are unique, allowing the design of junctional region‐specific oligonucleotides for the detection of minimal residual disease and recurrence of T cell lymphomas.8,9,10 The grade of clonality may be of importance in disease progress, with monoclonality considered to be the highest grade.
As the TCRγ gene consists of a few segments and the rearranged Vγ and Jγ segments include only one junctional N region, the recombination potential is relatively restricted. This makes the TCRγ gene particularly suitable for PCR amplification compared with the TCRβ gene, which is more complex. Because of the relatively small differences in the length of the rearranged segments, it may, however, be difficult to distinguish between rearrangements on lengths alone by using conventional agarose gels, making capillary electrophoresis an excellent option for clonality analysis. This technique permits discrimination between different clones, in that it separates PCR amplicons on the basis of a single nucleotide.
In this study, we assessed the usefulness and the quality of molecular testing in identifying clonal rearrangements in the TCRγ gene in DNA derived from frozen and FFPE tissues of lymphoproliferative T cell lesions, using capillary electrophoresis (GeneScan analysis and sequencing, ABI 310 Genetic Analyser, Foster City California, USA).
It is concluded that capillary electrophoresis (GeneScan analysis and sequencing) is a useful adjuvant to routine histological and immunohistochemical studies in the diagnosis of biopsy specimens from suspected T cell lymphomas. Such lymphoproliferative lesions, however, should be classified by a combination of clinical, histological and immunohistochemical data, and the TCRγ gene arrangement analysis should be carried out only in cases with less conclusive findings.
Materials and methods
Patients and DNA samples
The cases examined were retrieved from the files of the University Institute of Pathology, Århus University Hospital, Denmark, and only cases with frozen biopsy specimens available were included in the study. The T cell lymphomas were classified according to the World Health Organization (WHO) classification. Tumour samples from 21 patients (9 women and 12 men), diagnosed in the period from June 1996 to August 2000, were retrospectively selected for analysis of clonal TCRγ gene rearrangement. The age range for the women was 36–71 (median 55.5) years and for the males 5–79 (median 40.3) years.
The tissue samples were divided into two equal groups. One group was pre‐fixed in formalin followed by embedding in paraffin wax and the other was frozen at −80°C until use.
Five sections of 20 μm thickness were cut twice from each block in sequence (frozen and FFPE tissue) and collected in two tubes. Special attention was taken to avoid any potential contamination during the cutting procedure.
Verification of the presence of tumour cells in the tissue samples was carried out on frozen sections in all cases. FFPE tissue was not available from cases 1, 5 and 16 because of poor quality DNA, and only one PCR amplicon (frozen tissue) was obtained from case 13. In all, 22 hyperplastic lymph nodes (frozen 13; FFPE 9) and 15 B cell lymphomas (frozen 0; FFPE 15) were included as negative controls. Four clonal T cell lines were used as positive controls. The T cell lines were isolated from patients with Sezary's syndrome, mycosis fungoides, atopic dermatitis and psoriasis vulgaris. Peripheral blood was obtained from a healthy volunteer.
Extraction of DNA from frozen tissue, FFPE tissue, blood and T cell lines
Extraction of DNA from frozen tissue
Five sections of 20 μm thickness were cut in sequence and collected in a tube, 100 μl 1 mg/ml proteinase K was added and mixed overnight at 55°C. Proteinase K was dissolved in sterile water. Subsequently, the enzyme was inactivated at 98°C for 10 min.
Extraction of DNA from FFPE tissue
Five sections of 20 μm thickness were cut in sequence and collected in a tube followed by treatment with xylene at 60°C for 20 min to remove the paraffin wax. The sample was then centrifuged and washed with 99.9% ethanol, followed by addition of 2–3 drops acetone and incubation at 60°C for 20 min. Finally, the proteinase K digestion was carried out as described above.
Extraction of DNA from peripheral blood and T cell lines
The extraction procedure was carried out according to the manufacturers protocol (PureGene, D‐5000, Isolation kit, The Biotech Line, Minneapolis, Minnesota, USA).
PCR
All samples were subjected to PCR amplification. PCR was carried out in a 50 μl volume containing 10×PCR buffer (100 mM Tris–HCl, 15 mM MgCl2, 500 mM KCl, pH 8.3), dNTP (0.25 mmol/l of each), 1 U Taq DNA polymerase (Roche, Mannheim, Germany) and 5 μl DNA (concentration unknown). The primers used were the variable segment primers Vγ1–8, Vγ9, Vγ10 or Vγ11 (50 pmol) in combination with a mixture of primers covering the joining segments JγP1/JγP/JγP2/Jγ2 (30 pmol of each). For primer sequences see Greiner et al.11 The Vγ primers were 5′ labelled with FAM (6‐carboxyfluorescin). In cases where it was difficult to obtain a PCR product 2 U AmpliTaq Gold (Applied Biosystems, Foster City, California, USA) to some extent successfully replaced the Taq DNA polymerase.
The four sets of primers were used on each sample. The size of the PCR products was approximately 260 bp for Vγ1–8, 180 bp for Vγ9, 160 bp for Vγ10, and 140 bp for Vγ11. Primers purified by high‐performance liquid chromatography were purchased from Hobolth Synthesis, Denmark.
DNA was amplified in a GeneAmp9700 Thermal Cycler (Applied Biosystems). PCR was carried out as follows: 95°C for 5 min (1 cycle), 95°C for 1 min, 59°C for 1 min, 72°C for 1 min (40 cycles) and a final extension at 72°C for 7 min.
PCR was carried out at least twice to exclude pseudoclonality. To assess the quality of the DNA, the human β globin gene (268 bp) was amplified in each sample run.12 Hyperplastic lymph nodes, B cell lymphomas and a non‐template PCR were included as negative controls. The T cell lines served as positive controls.
Before the GeneScan analysis, the amplicons and a size standard were run in a gel (VisiGel Separation Matrix, Stratagene, Belgium) and visualised on a ultraviolet transilluminator (Vilber Lourmat, UVP, Cambridge, UK). In cases of heavy bands, the amplicons were diluted before GeneScan analysis to avoid excessive fluorescent signalling.
Capillary electrophoresis (GeneScan analysis)
GeneScan analysis was carried out using an ABI 310 Sequence Analyser (Applied Biosystems). Four microlitres of PCR product were mixed with 24 μl deionised formamide and 1 μl molecular weight marker, labelled with TAMRA (N,N,N′N′ tetram‐ethyl‐6‐carboxyrhodamin, GeneScan‐TAMRA‐500, Applied Biosystems). The samples were heated at 95°C for 2 min and immediately cooled on ice. The GeneScan procedures were carried out according to the manufacturer's recommendations. Data were collected and analysed using the GeneScan program (GeneScan V.3.1.2).
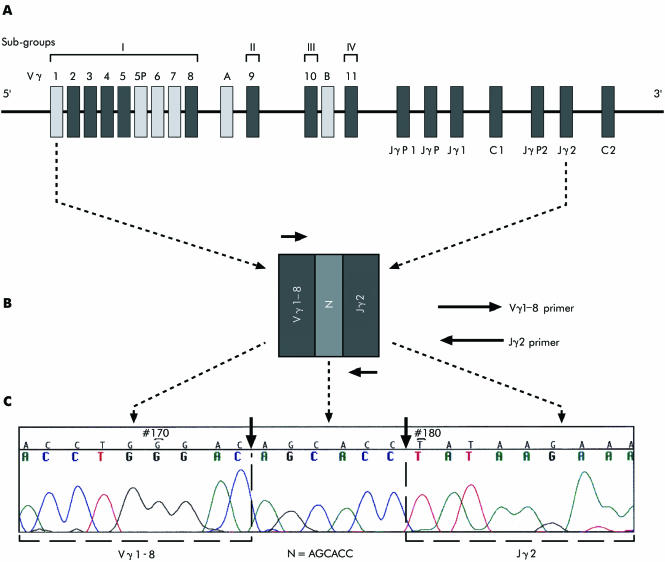
Figure 1A outlines the organisation of the human germline TCRγ gene. The mixture of Vγ and Jγ segment primers used made it possible to detect a broad range of clonal TCRγ rearrangements. Figure 1B shows monoclonal rearrangement of the Vγ1–8 and the Jγ2 segments. The Vγ1–8 primer covers all the genes in group I.
Figure 1 The organisation of the human T cell receptor γ (TCRγ) locus, 7p15. (A) Germline configuration of the human TCRγ gene. (B) Rearranged configuration of the Vγ and Jγ segments and the N region. (C) Sequence of the rearranged configuration shown in B (frozen tissue). Boxes indicate the type of gene segment. Functional genes (black), pseudogenes (light grey) Vγ, variable segment; Jγ, joining segment; C, constant segment; N (dark grey) indicates the nucleotides intercalated between the Vγ and Jγ segments. The distances between segments are not drawn to scale.
Table 1 summarises the morphological and clinical characteristics of each patient included in this study.
Table 1 Clinical characteristics of patients.
| Case no | Stage | Sex | Age (years) | Location | Diagnosis | Course |
|---|---|---|---|---|---|---|
| 1 | 3A | M | 71 | Neck | Peripheral T cell lymphoma, unspecified | D |
| 2 | 4A | F | 36 | Thorax | Precursor T cell lymphoblastic lymphoma | L |
| 3 | 4B | M | 20 | Arm | Precursor T cell lymphoblastic lymphoma | L |
| 4 | 4B | M | 79 | Armpit | Peripheral T cell lymphoma, unspecified | D |
| 5 | 3A | M | 49 | Groin | Peripheral T cell lymphoma, unspecified | D |
| 6 | 4B | F | 46 | Groin | Anaplastic large T cell lymphoma | L |
| 7 | 4B | F | 64 | Groin | Peripheral T cell lymphoma, unspecified | L |
| 8 | 4A | M | 40 | Skin, subcortex | Peripheral T cell lymphoma, unspecified | D |
| 9 | 3B | M | 29 | Neck | Anaplastic large T cell lymphoma | L |
| 10 | 4B | F | 71 | Neck, armpit | Peripheral T cell lymphoma, unspecified | L |
| 11 | 3A | M | 16 | Neck | Precursor T cell lymphoblastic lymphoma | L |
| 12 | 4B | F | 67 | Intestine | Enteropathy‐type T cell lymphoma | D |
| 13 | 3A | M | 5 | Mediastinum | Precursor T cell lymphoblastic lymphoma | L |
| 14 | 4A | F | 54 | Neck | Anaplastic large T cell lymphoma | D |
| 15 | 3A | F | 45 | Neck | Anaplastic large T cell lymphoma | D |
| 16 | 1A | M | 48 | Nose | Mucosa‐associated T cell lymphoma | L |
| 17 | 4B | M | 41 | Mediastinum | Precursor T cell lymphoblastic lymphoma | L |
| 18 | 4B | M | 54 | Neck | Precursor T cell lymphoblastic lymphoma | D |
| 19 | 4A | M | 61 | Neck | Peripheral T cell lymphoma, unspecified | D |
| 20 | 4B | F | 51 | Axillae | Anaplastic large T cell lymphoma | L |
| 21 | 4B | F | 66 | Neck | Peripheral T cell lymphoma, unspecified | D |
F, female; M, male; D, dead; L, alive.
Sequencing
When a pure monoclonal/monoallelic pattern—that is, one distinct peak—was identified the sample was subjected to additional PCR using separate Vγ primers (unlabelled) with separate Jγ primers (unlabelled) to identify the Vγ or Jγ segment present. The use of unlabelled primers greatly improved the sequencing results.
The monoclonal/monoallelic products were purified before cycle sequencing according to the manufacturer's protocol (Qiaquick, PCR purification kit, Hilden, Germany). The Big Dye Terminator Kit, V.1.1 (Applied Biosystems) was used for cycle sequencing (96°C for 10 s, 50°C for 5 s and 60°C for 4 min, 25 cycles). The amplified products were subsequently purified according to a slightly modified protocol (Applied Biosystems). In short, the cycle sequencing products were added to 3 M sodium acetate (NaOAc) and 95% ethanol to precipitate the extension products (20 min), then centrifuged for 40 min at 13 000 rpm and washed twice with 70% ethanol. Finally, the products were re‐suspended in 25 μl template suppressor reagent. (Applied Biosystems).
For the cases showing monoclonal/biallelic patterns—that is, two distinct peaks—the bands were excised from the gel and purified according to the manufacturer's protocol (Amersham Biosciences, Freiburg, Germany) and sequenced.
The sequences were analysed using the Sequenche Analysis program V.3.4.1 (Applied Biosystems) and the Sequencher program V.4.1.2 (MedProbe AS, Oslo, Norway). The analysed sequences were compared with the germline TCRγ gene sequences downloaded from http://www.ncbi.nlm.nih.gov/ yielding the TCRγ gene rearrangements in the Vγ and Jγ families.
Analytical sensitivity
To assess the sensitivity of the assay:
DNA from the monoclonal T cell lines was serially diluted into DNA isolated from hyperplastic lymph nodes (polyclonal; n = 8);
DNA from patient specimens that had previously shown a monoclonal pattern (n = 13) was serially diluted into DNA isolated from peripheral blood (polyclonal).
The dilutions were 0%, 0.1%, 0.5%, 1%, 2%, 5% and 10%. PCR was carried out using the Vγ primers with a mixture of Jγ primers according to the protocol.
Specificity
To test the specificity of the assay, PCR was carried out on DNA isolated from B cell lymphomas (n = 15) and hyperplastic lymph nodes (n = 22) using all four sets of primers.
Ethics
The local ethical committee (Århus) approved the project. The patients included in the project, living and deceased, are anonymous and cannot be identified.
Results
Capillary electrophoresis (GeneScan analysis)
The quality of DNA was confirmed in (almost) all samples by successful amplification of the β globin gene. In all cases with no amplification of the gene, no amplification of the Vγ or Jγ segments was seen. The size of the PCR amplicons for each subgroup (I–IV) ranged from 231 to 283 bp for Vγ1–8, 147 to 207 bp for Vγ9, 132 to 182 bp for Vγ10 and 116–165 bp for Vγ11.
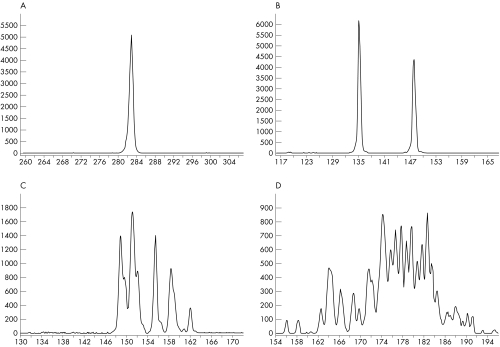
Clonal TCRγ gene rearrangements were interpreted as follows: one or two distinct peaks, monoclonality (monoallelic or biallelic patterns, respectively; figs 2A,B); 3–5 peaks, oligoclonality, fig 2C; and more than five peaks, polyclonality, fig 2D. In oligoclonal and polyclonal patterns, each peak corresponds to the amplification of rearrangements of different clones, sharing the same Vγ and Jγ region, but having distinct N regions.
Figure 2 T cell receptor γ (TCRγ) gene rearrangements. Electrophoretic profiles of PCR products of the TCRγ gene using DNA extracted from T cell lymphomas (frozen tissue). (A) A monoclonal (monoallelic) pattern is present as a single peak at 282 bp. (Vγ1–8 primer and a mixture of Jγ primers). (B) A monoclonal (biallelic) pattern is shown as two peaks at 148 bp and 135 bp, respectively. (Vγ11 primer and a mixture of Jγ primers). (C) An oligoclonal pattern. (Vγ10 primer and a mixture of Jγ primers) and in (D) a polyclonal pattern. (Vγ9 primer and a mixture of Jγ primers). In (C) and (D), each peak corresponds to the amplification of rearrangements from different clones, sharing the same V and J regions but having distinct junctional sequences. The y axes indicate the relative fluorescence intensities and the x axes the size (bp) of the PCR products.
The monoclonal group was characterised by two different patterns: (1) the pure monoallelic or biallelic patterns and (2) the mixed monoallelic or biallelic patterns that are superimposed on a polyclonal cell background by one or two peaks.
To exclude pseudospikes, the relative height of the distinct peaks was estimated. The height of the peaks should have a minimum ratio of at least twice the height of the normal distribution of the polyclonal peaks to be considered a true monoclonal peak.13
In all, 156 samples (the total 168 samples minus 12 FFPE tissue samples not available) were subjected to PCR amplification. Of these, 137 (88%) were successfully amplified. Of the 19 samples not amplified, 15 (79%) originated from FFPE tissue and 4 (21%) from frozen tissue. In 56 of 137 (41%) samples, DNA from both frozen and FFPE tissues was amplified, and 38 (68%) samples correlated; table 2.
Table 2 T cell receptor γ gene rearrangements.
| Case | Vγ1–8 | Vγ9 | Vγ10 | Vγ11 |
|---|---|---|---|---|
| 1 | B/NT | P/NT | P/NT | P/NT |
| 2 | M/M | P/P | NP/P | M/M |
| 3 | P/MP | P/P | P/P | P/P |
| 4 | BP/MP1‐P2 | P/P | P/P | MP/MP |
| 5 | P/NT | P/NT | P/NT | BP/NT |
| 6 | P/P | P/Oligo | P/P | P/MP |
| 7 | MP/P | P/P | P/P | MP/MP |
| 8 | P/M | P/MP | P/Oligo | MP/Oligo |
| 9 | P/P | P/P | P/P | MP/P |
| 10 | P/P | P/P | MP/P | M/M |
| 11 | M/M | MP/P | P/P | MP/P |
| 12 | P/P | P/Oligo | MP/MP | MP/Oligo |
| 13 | M/NP | NP/NP | NP/NP | NP/NP |
| 14 | P/P | P/P | MP/ M1‐P2 | B/B |
| 15 | M/P | P/P | M/M | P/P |
| 16 | P/NT | M/NT | P/NT | P/NT |
| 17 | M/M | Oligo/NP | P/NP | P/NP |
| 18 | M/M | P/P | P/P | P/P |
| 19 | M/NP | P/NP | P/NP | P/NP |
| 20 | M/NP | P/NP | P/NP | P/NP |
| 21 | P/P | P/P | M/MP | M/M |
Data are for frozen/formalin‐fixed paraffin wax‐embedded tissues, respectively.
Data in bold indicate notable differences.
B, pure monoclonal (biallelic); BP (mixed clonal), two distinct peaks in a polyclonal cell background; M, pure monoclonal (monoallelic); MP (mixed clonal), one distinct peak in a polyclonal cell background; M1‐P2, the first PCR showed a pure monoclonal (monoallelic) and the second a polyclonal pattern; MP1‐P2, the first PCR showed a mixed clonal (monoallelic) and the second a polyclonal pattern; NP, no PCR product; NT, no FFPE tissues available; Oligo, oligoclonal; P, polyclonal.
The monoallelic and biallelic patterns were always identical when repeating PCR using DNA from the same tube. We found differences between the first and the second PCR in cases 4 and 14 in FFPE tissue. The oligoclonal patterns differed slightly between PCRs. Of the 56 samples, discrepancies were documented in 18 (32%) samples when comparing patterns from frozen and FFPE tissues. The most notable differences were found in cases 8 and 15. Case 8 showed a polyclonal pattern in frozen tissue and a monoclonal in FFPE tissue, and case 15 showed the opposite (table 2). In one sample (case 2) there was a pure monoclonal/monoallelic pattern in two segments in both frozen and FFPE tissues. A mixed clonal and polyclonal pattern was found in eight cases. Mixed patterns in both frozen and FFPE tissues were found in three cases. Samples showing neither pure monoallelic nor biallelic patterns always showed a mixed pattern with either one or two distinct peaks corresponding to the size of the segment of interest (seven cases; table 2). Oligoclonal patterns were found in five FFPE and one frozen tissue sample (table 2).
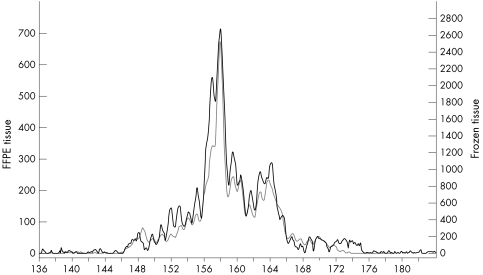
We found that the pure monoallelic peaks had identical sizes when comparing frozen and FFPE tissues from patients, except for case 18 (Vγ1–8, frozen, 265 bp; FFPE, 258 bp). In the three samples showing a mixed monoclonal/monoallelic pattern in both frozen and FFPE tissues the size of the distinct peaks correlated (fig 3). In this study, the monoclonal (monoallelic/biallelic) peak size was specific for each patient.
Figure 3 Monoclonal peaks superimposed on a polyclonal cell background. Electrophoretic profiles of PCR products of the T cell receptor γ chain gene using DNA from a T cell lymphoma. The profiles from frozen (grey line) and FFPE (black line) tissue are placed on top of each other showing identical patterns. (Vγ10 primer and a mixture of Jγ primers). The y axis indicates the relative fluorescence intensities and the x axis the size (bp) of the PCR products.
The joining segment involved Jγ2 in combination with Vγ1–8, Vγ9, Vγ10 or Vγ11 in all but three cases, in which JγP was involved in combination with Vγ1–8 (cases 19 and 20) and Vγ9 (case 16).
Table 3 shows the frequencies of the patterns of clonality in specific segments.
Table 3 Patterns of clonality in the T cell receptor γ chain gene.
| Vγ1–8 | Vγ9 | Vγ10 | Vγ11 | |
|---|---|---|---|---|
| Monoclonal (monoallelic) | 13/36 (36.1) | 1/34 (2.9) | 3/33 (9.1) | 6/34 (17.6) |
| Monoclonal (biallelic) | 1/36 (2.8) | 0/34 (0) | 0/33 (0) | 2/34 (5.9) |
| MP or BP | 4/36 (11.1) | 2/34 (5.9) | 5/33 (15.2) | 10/34 (29.4) |
| M1‐P2 | 0/36 (0) | 0/34 (0) | 1/33 (3) | 0/34 (0) |
| MP1‐P2 | 1/36 (2.8) | 0/34 (0) | 0/33 (0) | 0/34 (0) |
| Oligoclonal | 0/36 (0) | 3/34 (8.8) | 1/33 (3) | 2/34 (5.9) |
| Polyclonal | 17/36 (47.2) | 28/34 (82.4) | 23/33 (69.7) | 14/34 (41.2) |
Values in parentheses are percentages.
BP (mixed clonal), two distinct peaks in a polyclonal cell background; MP (mixed clonal), one distinct peak in a polyclonal cell background; M1‐P2, the first PCR showed a pure monoclonal (monoallelic) and the second a polyclonal pattern; MP1‐P2, the first PCR showed a mixed clonal (monoallelic) and the second a polyclonal pattern.
Of 21 patients, 10 (48%) were dead at the time of inclusion. There was no evident linkage between death and clonality in specific segments (tables 1 and 2).
The pure monoallelic or biallelic patterns were most often observed in the Vγ1–8/Jγ2 (39%) and the Vγ11/Jγ2 (24%) segments. Including the mixed monoclonal patterns, however, the Vγ11/Jγ2 (53%) segment became slightly more frequent than Vγ1–8/Jγ2 (50 %). Despite the observed discrepancies there was total agreement between the immunohistopathological diagnosis and the molecular test in all cases investigated.
Sensitivity
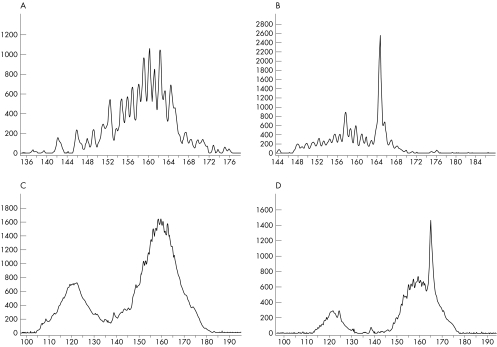
In the sensitivity test, observable peaks were present when DNA from a clonal cell line represented 0.5–5% and DNA from monoclonal patient specimens represented 1–10% of the entire sample. Figure 4 shows an example.
Figure 4 Serial dilutions of DNA from a cell line into DNA from a hyperplastic lymph node (frozen tissue; A,B) and a monoclonal specimen (frozen tissue) diluted into DNA from peripheral blood (C,D). Electrophoretic profiles of PCR products of the T cell receptor γ gene. A (0), B (0.5), C (0), D (1). The values in parentheses indicate the percentage of monoclonal DNA diluted into polyclonal DNA. (Vγ10 primer and a mixture of Jγ primers). The y axes indicate the relative fluorescence intensities and the x axes the size (bp) of the PCR products.
Specificity
The specificity of the assay was 100% as no monoclonal T cell clones were identified in any of the hyperplastic lymph nodes or B cell lymphomas tested (37 samples).
Sequencing
Pure monoallelic or biallelic PCR products were sequenced. Sequencing was repeated to confirm the result. Because of the rather weak and close bands in the biallelic patterns, it was possible only to obtain an identifiable N region in one case. The excision and purification procedure was carried out several times. Identifying the N region in PCR products derived from FFPE tissue was difficult, in most cases not possible at all. The N region was identified in PCR products in only 4 of 13 (31%) samples from FFPE tissue compared with 14 of 16 (88%) samples from frozen tissue. Figure 1C shows a sequence including the N region. The N regions were similar when sequences from frozen and FFPE tissues were compared, except for case 18 in which the region Vγ1–8–N–Jγ2 diverged (table 4).
Table 4 Identified junctional N regions.
| Case | |
| Vγ1–8 | |
| 1 | (F) monoclonal (biallelic), nd |
| (P) NT | |
| 2 | (F) AGC ACC |
| (P) AGC ACC | |
| 8 | (F) polyclonal |
| (P) nd | |
| 11 | (F) AGC XGC C |
| (P) nd | |
| 13 | (F) TTC AT |
| (P) nd | |
| 15 | (F) TTG TAG G |
| (P) polyclonal | |
| 17 | (F) TTC CCT ACA CCC CCA GAT TAA GAT ACG GAG CT |
| (P) nd | |
| 18 | (F) AGT CCA TGA GGG GGG G |
| (P) AGT CAT GAT GGG GGG GTA | |
| 19 | (F) TAA ATT TTA TCC GG C |
| (P) nd | |
| 20 | (F) CAA |
| (P) nd | |
| Vγ9 | |
| 16 | (F) CAA GAG TTG GGC AAA AAA ATC AAG |
| (P) NT | |
| Vγ10 | |
| 15 | (F) GCC GGT TTG GA |
| (P) GCC GGT TTG GA | |
| 21 | (F) TAA |
| (P) mixed clonal | |
| Vγ11 | |
| 2 | (F) nd |
| (P) nd | |
| 10 | (F) GCT TAT TA |
| (P) GCT TAT TA | |
| 14 | (F) monoclonal (biallelic) 1. band. nd 2. band. CCG GGG GTG T |
| (P) nd | |
| 21 | (F) ATT ATA A |
| (P) nd |
F, frozen tissue; mixed clonal, one distinct peak in a polyclonal cell background; NT, no FFPE (formalin‐fixed paraffin wax‐embedded) tissue available; nd, not determined; P, FFPE tissue; X, not identifiable . PCR products from cases 3, 4, 5, 6, 7, 9 and 12 all showed a mixed clonal pattern and were consequently not sequenced. Values in bold indicate notable differences.
It was, however, shown that irrespective of the segment investigated, each monoclonal/monoallelic TCRγ gene rearrangement was unique for each patient (table 4).
Discussion
Molecular investigations of clonal TCR gene rearrangements are essential in lymphoproliferative lesions as morphological and immunohistochemical studies occasionally fail. For several years, Southern blotting analysis has been the gold standard for molecular detection of clonal TCRγ gene rearrangements.14,15 Relatively large amounts of DNA isolated from fresh or frozen tissue, however, are required in addition to radioactively labelled probes and the process is laborious. The use of other methods such as single strand conformation polymorphism or heteroduplex analysis may result in poorer interpretation because of frequently incomplete separation of fragments during electrophoresis.16,17,18,19 In the present study, we focus on whether capillary electrophoresis (GeneScan analysis and sequencing) of the TCRγ gene rearrangements can be established as a sensitive and specific diagnostic procedure by comparing TCRγ gene rearrangements in lymphoproliferative lesions in frozen and FFPE tissue samples. Application on FFPE tissue is of particular importance because in many cases it is the only obtainable diagnostic tissue.
High‐resolution capillary electrophoresis was selected for this investigation because the method is highly sensitive and provides reliably distinguishable results.20,21,22 The use of an internal standard in each run provides an exact base size calling as the PCR products are separated in length of just one base, altogether resulting in more accurate interpretation than in conventional gels. The outlined post PCR handling may seem expensive and time consuming. We, however, recommend carrying out this test only in a few cases in which it is not possible to reach a diagnosis by morphological and immunohistochemical methods. Reproduction of the PCR amplification was carried out to ensure reliable interpretation of the data and is obligatory for the exclusion of pseudoclonality. PCR assays are very sensitive and detection of false‐positive clonal rearrangements may occur in cases of contamination. Sizing of the PCR fragments minimises the risk of false‐positive results, but does not exclude them. The European BIOMED‐2 collaborative study suggests that using GeneScan analysis alone increases the risk of false‐positive results because of overinterpretation of minor peaks and recommends reducing the false‐positive results by using GenScan analysis in combination with heterodulpex analysis.23 We, however, speculate that excluding pseudospikes based on a previously described calculation may reduce or even eliminate the problem of false‐positive results that may be caused by GeneScan analysis.13,24,25 False‐negative results may occur because of ineffective amplification caused by poor‐quality DNA, primers that fail to anneal to the TCRγ sequence (eg, imperfect primers, mutations in the region) or a sample that is too diluted (ie, the clone is superimposed on a polyclonal cell background). Avoiding a high rate of false‐negative results may include (1) selective sampling of tumour cells, (2) designing appropriate primers and (3) testing more than one DNA concentration.
As expected, a relatively lower proportion of detectable PCR products was obtained from archival, FFPE tissue samples compared with frozen tissue as pre‐fixation in formalin severely degrades the DNA.26,27 The design of oligonucleotides producing shorter PCR products may improve the proportion of identifiable PCR products in FFPE tissue.
On comparing PCR products from two tubes (tissue sections cut sequentially), discrepancies were found only in DNA from FFPE tissues. When comparing PCR products from frozen and FFPE tissues, relatively many (18) discrepancies were found. This shows that if the TCRγ rearrangement analysis had been carried out exclusively on FFPE tissues, 4 of 18 (22%) cases would have been missed and not classified as monoclonal. Therefore, if only FFPE tissue is available, the negative results should be interpreted with caution. In cases where different or negative results are obtained, the sampling procedure should also be taken into consideration. It may be necessary to carry out selective sampling from the lymphoid tissue, guided by either the microscopic evaluation or microdissection.
Slightly dissimilar oligoclonal patterns were observed when repeating the PCR, which probably is due to amplification of different clones constituting the oligoclone (data not shown). The findings of most oligoclonal patterns in FFPE tissue, the different patterns between the first and second PCR in cases 4 and 14 in FFPE tissue and the discordance between frozen and FFPE tissue in case 15 are probably because of lower sensitivity of the assay in detecting monoclonal peaks from FPPE tissue.
Case 8 was further validated by immunohistochemical studies (CD4, CD56) that confirmed the diagnosis. We suggest that sampling may be the reason for the divergence observed.
In this study, it was shown that samples showing neither a pure monoallelic nor a biallelic pattern always showed distinct monoallelic or biallelic peaks superimposed on a polyclonal background. Criteria for the characterisation of clonality are definitely needed and some have already been proposed.24,25 The criteria used in this study, however, will not function in every case.13 In detecting minimal residual disease it will be useless, as initially the clone rising in the polyclone background is barely visible.
It has been suggested that the size (bp) of the PCR product of a monoclonal TCRγ rearrangement may be of relevance, in that the size of the rearrangement seems to be patient specific and may be important for follow‐up.28,29 The specificity of the size also appeared to be the case in this investigation. We speculate that some patients, however, may have identically sized clones but variations in the composition of nucleotides in the N region. To monitor residual disease or recurrence of T cell lymphomas, it is mandatory to identify the junctional sequence by sequencing. Subsequently, the clone‐specific oligonucleotide can function as a patient‐specific probe in a real‐time PCR assay. This study confirms the specificity of the junctional sequences. In agreement with previously published observations, the Vγ1–8 segments are most commonly involved in monoclonality.30,31 Surprisingly, when including the mixed‐clonal patterns, the Vγ11 segment was observed more commonly, which is apparently in contrast with results in other studies.32 By using multiple primers, our detection rate of clonal rearrangements was 100%. As shown by others multiple primers covering most segments are needed to increase the detection rate.23,32 The different numbers of primers used, different interpretations of the peaks and the small number of samples investigated in this study may explain the divergent observations. The sensitivity of our assay was found to be comparable with that in previous studies.21 PCR amplification was highly specific. No false‐positive results in the control group (hyperplastic lymph nodes, B cell lymphomas) were obtained. Out of lineage, however, rearrangements have been reported previously. TCRγ gene rearrangements have been found in B lineage acute lymphoblastic leukaemia, immature B cells and B cell lymphomas.33,34,35,36,37,38 It turned out to be difficult to sequence DNA from FFPE tissue compared with DNA from frozen tissue, as seen by the very low rate of success which may be because of DNA degradation or introduction of artificial mutations into the FFPE tissue, making specific regions difficult to sequence.39 This may also cause the discrepancies observed in case 18. It has been shown that testing rearrangements in both the TCRγ and the TCRβ genes considerably enhances the sensitivity of detecting clonal rearrangements in T cell lymphoprolierative disorders.40,41,42,43 In cases with less conclusive histological analyses and no identified monoclonal rearrangements of the TCRγ gene, we also recommend examining the TCRβ gene. It may be required to study the arrangements in the TCRγ and TCRβ genes in supplementary biopsy specimens.
A negative result, however, does not necessarily exclude the presence of a malignant clone and a positive result does not necessarily indicate malignancy, as a clonal TCRγ rearrangement may be detected in non‐malignant diseases.44,45
Despite the uncertainties pointed out, we think that investigation of clonal rearrangements might be a useful adjuvant in diagnosing T cell lymphomas to ascertain the diagnosis in diagnostically difficult cases.
Take‐home messages
Capillary electrophoresis (GeneScan analysis and sequencing) is useful in the diagnosis of suspected T cell lymphomas.
GeneScan analysis is sensitive, reproducible and highly specific for detecting clonal T cell receptor γ (TCRγ) gene rearrangements in frozen and formalin‐fixed paraffin‐wax‐embedded tissue.
In all cases, the molecular testing (GeneScan analysis and sequencing) was in agreement with the histological diagnosis.
Lymphoproliferative lesions should be classified by using a combination of clinical, histological and immunohistochemical data and only in cases with less conclusive findings should TCRγ gene rearrangement analysis be performed.
In conclusion, capillary electrophoresis based on GeneScan analysis, and sequencing of PCR products from frozen and FFPE tissues from lymphoproliferative lesions are suitable tools for the determination of clonal TCRγ chain gene rearrangements, as the results correlate with the clinical and immunohistological diagnosis. This analysis, however, should not be carried out in all cases indiscriminately, but only in cases where morphological and immunohistochemical studies fail to provide a conclusive diagnosis of a T cell lymphoma.
Acknowledgements
We thank Associate Professor Brage S Andresen and Professor Niels Gregersen for their valuable scientific contributions, Marit Nyholm Nielsen for her excellent technical help at the Research Unit for Molecular Medicine, Århus University Hospital, Skejby Sygehus, and Associate Professor Keld Kaltofte, Department of Humane Genetics, Århus University, for providing the T cell lines.
Abbreviations
FFPE - formalin‐fixed and paraffin wax‐embedded
TCRγ - T cell receptor γ
Footnotes
Funding: This study was supported by the A P Møller and Spouse Chastine Mc‐Kinney Møller Foundation, the Clinical Institute of Århus University, the Johannes Fogh‐Nielsens and Mrs Ella Fogh‐Nielsens Foundation, the Research Foundation of Århus County Hospital, the Research Initiative for Århus County, and the Research Foundation of Århus University.
Competing interests: None declared.
References
- 1.Moller D R. T‐cell receptor genes in sarcoidosis. Sarcoidosis Vasculitis Diffuse Lung Dis 199815158–164. [PubMed] [Google Scholar]
- 2.Carding S R, Egan P J. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol 20022336–345. [DOI] [PubMed] [Google Scholar]
- 3.Forster A, Huck S, Ghanem N.et al New subgroups in the human T cell rearranging Vγ gene locus. EMBO J 198761945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefranc M ‐ P, Rabbitts T H. The human T‐cell receptor γ (TRG) genes. TIBS 198914214–218. [DOI] [PubMed] [Google Scholar]
- 5.Quertermous T, Murre C, Dialynas D.et al Human T‐cell γ chain genes: organization, diversity, and rearrangement. Science 1986231252–255. [DOI] [PubMed] [Google Scholar]
- 6.LeFranc M ‐ P, Forster A, Baer R.et al Diversity and rearrangement of the human T cell rearranging γ genes: nine germ‐line variable genes belonging to two subgroups. Cell 198645237–246. [DOI] [PubMed] [Google Scholar]
- 7.LeFranc M ‐ P, Forster A, Rabbitts T H. Rearrangment of two distinct T‐cell γ‐chain variable‐region genes in human DNA. Nature 1986319420–422. [DOI] [PubMed] [Google Scholar]
- 8.Steward C G, Goulden N J, Potter M N.et al The use of the polymerase chain reaction to detect minimal residual disease in childhood acute lymphoblastic leukemia. Eur J Cancer 199329A1192–1198. [DOI] [PubMed] [Google Scholar]
- 9.Sabesan V, Cairo M S, Lones M A.et al Assessment of minimal residual disease in childhood non‐Hodgkin lymphoma by polymerase chain reaction using patient‐specific primers. J Pediatr Hematol Oncol 200325109–113. [DOI] [PubMed] [Google Scholar]
- 10.Scrideli C A, de Paula Queiroz R G, Bernardes J E.et al PCR detection of clonal IgH and TCR gene rearrangements at the end of induction as a non‐remission criterion in children with ALL: comparison with standard morphologic analysis and risk group classification. Med Pediatr Oncol 20034110–16. [DOI] [PubMed] [Google Scholar]
- 11.Greiner T C, Raffeld M, Lutz C.et al Analysis of T cell receptor‐γ gene rearrangements by denaturing gradient gel electrophoresis of GC‐clamped polymerase chain reaction products. Correlation with tumor‐specific sequences. Am J Pathol 199514646–55. [PMC free article] [PubMed] [Google Scholar]
- 12.Greer C E, Peterson S L, Kiviat N B.et al PCR amplification from paraffin‐embedded tissues: effects of fixative and fixation time. Am J Clin Pathol 199195117–124. [DOI] [PubMed] [Google Scholar]
- 13.Greiner T C, Rubocki R J. Effectiveness of capillary electrophoresis using fluorescent‐labeled primers in detecting T‐cell receptor γ gene rearrangements. J Mol Diagn 20024137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diss T C, Watts M, Pan L X.et al The polymerase chain reaction in the demonstration of monoclonality in T cell lymphomas. J Clin Pathol 1995481045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreau E J, Langerak A W, van Gastel‐Mol E J.et al Easy detection of all T cell receptor gamma (TCRG) gene rearrangements by Southern blot analysis: recommendations for optimal results. Leukemia 1999131620–1626. [DOI] [PubMed] [Google Scholar]
- 16.Signoretti S, Murphy M, Cangi M G.et al Detection of clonal T‐cell receptor γ gene rearrangements in paraffin‐embedded tissue by polymerase chain reaction and nonradioactive single‐strand conformational polymorphism analysis. Am J Pathol 199915467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han X ‐ Q, He L, Shong L ‐ Y.et al Investigation of T‐cell receptor‐γ gene rearrangement in gastrointestinal lymphomas by PCR‐SSCP analysis. World J Gastroenterol 200410995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottaro M, Berti E, Biondi A.et al Heteroduplex analysis of T‐cell receptor γ gene rearrangements for diagnosis and monitoring of cutaneus T‐cell lymphomas. Blood 1994833271–3278. [PubMed] [Google Scholar]
- 19.Nirmala K, Rajalekshmy K R, Raman S G.et al PCR‐heteroduplex analysis of TCRγ, δ and TAL‐1 deletions in T‐acute lymphoblastic leukemias: implications in the detection of minimal residual disease. Leuk Res 200226335–343. [DOI] [PubMed] [Google Scholar]
- 20.Simon M, Kind P, Kaudewitz P.et al Automated high‐resolution polymerase chain reaction fragment analysis. A method for detecting T‐cell receptor γ‐chain gene rearrangements in lymphoproliferative diseases. Am J Pathol 199815229–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Dippel E, Assaf C, Hummel M.et al Clonal T‐cell receptor γ‐chain gene rearrangement by PCR‐based GeneScan analysis in advanced cutaneous T‐cell lymphoma: a critical evaluation. J Pathol 1999188146–154. [DOI] [PubMed] [Google Scholar]
- 22.Beaubier N T, Hart A P, Bartolo C.et al Comparison of capillary electrophoresis and polyacrylamide gel electrophoresis for the evaluation of T and B cell clonality by polymerase chain reaction. Diagn Mol Pathol 20009121–131. [DOI] [PubMed] [Google Scholar]
- 23.Dongen van J J M, Langerak A W, Brüggemann M.et al Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T‐cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED‐2 Concerted Action BMH4‐CT98‐3936. Leukemia 2003172257–2317. [DOI] [PubMed] [Google Scholar]
- 24.Lee S ‐ C, Berg K D, Racke F K.et al Pseudo‐spikes are common in histolgically benign lymphoid tissues. J Mol Diagn 20002145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprouse J T, Werling R, Hanke D.et al T‐cell clonality determination using polymerase chain reaction (PCR) amplification of the T‐cell receptor gamma‐chain gene and capillary electrophoresis of fluorescently labeled PCR products. Am J Clin Pathol 2000113838–850. [DOI] [PubMed] [Google Scholar]
- 26.Tokuda Y, Nakamura T, Satonaka K.et al Fundamental study on the mechanism of DNA degradation in tissues fixed in formaldehyde. J Clin Pathol 199043748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagi N, Satonaka K, Horio M.et al The role of DNase and EDTA on DNA degradation in formaldehyde fixed tissues. Biotech Histochem 199671123–129. [DOI] [PubMed] [Google Scholar]
- 28.Kerlan‐Cardon S, Soua Z, LeFranc M ‐ P.et al Detection of antigen receptor gene rearrangements in lymphoproliferative malignancies by fluorescent polymerase chain reaction. Tissue Antigens 19985120–29. [DOI] [PubMed] [Google Scholar]
- 29.Ayling J F, Iland H J. High‐resolution analysis of gene rearrangements in lymphoid malignancies. Pathology 199931252–256. [DOI] [PubMed] [Google Scholar]
- 30.Födinger M, Buchmayer H, Schwarzinger I.et al Multiplex PCR for rapid detection of T‐cell receptor‐gamma chain gene rearrangements in patients with lymphoproliferative diseases. Br J Haematol 199694136–139. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Bhawan J. New insights into the applicability of T‐cell receptor γ gene rearrangement analysis in cutaneous T‐cell lymphoma. J Cutan Pathol 200128412–418. [DOI] [PubMed] [Google Scholar]
- 32.Lawnicki L C, Rubocki R J, Chan W C.et al The distribution of gene segments in T‐cell receptor γ gene rearrangements demonstrates the need for multiple primer sets. J Mol Diagn 2003582–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Le Paslier D, Dausset J.et al Human T cell γ genes are frequently rearranged in B‐lineage acute lymphoblastic leukemias but not in chronic B cell proliferations. J Exp Med 19871651000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scrideli C A, Queiroz R G P, Kashima S.et al T cell receptor gamma (TCRG) gene rearrangements in Brazilian children with acute lymphoblastic leukemia: analysis and implications for the study of minimal residual disease. Leuk Res 200428267–273. [DOI] [PubMed] [Google Scholar]
- 35.Asou N, Matsuoka M, Hattori T.et al T‐cell γ gene rearrangements in hematologic neoplasms. Blood 198769968–970. [PubMed] [Google Scholar]
- 36.Subar M, Pelicci P G, Neri A.et al Patterns of T cell receptor gamma gene rearrangement and expression in B and T lymphoid malignancies. Leukemia 1988219–26. [PubMed] [Google Scholar]
- 37.Thériault C, Galoin S, Valmary S.et al PCR analysis of immunoglobulin heavy chain (IgH) and TCR‐γ chain gene rearrangements in the diagnosis of lymphoproliferative disorders: results of a study of 525 cases. Mod Pathol 2000131269–1279. [DOI] [PubMed] [Google Scholar]
- 38.Vega F, Medeiros L J, Jones D.et al A novel four‐color PCR assay to assess T‐cell receptor gamma gene rearrangements in lymphoproliferative lesions. Am J Clin Pathol 200111617–24. [DOI] [PubMed] [Google Scholar]
- 39.Williams C, Pontén F, Moberg C.et al A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol 19991551467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy K P, Sloane J P, Kabarowski J H S.et al A simplified method of detection of clonal rearrangements of the T‐cell receptor‐γ chain gene. Diagn Mol Pathol 19921173–179. [PubMed] [Google Scholar]
- 41.Krafft A E, Taubenberger J K, Sheng Z M.et al Enhanced sensitivity with a novel TCRgamma PCR assay for clonality studies in 569 formalin‐fixed paraffin‐embedded (FFPE) cases. Mol Diagn 19994119–133. [DOI] [PubMed] [Google Scholar]
- 42.Assaf C, Hummel M, Dippel E.et al High detection rate of T‐cell receptor beta chain rearrangements in T‐cell lymphoproliferations by family specific polymerase chain reaction in combination with the GeneScan technique and DNA sequencing. Blood 200096640–646. [PubMed] [Google Scholar]
- 43.Garcia M J, Martinez‐Delgado B, Granizo J J.et al IgH, TCR‐γ and TCR‐β gene rearrangement in 80 B‐ and T‐cell non‐Hodgkin's lymphomas: study of the association between proliferation and the so‐called “aberrant” patterns. Diagn Mol Pathol 20011069–77. [DOI] [PubMed] [Google Scholar]
- 44.Wood G S, Tung R M, Haeffner A C.et al Detection of clonal T‐cell receptor γ gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol 199410334–41. [DOI] [PubMed] [Google Scholar]
- 45.Idilman R, Colantoni A, de Maria N.et al Lymphoproliferative disorders in chronic hepatitis C. J Viral Hepat 200411302–309. [DOI] [PubMed] [Google Scholar]