Abstract
Objectives
To test whether magnetic resonance (MR) imaging can be used to assess differential lung blood flow as accurately as isotope lung perfusion studies in patients investigated for congenital heart disease.
Methods and results
Radionuclide lung perfusion and MR imaging were performed in 12 children with suspected unilateral branch pulmonary artery stenosis (mean age 12.1 (5.9) years, range 3.1–17.2 years). A non‐breath hold, fast gradient echo phase contrast MR sequence was used to measure flow in the pulmonary trunk and one pulmonary artery to calculate differential flow. Good agreement was shown between the two imaging methods by Bland‐Altman analysis. There was excellent correlation between the radionuclide and MR phase contrast calculated total lung blood flow (r = 0.98, p < 0.0001).
Conclusion
MR phase contrast is an accurate method for measuring differential total right and left lung blood flow. If MR imaging is performed to assess the branch pulmonary arteries, differential lung blood flow can be also measured, avoiding the need for an additional radionuclide lung perfusion scan and reducing the overall radiation burden to this group of patients.
Keywords: branch pulmonary stenosis, magnetic resonance imaging, phase contrast
The assessment and functional significance of patients with branch pulmonary artery stenosis is important in congenital heart disease. Conventional assessments include radionuclide lung perfusion scanning, and repeat investigations can be used to assess the effect of surgical or catheter based interventions.1 However, radionuclide lung perfusion imaging is associated with a radiation dose of about 1 mSv, equivalent to about 50 frontal chest radiographs or six months' background radiation exposure in the UK.2 Repeat investigations of this nature can therefore contribute significantly to the radiation burden for these children and adolescents.
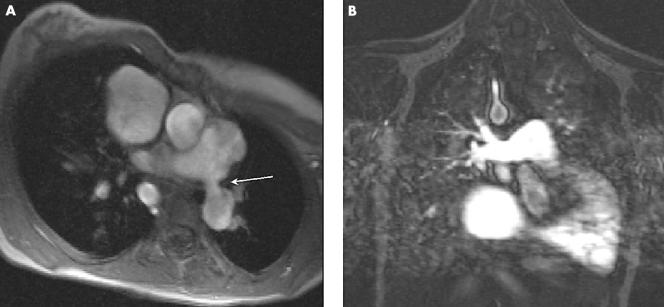
Magnetic resonance (MR) imaging is now increasingly used in the assessment of congenital heart disease, in both children and adults.3 MR is particularly good at imaging the morphology of branch pulmonary arteries, which may be difficult to image with echocardiography (fig 1).4 MR imaging can also be used to assess blood flow. This has been well documented for the measurement of aortic and pulmonary trunk blood flow for the assessment of aortic and pulmonary valve function, respectively.5,6 Other smaller vessels including the coronary arteries and branch pulmonary arteries have also been assessed.7,8,9
Figure 1 (A) Axial true fast imaging with steady state precession (FISP) of left pulmonary artery origin stenosis (arrow). (B) Coronal contrast enhanced magnetic resonance (MR) angiography of widely patent right pulmonary artery in the same subject.
Correlation between the assessment of differential blood flow MR imaging and radionuclide lung perfusion has been shown to be accurate in adults with normal branch pulmonary artery anatomy.8 More recently, branch pulmonary artery blood flow has been well documented in patients with pulmonary incompetence.9
To date no data have been collected assessing differential blood flow in patients with pulmonary artery stenosis. The objective of our study was to compare MR imaging measurements of differential right and left lung blood flow with radionuclide lung perfusion data in children with congenital heart disease and potential unilateral branch pulmonary artery stenosis. Importantly, branch pulmonary blood flow can be assessed with MR imaging at the same time as anatomical visualisation in a minimally invasive fashion without exposure to x ray radiation.
PATIENTS AND METHODS
Subject population
Twelve consecutive patients who were referred for MR imaging for suspected unilateral branch pulmonary artery stenosis as defined by echocardiography and who had previously undergone radionuclide lung perfusion were enrolled in the study (mean age 12.1 (5.9) years, range 3.1–17.2 years, five girls). Patients with bilateral pulmonary artery stenosis were excluded from the study. No patient was included more than once in the study.
All investigations were performed as part of routine clinical assessment. The median duration between MR and radionuclide imaging was 74 days. The MR imagers were blinded to the nuclear medicine results. All patients (or their parents or guardians) gave informed consent for the MR imaging investigation.
MR imaging
MR imaging was performed at 1.5 T (Symphony, Maestro class; Siemens Medical Systems, Erlangen, Germany). Total scan duration was 45–60 minutes.
The clinical MR imaging protocol was as follows: localisers through the thorax; half Fourier single shot turbo spin echo “black blood” axial images through the heart and great vessels; balanced steady state free precession cine images through the heart (long axis, short axis, right and left ventricular outflow tracts in two planes); black blood turbo spin echo images in two perpendicular planes through the pulmonary trunk and both branch pulmonary arteries; and gadolinium enhanced MR angiography of the pulmonary vasculature (Magnevist (gadopentetate dimeglumine); Schering AG, Germany) 0.2–0.4 ml/kg). Contrast agent was delivered by an automatic injector with triggering of image acquisition in real time. All of these images were acquired during a single breath hold of between 8–20 seconds.
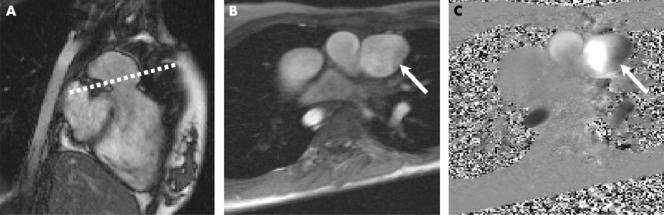
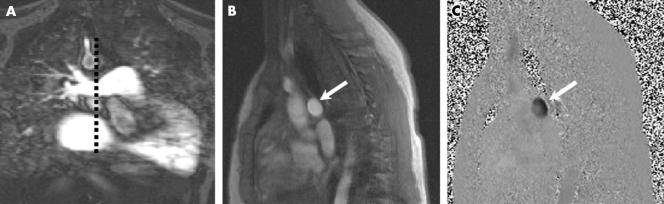
Phase contrast imaging was performed in the pulmonary trunk and the larger of the two branch pulmonary arteries (that is, the non‐stenosed branch pulmonary artery) during free respiration (scan duration 2–4 minutes). A fast low angle shot sequence was used with the following imaging parameters: repeat time 23 ms, echo time 4.8 ms, flip angle 15°, slice thickness 5 mm, matrix 256 × 256, field of view 340 mm, three averages, velocity encode gradient 1–4 m/s, and 40 phases. Through plane flow through the pulmonary trunk was performed just above the pulmonary valve, prescribed from two perpendicular pulmonary trunk views, as described previously10 (fig 2). Branch pulmonary artery flow was measured through plane in the least stenosed branch pulmonary artery, prescribed from two perpendicular views10 (fig 3).
Figure 2 Flow assessment in the pulmonary trunk. (A) Right ventricular outflow tract true FISP image. (B) Modulus image and (C) phase contrast image of pulmonary trunk through plane (arrows) prescribed from image A (dotted line).
Figure 3 Flow assessment in the right pulmonary artery (RPA). (A) RPA coronal contrast enhanced MR angiography. (B) Modulus image and (C) phase contrast image of RPA through plane prescribed from image A (dotted line).
MR imaging was performed without anaesthesia or sedation in children older than 8 years. Clear operator instructions and the use of a video within the scanner, playing a movie selected by the patient, allowed for greater compliance with the MR protocol.
For three children under 8 years of age, cardiac MR imaging was performed under general anaesthesia according to departmental protocol.
MR image data analysis
Blood flow was calculated from the phase contrast images by using a semi‐automated vessel edge detection algorithm, with minor operator corrections made by Argus flow analysis software (Siemens Medical Systems). The net forward flow was used to enable inclusion of patients with pulmonary incompetence in the study, where net forward flow (ml) = total forward flow (ml) – total backward flow (ml).
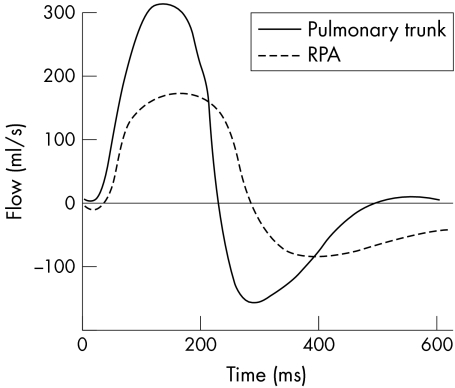
The percentage total blood flow to the measured lung was calculated as [net forward branch pulmonary artery blood flow (ml) × 100] divided by [net forward pulmonary trunk blood flow (ml)] (fig 4).
Figure 4 Plot of instantaneous flow versus time for the pulmonary trunk and RPA. Severe pulmonary incompetence is noted. The net forward flow for each vessel is calculated, and the percentage of net RPA forward flow to net pulmonary trunk flow gives the differential lung blood flow: right lung 65%, left lung 35%.
The percentage total blood flow to the non‐measured lung was 100% minus the measured lung blood flow calculated above.
A single blinded experienced investigator analysed and processed the MR phase contrast data. The diameters of the proximal and distal branch pulmonary arteries were also recorded by a combination of the black blood turbo spin echo and MR angiography images. The MR angiogram images were used in the first instance to assess diameter, as this is a three dimensional dataset. However, for severe stenoses, signal may be lost in the MR angiography gradient echo images, leading to an overestimation of stenosis severity. The black blood spin echo images are not affected by this signal loss and, although the images are two dimensional, they can give an accurate assessment when stenoses are severe.
Radionuclide lung perfusion imaging
Radionuclide lung perfusion scanning was performed with technetium‐99m macroaggregated albumin as part of routine clinical assessment. The dose of 99mTc used for each patient was based on a percentage of the standard adult dose of 80 MBq, calculated with the use of a surface area scaling factor.
Dynamic views were obtained initially for the first three minutes of each perfusion study at 1 frame/s to assess blood flow and enable imaging of any possible shunts. Posterior images were then acquired over a 5–8 minute period to assess blood pooling in the lungs. Left posterior oblique, right posterior oblique, and anterior images were then also acquired (acquisition period 5–8 minutes each).
The scan duration was 25–35 minutes, with oral sedation required for children less than 5 years of age.
The images were analysed with a differential filter. Percentage counts to each lung were measured to give total right and left lung perfusion. Images were reviewed by a radiographer and formally reported by a radiologist.
Statistical analysis
The percentage differential right and left blood flow MR and radionuclide lung perfusion data were compared for the right lung by Spearman rank correlation and a Bland‐Altman plot.11 Spearman rank correlation was also assessed between the ratio of the mean cross sectional diameters of the stenosed to the non‐stenosed branch pulmonary arteries and the ratio of the right to left lung blood flows.
Data were statistically analysed with SPSS version 12 (SPSS Inc, Chicago, Illinois, USA). A value of p < 0.05 was taken to be significant.
RESULTS
Table 1 shows patient demographic data and table 2 the flow data. MR phase contrast imaging was successfully performed in all patients. Six of the 12 patients had a proximal left pulmonary artery stenosis.
Table 1 Flow data and magnetic resonance (MR) measurements.
| Patient | Age (years) | Sex | Diagnosis | MR | NM | Diff (R) | Mean | MR measurements | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R lung | L lung | R lung | L lung | PT (mm) | Proximal LPA (mm) | Proximal RPA (mm) | Distal LPA (mm) | Distal RPA (mm) | ||||||
| 1 | 16.5 | M | Repaired TOF | 32 | 68* | 37 | 63 | −5 | 34.5 | 16×18 | 13×14 | 6×7 | 13×14 | 10×14 |
| 2 | 15.1 | F | TGA/arterial switch | 78* | 22 | 79 | 21 | −1 | 78.5 | 18×18 | 5×8 | 12×13 | 10×11 | 12×13 |
| 3 | 16.1 | F | TGA/arterial switch | 20 | 80* | 8 | 92 | 12 | 14 | 18×18 | 7×12 | 5×6 | 10×12 | 7×10 |
| 4 | 4.9 | M | Disconnected RPA | 20 | 80* | 21 | 79 | −1 | 20.5 | 15×17 | 11×11 | 2×3 | 11×11 | 4×6 |
| 5 | 10.6 | F | Isolated LPA stenosis | 65* | 35 | 68 | 32 | −3 | 66.5 | 13×15 | 4×6 | 9×9 | 8×9 | 9×9 |
| 6 | 5.6 | M | Repaired TOF | 55 | 45* | 51 | 49 | 4 | 53 | 8×8 | 9×12 | 7×11 | 12×12 | 12×12 |
| 7 | 3.0 | M | Anomalous pulmonary venous drainage R lung | 8 | 92* | 5 | 95 | 3 | 6.5 | – | 9×9 | 4×5 | 9×9 | 5×5 |
| 8 | 14.9 | F | Repaired TOF | 49* | 51 | 55 | 45 | −6 | 52 | 28×39 | 9×10 | 10×11 | 10×10 | 12×17 |
| 9 | 19.2 | M | Repaired TOF | 80* | 20 | 88 | 12 | −8 | 84 | 35×39 | 2×3 | 8×12 | 8×12 | 12×16 |
| 10 | 14 | M | TGA/arterial switch | 30 | 70* | 28 | 72 | 2 | 29 | 17×17 | 4×5 | 7×9 | 9×9 | 16×16 |
| 11 | 15.8 | M | Common arterial trunk | 35 | 65* | 30 | 70 | 5 | 32.5 | 20×20 | 11×13 | 5×7 | 15×15 | 9×10 |
| 12 | 14 | M | Repaired TOF | 48* | 52 | 44 | 56 | 4 | 46 | 17×17 | 14×15 | 16×17 | 18×22 | 16×17 |
*Directly measured flow data.
Diff, difference; F, female; L, left; LPA, left pulmonary artery; M, male; NM, radionuclide lung perfusion; PT, pulmonary trunk; R, right; RPA, right pulmonary artery; TGA, transposition of the great arteries; TOF, tetralogy of Fallot.
Table 2 Right ventricular volumes and flow analysis.
| Patient | Age (years) | Sex | Diagnosis | RV SV (ml) | PT FF (ml) | PT BF (ml) | PT SV (ml) | PT RF (%) | RPA flow (ml) | LPA flow (ml) | Ratio R:L lung perfusion from MR | Ratio of R:L proximal PA diameter |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16.5 | M | Repaired TOF | 92 | 94 | 17 | 76 | 18 | 24 | 52* | 0.47 | 0.48 |
| 2 | 15.1 | F | TGA/arterial switch | – | – | – | 51 | – | 40* | 11 | 3.55 | 1.92 |
| 3 | 16.1 | F | TGA/arterial switch | 71 | 74 | 0 | 74 | 0 | 15 | 59* | 0.25 | 0.58 |
| 4 | 4.9 | M | Disconnected RPA | 33 | 30 | 0 | 30 | 0 | 6 | 24* | 0.25 | 0.23 |
| 5 | 10.6 | F | Isolated LPA stenosis | – | 41 | 1 | 40 | 2.5 | 26* | 14 | 1.86 | 1.8 |
| 6 | 5.6 | M | Repaired TOF | 26 | 25 | 7 | 18 | 30 | 10 | 8* | 1.22 | 0.86 |
| 7 | 3.0 | M | Anomalous pulmonary venous drainage R lung | 21 | 25 | 0 | 25 | 0 | 2 | 23* | 0.09 | 0.50 |
| 8 | 14.9 | F | Repaired TOF | 129 | 130 | 65 | 65 | 50 | 32* | 33 | 0.96 | 1.11 |
| 9 | 19.2 | M | Repaired TOF | 120 | 120 | 47 | 73 | 40 | 58* | 15 | 4.00 | 4.00 |
| 10 | 14 | M | TGA/arterial switch | 78 | 80 | 0 | 80 | 0 | 56 | 24* | 0.43 | 1.78 |
| 11 | 15.8 | M | Common arterial trunk | 64 | 65 | 11 | 54 | 17 | 19 | 35* | 0.54 | 0.50 |
| 12 | 14 | M | Repaired TOF | 87 | 92 | 2 | 90 | 2 | 43* | 47 | 0.92 | 1.14 |
*Directly measured flow data.
BF, backward flow; FF, forward flow; RF, regurgitant fraction; RV, right ventricle; SV, stroke volume.
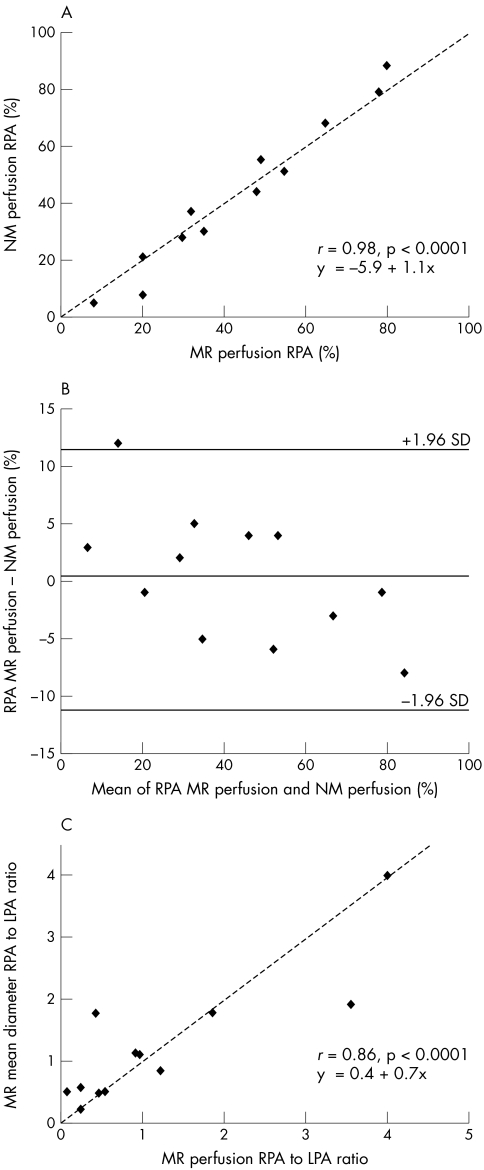
There was excellent agreement between the radionuclide and phase contrast MR calculated differential lung blood flow (r = 0.98, p < 0.0001) (fig 5A), with systematic bias between the two methods shown on Bland‐Altman analysis (fig 5B).
Figure 5 (A) Plot of radionuclide (NM) versus MR phase contrast measurement of percentage right lung blood flow, showing good correlation. (B) Bland‐Altman plot of the same data. (C) Correlation between the ratio of right to left proximal pulmonary artery (LPA) cross sectional diameter and the ratio of right to left lung blood flow.
There was also a good correlation between the ratio of the right to the left proximal branch pulmonary artery diameters and the ratio of the right to the left lung blood flows (r = 0.86, p < 0.0001). However, there were two notable outliers, in whom assessment of the ratio of the cross sectional diameters alone would have led to either an underestimation or overestimation of the functional significance of the stenosis (fig 5C, table 2).
DISCUSSION
MR assessment of the pulmonary arteries provides detailed morphological images and flow information to diagnostic standards.12,13,14 MR imaging, unlike echocardiography, is not restricted by conventional acoustic imaging planes, allowing the display of the entire flow jet in any plane or direction. Phase contrast MR techniques are the best available in vivo tests of flow.5,6
The validity of phase contrast MR to calculate differential branch pulmonary perfusion has been previously documented in adults, without evidence of pulmonary artery branch stenosis.8 Phase contrast MR has been validated in the assessment of differential pulmonary incompetence in branch pulmonary arteries after surgical repair of tetralogy of Fallot.9 In this study, the sum of the net forward flows in the individual branch pulmonary arteries was shown to be equal to the net forward flow measured in the pulmonary trunk. However, we did not compare data from a further imaging modality to confirm the differential pulmonary blood flow.
A recent report comparing both imaging modalities in the setting of congenital heart disease showed a mean difference of 17.9% between techniques in nearly 25% of subjects assessed.15 Critical analysis of the sources of these discrepant results showed that they were largely due to technical errors in the phase contrast assessment. In particular, a too distal or imprecise prescription of the phase contrast MR imaging plane resulted in underestimation of blood flow volume through that branch pulmonary artery. Furthermore, this group found that turbulent flow at the site of stenosis produced de‐phasing artefact with signal loss and an initially unrecognised aliasing artefact.15
We have overcome this potential problem in our study by assessing the less stenosed branch pulmonary artery. This allowed for an accurate but indirect assessment of blood flow to the lung perfused by the stenosed branch pulmonary artery by subtracting the flow volume of the opposite lung from the main pulmonary artery flow volume.
In our study we have shown that phase contrast MR has excellent agreement with radionuclide lung perfusion imaging in the assessment of differential branch pulmonary artery blood flow, even when unilateral branch pulmonary stenosis exists. Thus, MR can be used to assess the significance of branch pulmonary stenosis without the need for nuclear imaging, reducing the overall radiation burden to this patient group. Furthermore, in one patient with right pulmonary artery stenosis, both phase contrast MR and radionuclide perfusion imaging showed a 25% total differential flow to the right lung; after subsequent stenting of the stenosed right pulmonary artery and relief of stenosis, right pulmonary artery flow on phase contrast MR imaging returned to normal (equal blood flow to both lungs). This example highlights the value of phase contrast MR imaging to assess these patients serially and the results of catheter or surgical based interventions.
Study limitations
The small number of this patient cohort is the main limitation of this study. However, the very good agreement between MR and nuclear imaging has led to a rapid change in our clinical practice. Thus, for patients with congenital heart disease investigated with MR imaging as part of routine clinical assessment, differential pulmonary blood flow is assessed by phase contrast MR assessment without radionuclide imaging. It is difficult to justify the additional radiation burden associated with radionuclide lung perfusion imaging where a comparable, non‐ionising strategy exists.
We have not assessed inter‐ or intraobserver variability in this study, as phase contrast MR is well validated,6,7,8,9,15 the data analysis is not very operator dependent (semiautomated contour drawing and automated flow calculation), and, lastly, we have shown good agreement between right ventricular stroke volume (ventricular function data) and total forward pulmonary flow (flow data), which serves as an internal control for the reliability of our flow measurements (table 2).
Conclusion
Phase contrast MR has excellent correlation with radionuclide perfusion imaging as a method for measuring differential total right and left lung blood flow. This information can be used to assess the functional significance of branch pulmonary artery stenoses and guide subsequent interventional management. If MR imaging is performed to assess the branch pulmonary arteries, differential lung blood flow should also be measured, avoiding the need for an additional radionuclide lung perfusion scan and reducing the overall radiation burden to this group of patients.
We have now changed our clinical practice in this patient group. However, if MR data appear to contradict other clinical data, such as flow velocities, as assessed by echocardiography or other lung perfusion data (peripheral regional lung perfusion or ventilation data), radionuclide lung imaging may still be appropriate.
ACKNOWLEDGEMENTS
Dr Andrew Taylor is funded by the Higher Education Funding Committee for England (HEFCE).
References
- 1.Moore P, Lock J E. Catheter intervention: balloon angioplasty: experimental studies, technology and methodology. In: Lock JE, Keane JF, Perry SB, eds. Diagnostic and interventional catheterization in congenital heart disease. Boston: Kluwer Academic, 2001119–149.
- 2.Royal College of Radiologists Making the best use of a department of clinical radiology: guidelines for doctors. 5th ed. London: Royal College of Radiologists, 2003
- 3.Murthurangu V, Razavi R, Bogaert J.et al Congenital heart disease. In: Bogaert J, Dymarkowski S, Taylor AM, eds. Clinical cardiac MRI. Heidelberg: Springer, 2005439–473.
- 4.Canter C E, Gutierrez F R, Mirowitz S A.et al Evaluation of pulmonary arterial morphology in cyanotic congenital heart disease by magnetic resonance imaging. Am Heart J 1989118347–354. [DOI] [PubMed] [Google Scholar]
- 5.Kondo C, Caputo G R, Semelka R.et al Right and left ventricular stroke volume measurements with velocity encoded cine NMR imaging: in vivo and in vitro evaluation. AJR Am J Roentgenol 19911579–16. [DOI] [PubMed] [Google Scholar]
- 6.Firmin D N, Nayler G L, Klipstein R H.et al In vivo validation of MR velocity imaging. J Comput Assist Tomogr 198711751–756. [DOI] [PubMed] [Google Scholar]
- 7.Hundley W G, Hillis L D, Hamilton C A.et al Assessment of coronary arterial stenosis with phase‐contrast magnetic resonance imaging measurements of coronary flow reserve. Circulation 20001012375–2381. [DOI] [PubMed] [Google Scholar]
- 8.Silverman J M, Julien P J, Herfkens R J.et al Quantitative pulmonary perfusion: MR imaging versus radionuclide lung scanning. Radiology 1993189699–701. [DOI] [PubMed] [Google Scholar]
- 9.Kang I S, Redington A N, Leland N.et al Differential regurgitation in branch pulmonary arteries after repair of tetralogy of Fallot: a phase contrast cine magnetic resonance study. Circulation 20031072938–2943. [DOI] [PubMed] [Google Scholar]
- 10.Taylor A M, Bogaert J. Cardiovascular imaging planes and segmentation. In: Bogaert J, Dymarkowski S, Taylor AM, eds. Clinical cardiac MRI. Heidelberg: Springer, 200585–98.
- 11.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986i307–310. [PubMed]
- 12.Hernandez R J. Magnetic resonance imaging of the mediastinal vessels. Magn Reson Imaging Clin North Am 200210237–251. [DOI] [PubMed] [Google Scholar]
- 13.Powell A J, Chung T, Landzberg M J.et al Accuracy of MRI evaluation of pulmonary blood supply in patients with complex pulmonary stenosis or atresia. Int J Card Imaging 200016169–174. [DOI] [PubMed] [Google Scholar]
- 14.Vogt F M, Goyen M, Debatin J F. MR angiography of the chest. Radiol Clin North Am 20034129–41. [DOI] [PubMed] [Google Scholar]
- 15.Roman K S, Kellenberger C J, Farooq S.et al Comparative imaging of differential pulmonary blood flow in patients with congenital heart disease: magnetic resonance imaging versus lung perfusion scintigraphy. Pediatr Radiol 200535295–301. [DOI] [PubMed] [Google Scholar]