Abstract
Background
Grade‐III invasive ductal carcinomas of no special type (IDCs‐NST) constitute a heterogeneous group of tumours with different clinical behaviour and response to chemotherapy. As many as 25% of all grade‐III IDCs‐NST are known to harbour a basal‐like phenotype, as defined by gene expression profiling or immunohistochemistry for basal cytokeratins. Patients with basal‐like breast carcinomas (BLBC) are reported to have a shorter disease‐free and overall survival.
Material and methods
A retrospective analysis of 49 patients with BLBC (as defined by basal cytokeratin expression) and 49 controls matched for age, nodal status and grade was carried out. Histological features, immunohistochemical findings for oestrogen receptor (ER), progesterone receptor (PgR) and HER2, and clinical outcome and survival after adjuvant chemotherapy were compared between the two groups.
Results
It was more likely for patients with BLBCs to be found negative for ER (p<0.0001), PgR (p<0.0001) and HER2 (p<0.01) than controls. Patients with BLBCs were found to have a significantly higher recurrence rate (p<0.05) and were associated with significantly shorter disease‐free and overall survival (both p<0.05). In the group of patients who received anthracycline‐based adjuvant chemotherapy (BLBC group, n = 47; controls, n = 49), both disease‐free and overall survival were found to be significantly shorter in the BLBC group (p<0.05).
Conclusions
BLBCs are a distinct clinical and pathological entity, characterised by high nuclear grade, lack of hormone receptors and HER2 expression and a more aggressive clinical course. Standard adjuvant chemotherapy seems to be less effective in these tumours and new therapeutic approaches are indicated.
Breast cancer is a heterogeneous disease, encompassing many morphological and molecular genetic entities. Invasive ductal carcinomas of no special type (IDCs‐NST) are the most prevalent histological type, accounting for as many as 85% of all malignant breast neoplasms. Although several markers have been used to identify specific prognostic groups and predict response to treatment, histological grade still remains one of the best predictors of tumour behaviour in IDCs.1
Poorly differentiated (grade‐III) IDCs are strongly associated with shorter recurrence‐free and overall survival than grade‐I and grade‐II tumours.1,2,3 Heterogeneity is found even within the group of poorly differentiated IDCs, and a proportion shows a more aggressive pattern of disease.4 Molecular markers have provided additional prognostic and predictive data for poorly differentiated IDCs. In particular, immunohistochemical staining for hormone receptors (oestrogen receptor (ER) and progesterone receptor (PgR), oncogenes (HER2), tumour suppressor genes (p53) and the proliferative marker MIB‐1) has proved to be an important tool for defining groups of patients with more aggressive tumours.1,3,5,6,7
Recently, gene expression microarray analysis redefined breast cancer taxonomy and identified five groups associated with distinct overall survival patterns.8,9,10,11,12 One of the subgroups identified had some characteristics of basal or myoepithelial cells in the normal breast and was associated with a more aggressive clinical behaviour.8,9,10,11,12 The expression of basal or myoepithelial markers has been observed in 2–18% of all IDCs and in as many as 25% of grade‐III carcinomas.13,14 Most of these are high‐grade tumours, have large central acellular or necrotic zones and a high proliferation index, and are negative for hormone receptors (ER and PgR) and HER2.4,14,15,16,17,18,19,20,21,22 It has been reported that this group is associated with a higher risk for brain and lung metastases19 and for cancer‐related death, independent of nodal status and tumour size.13
Although several definitions of basal‐like carcinomas have been proposed,4,8,9,10,11,13,14,15,16,17,21,22,23,24 there is no internationally accepted consensus on this. The expression of cytokeratins (Cks) of high molecular weight, including Ck5, Ck14 and Ck17, can be used to identify these lesions reliably on tissue sections fixed in formalin and embedded in paraffin wax.4,13,14,15,17,25 By contrast, Nielsen et al8 have developed an immunohistochemical panel for identifying basal‐like carcinomas on the basis of a comparison between the transcriptomic and immunohistochemical profiles. According to this definition,8 basal‐like carcinomas are negative for ER and HER2 and positive for basal cytokeratins, epidermal growth factor receptor (HER1 or EGFR) or c‐KIT. On the other hand, others have proposed that a proportion of basal‐like breast carcinomas (BLBCs) may be positive for ER.17,21 Furthermore, overexpression and amplification of HER2 has also been accepted as part of the definition of basal‐like carcinomas.22,23
Anecdotal experience of treating patients with a basal‐like phenotype is that they have a shorter disease‐free survival than patients with other grade‐III tumours after adjuvant chemotherapy, but only limited data are available on the response of these tumours to different neoadjuvant therapeutic regimens.26,27 We present a retrospective analysis of the natural history, outcome and survival after chemotherapy of patients with BLBCs diagnosed and managed at the Royal Marsden Hospital, London, UK.
Material and methods
Patient selection
A cohort of 282 patients with grade‐III2 primary breast carcinomas, diagnosed and treated at the Royal Marsden Hospital between 1993 and 2000, were screened for the expression of Ck5/Ck6, Ck14 and Ck17. In all, 49 (17.4%) cases were positive for at least one of these markers and were therefore considered to be of the basal‐like subtype. The control group consisted of 49 patients matched with the group with basal‐like tumours, according to the following parameters: age (SD 10 years), nodal status (negative, 1–4 nodes; positive, >5 nodes) and grade (grade III). When more than one matching control was available, that with the date of diagnosis closest to that of the basal‐like tumour was selected. All participants in both groups were diagnosed and treated at the Royal Marsden Hospital.
Clinical data were obtained retrospectively by using the electronic patient record system, medical notes and the database at the RMH. The clinical parameters documented included disease relapse, response to treatment, disease progression and disease‐related death.
Histological and immunohistochemical analysis
All cases were independently reviewed by two of the authors (JSRF and SRL) and representative blocks were chosen. Tumours were graded according to a modified Bloom–Richardson–Scarff grading system.2 Sections of 2 μm thickness were cut and mounted on polylysine‐coated slides. Immunohistochemical analysis was carried out as described previously,4,28 with antibodies against ER (clone ID5, Dakocytomation (Glostrup, Denmark), 1:40, antigen retrieval: 2 min pressure cooker), PgR (clone PgR636, Dakocytomation, 1:150, antigen retrieval: 2 min pressure cooker), HER2 (polyclonal, Dakocytomation, 1:1200, 40 min, antigen retrieval: water bath), Ck5/Ck6 (clone D5/16B4, Chemicon (Temecula, CA, USA), 1:600, antigen retrieval: 18 min microwave oven, citrate buffer, pH 6), Ck14 (clone LL002, Vector, 1:40, antigen retrieval: 18 min microwave oven, citrate buffer, pH 6) and Ck17 (clone E3, Dakocytomation, 1:100, antigen retrieval: 2 min pressure cooker). Positive and negative controls were included in each slide run and showed appropriate results.
Tumours were considered to be positive for ER and PgR when nuclear reactivity was observed in >10% of neoplastic cells at any intensity.29 For Ck5, Ck14 and Ck17, any cytoplasmic expression in definite neoplastic cells or tissue was considered to be positive.17 Evaluation of HER2 activity was carried out according to the system used for scoring HercepTest30 (1+, faint or barely perceptible incomplete membrane staining in >10% of the tumour cells; 2+, weak to moderate complete membrane staining in >10% of the tumour cells; 3+, strong and complete membrane staining in 10% of cells). Scores of 0 and 1+ were considered to be a negative HER2 result. Tumours scored as 3+ were considered to be positive for HER2 overexpression. Tumours scored as 2+ were further analysed for HER2 amplification by means of chromogenic in situ hybridisation, as described previously.25,28
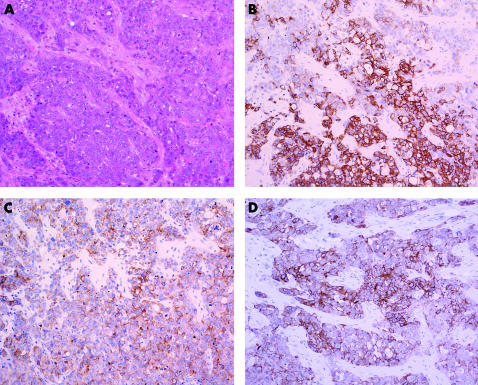
For the purpose of this study, we used the definition of basal‐like carcinoma proposed by Jones et al,4,14 van de Rijn et al,13 Laakso et al21 and Fulford et al,17 in which these tumours are characterised by the expression of basal markers (Ck5 or Ck14 or Ck17), regardless of the expression of hormone receptors or HER2 (fig 1). The pathological features noted included tumour size, nodal status, presence of vascular invasion and the immunohistochemical profile for ER, PgR, HER2, Ck5/Ck6, Ck14 and Ck17.
Figure 1 Photomicrograph of a basal‐like breast carcinoma showing a high‐grade invasive ductal carcinoma not otherwise specified, composed of sheets and cords of polygonal, epithelioid atypical cells, with marked pleomorphism, moderate eosinophilic cytoplasm and conspicuous mitotic activity. (A) Staining with haematoxylin–eosin; (B) cytokeratin (Ck) 14; (C) Ck5/Ck6; and (D) Ck17.
Statistical analysis
Tumour characteristics were compared between groups with basal‐like and non‐basal‐like (control) carcinomas by means of the Fisher exact test or Mann–Whitney U test. Survival and disease‐free survival were measured from the date of diagnosis until death or relapse in any site, or date of last follow‐up. Survival curves were drawn by the Kaplan–Meier method and differences assessed by the log‐rank test. All tests were two tailed, with a confidence interval of 95%.
Multivariate analysis was carried out with Cox's multiple hazards model and p = 0.1 in the univariate survival analysis was adopted as the limit for inclusion in the multivariate model. Cases with missing values were excluded. Data were statistically analysed with SPSS and Statview.
Results
Table 1 summarises the histopathological and immunohistochemical features of the basal‐like and control carcinomas. Briefly, basal‐like carcinomas were more frequently ER negative (p<0.0001), PgR negative (p<0.0001) and HER2 negative (p<0.01) than controls. Table 2 gives the tumour characteristics of the group with basal‐like carcinoma and of the matched controls. The median follow‐up time for the group with basal‐like carcinoma was 68.1 (range 9.0–134.2) months, which was similar to that for the controls (median 72.3, range 7.5–191.1 months).
Table 1 Histopathological and immunohistochemical features of basal‐like carcinomas and matched controls.
| Basal‐like | Control | Significance | ||
|---|---|---|---|---|
| Pathological variables | ||||
| Tumour size (mm) | Median (range) | 20 (5–68) | 20 (4–60) | p = 0.5 (Mann–Whitney) |
| Vascular invasion | Negative | 23 | 17 | p>0.1 |
| Positive | 26 | 32 | ||
| Tumour markers | ||||
| Ck14 | Positive | 32 | 0 | ND |
| Negative | 17 | 49 | ||
| Ck5/Ck6 | Positive | 32 | 0 | ND |
| Negative | 17 | 49 | ||
| Ck17 | Positive | 31 | 0 | ND |
| Negative | 18 | 49 | ||
| Ck14 and Ck5/Ck6 | Positive | 25 | 0 | ND |
| Negative | 24 | 49 | ||
| Ck14 and Ck17 | Positive | 31 | 0 | ND |
| Negative | 18 | 49 | ||
| Ck5/Ck6 and Ck17 | Positive | 17 | 0 | ND |
| Negative | 32 | 49 | ||
| All Ck | Positive | 15 | 0 | ND |
| Negative | 34 | 49 | ||
| ER status | Negative | 40 | 11 | p<0.0001 |
| Positive | 9 | 38 | ||
| PgR status | Negative | 39 | 14 | p<0.0001 |
| Positive | 10 | 35 | ||
| HER2 status* | Negative | 45 | 34 | p<0.01 |
| Positive | 4 | 15 | ||
Ck, cytokeratin; ER, oestrogen receptor; ND, not done (definition criteria); PgR: progesterone receptor.
*One basal‐like carcinoma and one control were scored as HER2 2+ by immunohistochemical analysis; however, both failed to show HER2 amplification by chromogenic in situ hybridisation (data not shown). Therefore, these cases were considered to be negative for HER2 amplification or overexpression.
Table 2 Summary of the clinical features of basal‐like carcinomas and matched controls.
| Matching variables | Basal‐like (n = 49) | Control (n = 49) | Significance | |
|---|---|---|---|---|
| Age (years) | Median (range) | 45 (25–64) | 48 (35–64) | |
| Nodal status | Negative | 28 | 27 (1 ND) | |
| 1–4 nodes Positive | 11 | 11 | ||
| >5 nodes | 10 | 10 | ||
| Primary treatment | ||||
| Surgery | Conservative | 35 | 44 | p<0.05 |
| Mastectomy | 14 | 5 | ||
| Adjuvant chemotherapy | None | 2 | 0 | p = 0.5 |
| Anthracycline regimen | 47 | 49 | ||
| Endocrine | None | 19 | 6 | p<0.005 |
| Tamoxifen | 30 | 43 | ||
| Radiotherapy | None | 9 | 5 | p = 0.4 |
| Yes | 40 | 44 |
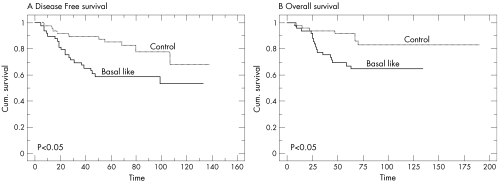
Basal‐like carcinomas showed a higher recurrence rate (42.8%) than controls (20.4%; p<0.05). These tumours also showed a significantly shorter disease‐free survival (fig 2A; p<0.05) and overall survival (fig 2B; p<0.05). At 5 years, the actuarial disease‐free survival was significantly different in patients with basal‐like and control carcinomas (61.1% v 89.8%, respectively; p<0.05). The actuarial overall survival at 5 years was also significantly lower in patients with basal‐like carcinomas than in the control group (69.3% v 91.8%, respectively; p<0.05).
Figure 2 Kaplan–Meier curves for (A) disease‐free and (B) overall survival for all patients.
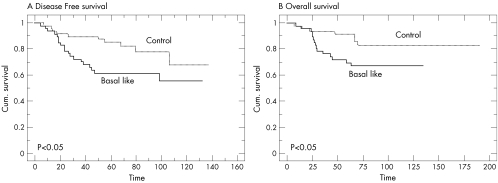
The number of patients receiving anthracycline‐based adjuvant chemotherapy—namely 5‐fluorouracil and cyclophosphamide and either epirubicin or adriamycin—was similar in both groups (basal‐like, n = 47; controls, n = 49). Taking into account only those patients who received the same type of adjuvant chemotherapy, the disease‐free survival and overall survival were also significantly shorter in patients in the BLBC group (figs 3A,B; p<0.05). In multivariate analysis including ER, PgR, lymph–vascular invasion, endocrine therapy and basal‐like status, none of the variables reached statistical significance for disease‐free and overall survival.
Figure 3 Kaplan–Meier curves for (A) disease‐free and (B) overall survival for patients treated with adjuvant chemotherapy.
Recurrent disease
Twenty patients in the BLBC group developed relapsed disease. Table 3 outlines the sites of disease relapse. Fifteen patients were treated with chemotherapy either alone or in combination with radiotherapy, endocrine therapy or surgery for metastatic disease. The response to treatment was documented in 4 (27%) of these patients; response duration ranged from 1 to 6 months (table 4). Table 5 summarises the clinical course of the patients who did not respond to chemotherapy. Two patients received radiotherapy alone for metastatic disease to the brain. Both patients died within 4 months of the diagnosis of metastatic disease. In addition, three patients received treatment for local relapse.
Table 3 Site distribution of disease relapse, including locoregional recurrence and distant metastases.
| Site of relapse | Initial event* | Total events | ||
|---|---|---|---|---|
| Basal‐ like | Control | Basal‐ like | Control | |
| Local recurrence† | 8 | 2 | 8 | 2 |
| Soft tissue and skin | 0 | 0 | 4 | 0 |
| Lymph nodes (non‐regional) | 6 | 1 | 9 | 1 |
| Bone | 4 | 1 | 6 | 1 |
| Liver | 3 | 1 | 5 | 1 |
| Lung and pleura | 6 | 3 | 11 | 3 |
| Brain | 3 | 0 | 5 | 0 |
| Total number of events | 30 | 8 | 48 | 8 |
*All concurrent metastatic events were considered to be independent events.
†Local recurrence was defined as recurrence in the ipsilateral breast, skin and regional nodes.
Table 4 Summary of clinical histories of basal‐like tumours responding to chemotherapy.
| Case number | Disease‐free survival (months) | Relapse site | Treatment after relapse of disease | Response | Time from response to PD (months) | Site of PD | Treatment at progression | Time from relapse to death (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 19 | Bone, LN | MVP 6 cycles DXT (bone) | PR (post cycle 3 and 6, CT) | 6 | Breast LN | Doxorubicin (4 cycles) | 17 |
| 2 | 20 | Bone, liver | Doxorubicin 6 cycles | PR (post cycle 3 and 6, CT) | NA | NA | NA | 5 |
| 3 | 24 | Ipsilateral breast | Neoadjuvant vinorelbine | PR post 2 cycles PD post 4 cycles (clinical) | 1 | Chest wall, LN | Letrozole 2 months PD docetaxel (6 cycles) PD liver, sternum, pleural effusion capecitabine 2 months | 35 |
| 4 | 12 | LN neck, axilla | Surgery DXT PD on treatment docetaxel 4 cycles | PR (post 2 cycles, US) | 2 | LN | FTI 3 months PD chest wall vinorelbine+5FU (2 cycles) PD chest wall, LN MVP (7 cycles) PD lung | 14 |
5‐FU, 5‐fluorouracil; CT, computed tomography; DXT, radiotherapy; FTI, farnesyl transferase inhibitor; LN, lymph nodes; MVP, mitomycin, vinblastine, cisplatin; PD, progressive disease; PR, partial response; US, ultrasound.
Table 5 Summaries of clinical histories of patients with basal‐like tumours not responding to chemotherapy.
| Case number | Disease‐free survival (months) | Site of relapse | Treatment after relapse of disease | Time relapse to death (months) | Overall survival (months) |
|---|---|---|---|---|---|
| 1 | 8 | LN | MVP (2 cycles) PD lung, breast, LN | 5 | 13 |
| Ifosfamide (2 cycles) PD | |||||
| Paclitaxel (1 cycle) PD | |||||
| 5‐FU (1 cycle) PD | |||||
| Docetaxel (1 cycle) PD | |||||
| 2 | 27 | Liver, bone, LN | Vinorelbine (2 cycles) | 2 | 29 |
| 3 | 60 | Bone | Docetaxel (6 cycles) PD liver, lung, bone megestrol | 7 | 67 |
| 4 | 37 | Brain, lung | DXT brain docetaxel (4 cycles) | 8 | 45 |
| 5 | 48 | Bone | DXT bone | 15 | 63 |
| Letrozole 2 months PD | |||||
| Paclitaxel (6 cycles) PD | |||||
| Exemestane 13 months PD brain | |||||
| DXT brain | |||||
| Capecitabine | |||||
| 6 | 19 | Bilateral breast, axilla | Docetaxel (4 cycles) | 7 | 26 |
| MVP (1 cycle) | |||||
| 7 | 5 | Lung | Docetaxel (2 cycles) | 5 | 10 |
| 8 | 16 | Skin, axilla | FTI PD pericardial effusion | 11 | 27 |
| Vinorelbine (2 cycles) PD chest wall | |||||
| Docetaxel (4 cycles) PD lung | |||||
| MVP (1 cycle) | |||||
| 9 | 28 | Liver, lung | Vinorelbine/epirubicin (1 cycle) | 0.8 | 28.8 |
| 10 | 7 | Ipsilateral LN | Surgery | 37 | 44 |
| Adjuvant FEC (6 cycles) | |||||
| PD skin | |||||
| Miftefosine 7 months PD | |||||
| Herceptin 10 months PD lung | |||||
| Taxoprexin (5 cycles) PD lung | |||||
| Vinorelbine (2 cycles) PD bone | |||||
| 11 | 7 | Ipsilateral LN | Surgery | 3 | 10 |
| Adjuvant CMF (2 cycles) PD lung | |||||
| Bleomycin (1 cycle) PD | |||||
| Doxorubicin (1 cycle) PD | |||||
| Megestrol (1 week) |
5‐FU, 5‐fluorouracil; CMF, cyclophosphamide, methotrexate, 5‐fluorouracil; FTI, farnesyl transferase inhibitor; LN, lymph nodes; MVP, mitomycin, vinblastine, cisplatin; PD, progressive disease, PR, partial response.
Discussion
BLBCs have attracted considerable attention over the past few years. The genes and molecular markers expressed in these tumours suggest that they are distinct biological entities, with an aggressive clinical behaviour and a characteristic pattern of metastatic spread. Our results corroborate those of others, in that basal‐like carcinomas were more frequently negative for ER, PgR and HER2 than non‐basal‐like carcinomas.4,13,15,16,21 Furthermore, patients with basal‐like tumours showed shorter disease‐free and overall survival when compared with patients matched for age, nodal status and grade of non‐BLBCs.4,8,13,15,16 van de Rijn et al13 also showed that basal‐like carcinomas (defined as those tumours expressing Ck17 or Ck5) were associated with a markedly worse prognosis than tumours negative for basal keratins. In the group of patients that was node negative, the expression of basal markers was a prognostic factor that was independent of tumour size, grade, HER2 and ER status.13
Take‐home messages
Basal‐like breast carcinomas (BLBCs) are a distinct clinical and pathological group, characterised by high histological grade, lack of hormone receptors and HER2 expression.
BLBCS have a significantly higher recurrence rate, shorter disease‐free and overall survival.
Adjuvant anthracycline‐based chemotherapy seems to be less effective for this group.
Other treatment approaches need to be identified for this group.
By using a different definition for basal‐like carcinomas, comprising negativity for ER and HER2 and expression of at least one basal marker, Nielsen et al8 showed that the disease‐specific survival of patients with basal‐like carcinomas is shorter than that of patients with ER‐positive tumours. Interestingly, in this study,8 patients with HER2‐positive tumours had a shorter overall survival than those with basal‐like tumours. This finding is in contrast with the results published by Sørlie et al,9 in which patients with basal‐like carcinomas showed the shortest overall survival. In our previous report on immunohistochemical and comparative genomic hybridisation analysis of grade‐III IDCs‐NST with extensive follow‐up, we showed that basal‐like tumours may be heterogeneous with “good” and “bad” prognosis groups,4 and this may partly account for some of the differences in outcome seen in different groups.
Basal markers are not routinely used in the standard histological diagnosis of breast cancer. As existing prognostic markers do not identify this group, patients with basal‐like and non‐basal‐like tumours are currently treated similarly. A potential explanation for the poorer clinical outcome of patients with basal‐like tumours is that this subset can be less responsive to chemotherapy. In our study we have shown data suggesting that adjuvant anthracycline‐based chemotherapy is less effective in patients with basal‐like carcinomas than in controls matched for age, nodal status and grade. Furthermore, we found that only a few of these tumours responded to chemotherapy after systemic relapse. Further data on the response of metastatic disease to chemotherapy are now needed, but it may be that patients with basal‐like carcinomas are less sensitive to standard adjuvant chemotherapy than those with other types of breast cancer, and that distinct, novel therapeutic approaches are required.
By contrast, data from other studies on the response to a neoadjuvant chemotherapy regimen in patients in the BLBC group suggested that the clinical response to adriamycin and cyclophosphamide was markedly higher among patients with basal‐like tumours than among those with non‐basal‐like tumours. Pathological complete response to neoadjuvant chemotherapy was higher in patients with basal‐like tumours than in those with non‐basal‐like tumours.26 In another study,27 responses to neoadjuvant chemotherapy (paclitaxel, doxorubicin, 5‐fluorouracil and cyclophosphamide) for basal‐like, HER2, luminal and normal BLBC showed that BLBCs and HER2 tumours were significantly more likely to have pathological complete response to chemotherapy (p<0.01) than the other groups.27 The differences between our findings and those from neoadjuvant studies26 may be due to differences in the cohorts analysed and in the definition of basal‐like tumours.4,8,9,10,11,13,14,15,16,17,21,22,23 Whereas Rouzier et al and Carey et al analysed cohorts with a mixture of breast carcinomas of grades I, II and III, we included only grade‐III tumours in our study. Furthermore, Carey et al26 defined basal‐like tumours as those lacking expression of ER, PgR and HER2, whereas Rouzier et al27 used a transcriptomic definition for these tumours. Neither of these studies used immunohistochemical analysis for basal cytokeratins in the definition.
It has been shown that basal‐like carcinomas consistently overexpress HER1 or EGFR8,31,32 and there may be a role for EGFR inhibitors in the treatment of this tumour subset. Tumours arising in carriers of the BRCA1 germline mutation often show transcriptomic and immunohistochemical profiles similar to those of basal‐like carcinomas.32,33,34 BRCA1‐associated tumours harbour defects in specific DNA double‐strand break–repair pathways and may show selective sensitivity to DNA cross‐linking chemotherapeutic agents such as cisplatin and carboplatin.34 Basal tumours showing hallmarks of “BRCAness”34 may show similar sensitivity and these drugs may be more effective in the treatment of this group.
In conclusion, basal‐like carcinomas constitute a discrete clinical and pathological entity, characterised by high nuclear grade and a propensity for lack of ER, PgR and HER2 expression. These tumours have a more aggressive clinical course, as shown by shorter disease‐free and overall survival after standard adjuvant anthracycline‐based chemotherapy, than otherwise matched non‐basal‐like tumours. New treatment options should be investigated for patients with this subtype of breast cancer.
Abbreviations
BLBC - basal‐like breast carcinoma
Ck - cytokeratin
EGFR - epidermal growth factor receptor
ER - oestrogen receptor
IDCs‐NST - invasive ductal carcinomas of no special type
PgR - progesterone receptor
Footnotes
Competing interests: None declared.
References
- 1.Ellis I O, Schnitt S J, Sastre‐Garau X.et al Invasive breast carcinoma. In: Devilee P, ed. Pathology and genetics. Tumours of the breast and female genital organs. Lyon: IAR Press, 200313–59.
- 2.Elston C W, Ellis I O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long‐term follow‐up, Histopathology 199119403–410. [DOI] [PubMed] [Google Scholar]
- 3.Silvestrini R, Daidone M G, Luisi A.et al Biologic and clinicopathologic factors as indicators of specific relapse types in node‐negative breast cancer. J Clin Oncol 199513697–704. [DOI] [PubMed] [Google Scholar]
- 4.Jones C, Ford E, Gillett C.et al Molecular cytogenetic identification of subgroups of grade III invasive ductal breast carcinomas with different clinical outcomes. Clin Cancer Res 2004105988–5997. [DOI] [PubMed] [Google Scholar]
- 5.Harvey J M, Clark G M, Osborne C K.et al Estrogen receptor status by immunohistochemistry is superior to the ligand‐binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999171474–1481. [DOI] [PubMed] [Google Scholar]
- 6.Menard S, Fortis S, Castiglioni F.et al HER2 as a prognostic factor in breast cancer. Oncology 20016167–72. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph P, Olsson H, Bonatz G.et al Correlation between p53, c‐erbB‐2, and topoisomerase II alpha expression, DNA ploidy, hormonal receptor status and proliferation in 356 node‐negative breast carcinomas: prognostic implications. J Pathol 1999187207–216. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen T O, Hsu F D, Jensen K.et al Immunohistochemical and clinical characterization of the basal‐like subtype of invasive breast carcinoma. Clin Cancer Res 2004105367–5374. [DOI] [PubMed] [Google Scholar]
- 9.Sørlie T, Perou C M, Tibshirani R.et al Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 20019810869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sørlie T, Tibshirani R, Parker J.et al Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 20031008418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perou C M, Jeffrey S S, van de Rijn M.et al Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA 1999969212–9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotiriou C, Neo S Y, McShane L M.et al Breast cancer classification and prognosis based on gene expression profiles from a population‐based study. Proc Natl Acad Sci USA 200310010393–10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Rijn M, Perou C M, Tibshirani R.et al Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 20021611991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C, Nonni A V, Fulford L.et al CGH analysis of ductal carcinoma of the breast with basaloid/myoepithelial cell differentiation. Br J Cancer 200185422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abd El‐Rehim D M, Pinder S E, Paish C E.et al Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 2004203661–671. [DOI] [PubMed] [Google Scholar]
- 16.Abd El‐Rehim D M, Ball G, Pinder S E.et al High‐throughput protein expression analysis using tissue microarray technology of a large well‐characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 2005116340–350. [DOI] [PubMed] [Google Scholar]
- 17.Fulford L G, Easton D F, Sofronis A.et al Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinomas of the breast. Pathol Int 200454A2–A3. [DOI] [PubMed] [Google Scholar]
- 18.Putti T C, El‐Rehim D M, Rakha E A.et al Estrogen receptor‐negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol 20051826–35. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda H, Takarabe T, Hasegawa F.et al Large, central acellular zones indicating myoepithelial tumor differentiation in high‐grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol 200024197–202. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda H, Takarabe T, Hasegawa T.et al Myoepithelial differentiation in high‐grade invasive ductal carcinomas with large central acellular zones. Hum Pathol 1999301134–1139. [DOI] [PubMed] [Google Scholar]
- 21.Laakso M, Loman N, Borg A.et al Cytokeratin 5/14‐positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol 2005181321–1328. [DOI] [PubMed] [Google Scholar]
- 22.Matos I, Dufloth R, Alvarenga M.et al p63, cytokeratin 5, and P‐cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Arch 2005447688–694. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum D, Bertucci F, Ginestier C.et al Basal and luminal breast cancers: basic or luminous? Int J Oncol 200425249–258. [PubMed] [Google Scholar]
- 24.Gusterson B A, Ross D T, Heath V J.et al Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res 20057143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isola J, Tanner M, Forsyth A.et al Interlaboratory comparison of HER‐2 oncogene amplification as detected by chromogenic and fluorescence in situ hybridization. Clin Cancer Res 2004104793–4798. [DOI] [PubMed] [Google Scholar]
- 26.Carey L A, Dees E C, Sawyer L R.et al The triple negative paradox: primary tumor chemosensitivity of the basal‐like breast cancer phenotype. Breast Cancer Res Treat 2004801023 [Google Scholar]
- 27.Rouzier R, Perou C M, Symmans W F.et al Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005115678–5685. [DOI] [PubMed] [Google Scholar]
- 28.Reis‐Filho J S, Simpson P T, Jones C.et al Pleomorphic lobular carcinoma of the breast: role of comprehensive molecular pathology in characterization of an entity. J Pathol 20052071–13. [DOI] [PubMed] [Google Scholar]
- 29.Ferrero‐Pous M, Trassard M, Le Doussal V.et al Comparison of enzyme immunoassay and immunohistochemical measurements of estrogen and progesterone receptors in breast cancer patients. Appl Immunohistochem Mol Morphol 20019267–275. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs T W, Gown A M, Yaziji H.et al Specificity of HercepTest in determining HER‐2/neu status of breast cancers using the United States Food and Drug Administration‐approved scoring system. J Clin Oncol 1999171983–1987. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda H, Morita D, Kimura M.et al Correlation of KIT and EGFR overexpression with invasive ductal breast carcinoma of the solid‐tubular subtype, nuclear grade 3, and mesenchymal or myoepithelial differentiation. Cancer Sci 20059648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakhani S R, Reis‐Filho J S, Fulford L.et al Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res 2005115175–5180. [DOI] [PubMed] [Google Scholar]
- 33.Foulkes W D, Stefansson I M, Chappuis P O.et al Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 2003951482–1485. [DOI] [PubMed] [Google Scholar]
- 34.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness' in sporadic cancers. Nat Rev Cancer 20044814–819. [DOI] [PubMed] [Google Scholar]