Abstract
Objective
To evaluate the growth of the pulmonary arteries after a Fontan procedure.
Design
Retrospective review.
Setting
Two paediatric cardiology tertiary care centres.
Patients
61 children who underwent a modified Fontan operation and had angiography suitable for assessment of pulmonary artery size before the Fontan procedure and during long term follow up. An atriopulmonary connection (APC) was present in 23 patients (37.7%) and a total cavopulmonary connection (TCPC) was present in 38 (62.3%). Postoperative angiograms were performed 0.5–121 months (median 19 months) after the Fontan operation.
Main outcome measure
Growth of each pulmonary artery measured just before the first branching point. The diameter was expressed as a z score with established nomograms used to standardise for body surface area.
Results
The mean change in the preoperative to postoperative z scores of the right pulmonary artery was −1.06 (p = 0.004). The mean change in the preoperative to postoperative z scores of the left pulmonary artery was −0.88 (p = 0.003). Changes in the preoperative to postoperative z scores were more pronounced in the patients undergoing APC than TCPC, especially for the right pulmonary artery.
Conclusion
After the Fontan operation, growth of the pulmonary arteries often fails to match the increase in body surface area.
Keywords: congenital heart disease, Fontan operation, pulmonary arteries
The modified Fontan procedure is being performed at an increasingly younger age at many institutions. Initial studies showed that pulmonary artery size was an important factor in determining the outcome for patients undergoing a Fontan‐type procedure.1,2,3,4 However, more recent studies have shown that the Fontan procedure can be safely performed in children with smaller pulmonary arteries.5,6 Yet little is known about the growth of the pulmonary arteries after the modified Fontan procedure. There is evidence that the rate of growth of the pulmonary arteries may be decreased after the bidirectional Glenn procedure.7,8,9,10 There also has been concern that the growth of the pulmonary arteries may not be proportional to the increase in body surface area after a modified Fontan procedure.11 We speculate that inadequate growth of the pulmonary arteries may be a contributing factor to late Fontan failure.
The objective of this study was to evaluate the growth of the pulmonary arteries after a modified Fontan procedure.
METHODS
Study design
All children who underwent a modified Fontan operation at Children's Hospital of Pittsburgh or the Milton S Hershey Medical Center between 1980 and 1994 and who had adequate angiographic imaging of the pulmonary arteries before and after the procedure were enrolled in this study. In the majority of cases during this era, follow up catheterisation and angiography were performed as part of the routine follow up evaluation of patients with Fontan repair at both institutions. When the patient had undergone multiple catheterisations since the Fontan procedure, the latest catheterisation was used for measurement purposes.
One of three surgeons performed the modified Fontan operation. Despite minor variations in surgical technique, the procedures could be classified as either a direct atriopulmonary connection (APC) or a total cavopulmonary connection (TCPC) with a lateral tunnel technique.
The left and right pulmonary arteries were measured immediately proximal to the first branching point of each pulmonary artery. This was performed during systole at the point in the cardiac cycle yielding the maximum size of the pulmonary arteries. Images were corrected for magnification by using filmed 1 cm markers (grid or sphere) when available or by known catheter diameter. A single investigator obtained all measurements. Measured pulmonary artery size was expressed as a z score based on published nomograms, which standardise pulmonary artery size for body surface area.12 Pulmonary artery index was also calculated from the same measurements as previously described.4
This study was performed in compliance with institutional ethics committee guidelines.
Statistical analysis
Descriptive data are expressed as a mean (SD) or median (range) as appropriate. Normally distributed continuous variables were compared by paired and unpaired Student's t tests. Mann‐Whitney U test was used for comparison of group means for variables that did not approximate to a normal distribution. Linear regression was used to assess the relation between continuous variables. Values of p < 0.05 were considered significant.
RESULTS
Of the 98 patients undergoing a modified Fontan operation between 1980 and 1994, 61 (62%) fulfilled inclusion criteria. Cardiac diagnoses of these patients were tricuspid atresia (19), double inlet left ventricle (17), hypoplastic left heart syndrome (13), pulmonary atresia with intact ventricular septum (six), and other diagnoses (six). Twenty eight patients were girls and 33 were boys. The age at the modified Fontan operation ranged from 7.3–252 months (mean 75.2 months, median 53 months). Eighteen (29.5%) were under 2 years of age at the time of surgery. Preoperative catheterisation was performed 0.1–19 months (mean 2.9 months, median two months) before the Fontan procedure. The weight at the time of the preoperative catheterisation ranged from 6–63.5 kg (mean 19.1 kg) and body surface area ranged from 0.32–1.5 m2 (mean 0.73 m2). The time from operation to postoperative follow up catheterisation ranged from 0.5–121 months (mean 24.6 months, median 19 months). The weight at the time of the postoperative catheterisation ranged from 8.3–69.2 kg (mean 25.5 kg) and body surface area ranged from 0.40–1.9 m2 (mean 0.89 m2). Compared with the patients undergoing TCPC, those undergoing APC were significantly older and had a larger body surface area at the time of the modified Fontan operation. They also underwent catheterisation after a longer follow up than did the patients undergoing TCPC (table 1).
Table 1 Comparison of atriopulmonary connection (APC) versus total cavopulmonary connection (TCPC) groups.
| APC | TCPC | p Value | |
|---|---|---|---|
| Number of patients | 23 (37.7%) | 38 (62.3%) | |
| Age at Fontan (months) | 105 (67) | 51 (67) | 0.0013 |
| BSA at Fontan (m2) | 0.89 (0.3) | 0.63 (0.3) | 0.0047 |
| Length of FU (months)* | 35 (25) | 20 (16) | 0.0006 |
Data are mean (SD).
*To latest postoperative catheterisation.
BSA, body surface area; FU, follow up.
APC was performed in 23 children (37.7%) and TCPC in 38 (62.3%), including four who had bilateral bidirectional Glenn procedures as a part of the TCPC. One or more prior palliative procedures had been performed in 54 patients (89%) including isolated systemic to pulmonary artery shunt (38 procedures), pulmonary artery banding (five procedures), and first stage Norwood procedure (12). One patient had a classic Glenn shunt, four had a bidirectional Glenn, and five had a hemi‐Fontan procedure. Some form of pulmonary artery reconstruction was done at the time of previous surgery in all patients undergoing these latter operations. Pulmonary arterioplasty was performed at the time of the modified Fontan procedure in 19 patients (31%). A 3 or 4 mm fenestration was created in the intra‐atrial baffle in 17 patients (28%) undergoing TCPC but not in patients with APC.
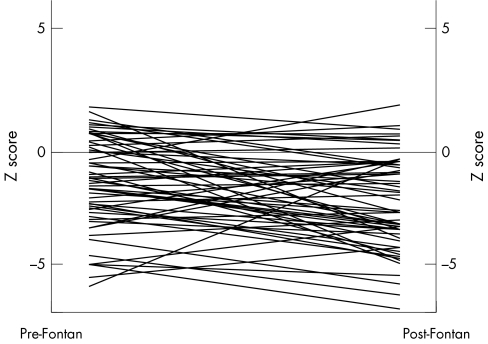
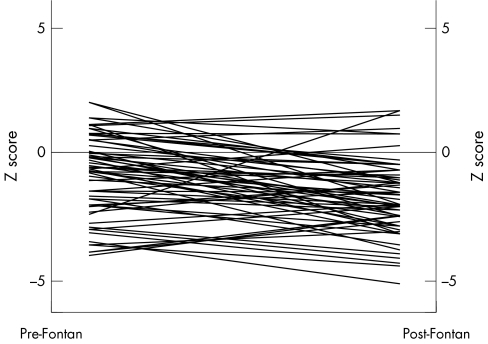
In almost all patients, growth of both the right and left pulmonary arteries failed to match the increase in body surface area after the modified Fontan operation. The pulmonary artery index decreased from 286.7 (131.8) at preoperative catheterisation to 206.6 (88.5) at postoperative catheterisation. The preoperative z score for the right pulmonary artery was −1.25 (2.0) and the postoperative z score was −2.31 (3.0) for a mean change in z score of −1.06 (p = 0.004) (fig 1). The preoperative z score for the left pulmonary artery was −0.87 (1.6) and the postoperative z score was −1.75 (1.5) for a mean change in z score of −0.88 (p = 0.003) (fig 2). Of the 61 patients only five had an increase in the z score for both pulmonary arteries. Seven patients had an increase in the left pulmonary artery z score only and 14 patients had an increase in the right pulmonary artery z score only. The remaining 35 patients had a decrease in the z score for both pulmonary arteries. Growth of either the left or right pulmonary artery (change in z score) was not correlated with age at modified Fontan procedure (r = 0.02 and r = −0.13, respectively), body surface area at the time of either procedure (r = 0.001 and r = −0.12), or the length of follow up after either procedure (r = −0.14 and r = −0.09). When comparing the two types of Fontan procedure, the patients undergoing APC had less growth of the pulmonary arteries (table 2). The mean change in the preoperative to postoperative z score for the right pulmonary artery was −0.58 (2.0) for the TCPC and −1.85 (1.9) for the APC group (p = 0.019). The mean change in the preoperative to postoperative z score for the left pulmonary artery was −0.83 (1.6) for the TCPC and −0.96 (1.5) for the APC group (p = 0.753).
Figure 1 Growth of the right pulmonary artery.
Figure 2 Growth of the left pulmonary artery.
Table 2 Changes in pulmonary artery z scores according to type of procedure.
| APC | TCPC | p Value | |
|---|---|---|---|
| Pre‐Fontan RPA z score | −1.78 (1.9) | −0.92 (1.9) | 0.103 |
| Post‐Fontan RPA z score | −3.63 (1.9) | −1.51 (1.7) | 0.001 |
| Δ RPA | −1.85 (1.9) | −0.58 (2.0) | 0.019 |
| Pre‐Fontan LPA z score | −1.28 (1.7) | −0.62 (1.6) | 0.127 |
| Post‐Fontan LPA z score | −2.25 (1.4) | −1.44 (1.5) | 0.041 |
| Δ LPA | −0.96 (1.5) | −0.83 (1.6) | 0.753 |
LPA, left pulmonary artery; RPA, right pulmonary artery.
DISCUSSION
Growth of the pulmonary bed has been shown to be strongly flow dependent in patients who are Fontan candidates.13,14 Early placement of a palliative shunt is important to promote sufficient bilateral pulmonary artery growth in cases of decreased pulmonary blood flow.15 Yet the optimal timing of subsequent operations to maximise the growth of the pulmonary arteries has not been established. There is evidence that the growth of pulmonary arteries does not match the increase in body size after a bidirectional Glenn anastomosis7,8,9,10 and after TCPC.11 Although Slavik et al8 suggested that short term pulmonary artery growth was satisfactory after a bidirectional Glenn anastomosis, they reported on overall decrease in z scores in the children they studied. Furthermore, they found that the rate of growth was lower in children undergoing the operation in the first year of life than in their older peers. The addition of pulsatile blood flow during the bidirectional Glenn procedure may be of some benefit in promoting pulmonary artery growth, as well as decreasing the likelihood of arteriovenous fistulas and temporarily increasing arterial oxygen saturations; however, results to date have been mixed.8,16,17,18,19,20,21
Performing a modified Fontan procedure in a very young patient has several benefits. Chief among these are relief of the ventricular volume overload and early separation of the right and left circulations to achieve near normal oxygen saturations. Although initially the Fontan procedure was rarely performed before the age of 4 due to worse outcomes,22,23 success with Fontan procedures performed in young children has been well documented in more recent series.23,24,25 There is a clear trend towards performing the Fontan operation at progressively younger ages, yet little is known about the growth of the pulmonary arteries after this procedure. Although modified Fontan procedures can be safely performed in children with a low pulmonary artery index,6 pulmonary arterial growth must be adequate for long term successful outcome. We speculate that poor pulmonary artery growth may lead to late Fontan failure.
Very few studies to date have analysed pulmonary artery growth after a Fontan procedure. Buheitel et al11 studied 32 patients who had undergone a Fontan procedure and underwent a catheterisation afterwards. The mean age of their patients at the time of the TCPC was 5.8 years and the mean time from operation to catheterisation was 3.5 years. They found that their patients' z scores increased significantly from the first year of life until the time of the modified Fontan procedure. However, after the Fontan procedure their z scores decreased for both pulmonary arteries.
In this study, the growth of both pulmonary arteries failed to match the increase in body surface area. The inadequate pulmonary arterial growth was more pronounced in the patients undergoing a direct APC than in those undergoing a TCPC, especially for the right pulmonary artery. The decrease in the pulmonary artery z score was comparable for the right and left pulmonary arteries in patients who underwent a TCPC. Patients undergoing a direct APC had a larger decrease in the z score for the right than the left pulmonary artery. When these two subsets of patients are compared, the APC group tended to be older at the time of operation, had their postoperative catheterisation performed after a longer time interval, and were operated on earlier in the series. These differences limit the interpretation of any statistical comparisons between the two groups. Aside from the difference between the two types of Fontan procedures, we did not find any factors that predicted the change in the z scores for their pulmonary arteries. In particular, the age at time of operation and length of follow up did not correlate with pulmonary artery growth. The impact of pre‐Fontan pulmonary blood flow on post‐Fontan pulmonary artery growth could not be investigated in this study, since the highly complex anatomy and variable sources of pulmonary blood flow precluded accurate measurement of pre‐Fontan pulmonary blood flow.
The patients in this study had their pulmonary arteries measured on average two years after the modified Fontan procedure. Buheitel et al11 performed catheterisation at a mean of 3.5 years after the Fontan surgery and found similar results to ours in a smaller cohort. Ideally, very long term follow up studies (> 10 years) to assess pulmonary artery growth throughout childhood are needed.
The number of patients who underwent the Fontan procedure at a very young age (< 2 years) was insufficient to allow for separate analysis of this subset of patients. DeGroff et al26 devised in vitro models of the TCPC that corresponded to the average vessel diameter of 3 and 15 year old patients. They found that the vessel size had a significant impact on flow efficiency. Since pulmonary artery growth is dependent on flow13,14 and as subsequent pulmonary arterial growth is of particular importance in younger children, further studies are needed to address this topic. Additionally, the underlying anatomy was very heterogeneous and the patients had undergone various types of palliative procedures (including pulmonary arterioplasty) before, or at the time of, the Fontan procedure. All of these factors can act as confounders; however, numbers were insufficient to allow for meaningful subgroup analysis.
No patients in this study underwent an extracardiac repair. Experimental data suggest that an extracardiac repair may have superior haemodynamic function than other Fontan procedures.27 Therefore, the results of this study may not be as applicable to patients undergoing more recent repairs. We did not include the more recent patients who have undergone extracardiac repairs, as they did not undergo routine postoperative catheterisation.
There are also factors that may affect the measurement of the pulmonary arteries. The data were collected retrospectively. The patients were from two centres and different techniques were used to measure the pulmonary arteries in different patients (that is, grids, spheres, and catheters). During the time period of this study, catheterisation offered the most reliable form of measuring pulmonary artery growth. As catheterisation is an invasive procedure, the majority of patients had undergone only a single post‐Fontan catheterisation. Thus, the number of data points for analysis was limited. Over 90% of the catheterisations performed were elective; therefore, the study should not be overly biased by the inclusion of patients undergoing catheterisation because of Fontan failure. For future studies, magnetic resonance imaging offers the capability for serial non‐invasive monitoring of pulmonary arterial growth.
In summary, the findings from this study are consistent with previous studies that have shown that the growth of pulmonary arteries does not match the increase in body size after a bidirectional Glenn anastomosis and after TCPC.7,8,9,10,11 Whether this is due to inadequate pulmonary blood flow and lack of pulsatility at a critical time in the growth and development of the pulmonary arteries or is due to some other cause remains to be fully elucidated. Nonetheless, we speculate that this poor pulmonary artery growth may be a contributing factor to late Fontan failure. These preliminary findings do not justify any changes in current practice but they do underline the need for long term follow up studies of pulmonary artery growth after the various modified Fontan procedures, especially when performed in very young patients.
Footnotes
Competing interest: No financial support was provided for the completion of this study. The authors of this study do not have any competing interests.
References
- 1.Choussat A, Fontan F, Besse P.et al Selection criteria for Fontan's procedure. In: Anderson RH, Shinebourne ED, eds. Pediatric cardiology. Edinburgh: Churchill‐Livingstone, 1978559–566.
- 2.Fontan F, Fernandez G, Costa F.et al The size of the pulmonary arteries and the results of the Fontan operation. J Thorac Cardiovasc Surg 198998711–724. [PubMed] [Google Scholar]
- 3.Senzaki H, Isoda T, Ishizawa A.et al Reconsideration of criteria for the Fontan operation: influence of pulmonary artery size on postoperative hemodynamics of the Fontan operation. Circulation 1994891196–1202. [DOI] [PubMed] [Google Scholar]
- 4.Nakata S, Imai Y, Takanashi Y.et al A new method for the quantitative standardization of crow‐sectional areas of the pulmonary arteries in congenital heart disease with decreased pulmonary flow. J Thorac Cardiovasc Surg 198488610–619. [PubMed] [Google Scholar]
- 5.Girod D, Rice M, Mair D.et al Relationship of pulmonary artery size to mortality in patients undergoing the Fontan operation. Circulation 198572(suppl)II93–II96. [PubMed] [Google Scholar]
- 6.Bridges N D, Farrell P E, Jr, Pigott J D.et al Pulmonary artery index: a nonpredictor of operative survival in patients undergoing modified Fontan repair. Circulation 198980(suppl)I216–I221. [PubMed] [Google Scholar]
- 7.Reddy V M, McElhinney D B, Moore P.et al Pulmonary artery growth after bidirectional cavopulmonary shunt: is there a cause for concern? J Thorac Cardiovasc Surg 19961121180–1192. [DOI] [PubMed] [Google Scholar]
- 8.Slavik Z, Webber S A, Lamb R K.et al Influence of bidirectional superior cavopulmonary anastomosis on pulmonary artery growth. Am J Cardiol 1995761085–1087. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn A M, Bove E L, Lupinetti F M.et al Central pulmonary artery growth pattern after the bidirectional Glenn procedure. J Thorac Cardiovasc Surg 19941071284–1290. [PubMed] [Google Scholar]
- 10.Penny D J, Pawade A, Wilkinson J L.et al Pulmonary artery size after bidirectional cavopulmonary connection. J Card Surg 19951021–26. [DOI] [PubMed] [Google Scholar]
- 11.Buheitel G, Hofbeck M, Tenbrink U.et al Changes in pulmonary artery size before and after total cavopulmonary connection. Heart 199778488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirklin J W, Baratt‐Boyes B G. Anatomy, dimensions and terminology. In: Cardiac surgery. 2nd edn. New York: Churchill Livingstone, 19933–60.
- 13.Borowski A, Reinhardt H, Schickendantz S.et al Pulmonary artery growth after systemic‐to‐pulmonary shunt in children with a univentricular heart and a hypoplastic pulmonary artery bed: implications for Fontan surgery. Jpn Heart J 199839671–680. [DOI] [PubMed] [Google Scholar]
- 14.Jarmakani J M M, Graham T P, Benson D W.et al In vivo pressure‐radius relationships of the pulmonary artery in children with congenital heart disease. Circulation 197143585–592. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa S, Takahashi T, Sato Y.et al Growth of the pulmonary arteries after systemic‐pulmonary shunt. Ann Thorac Cardiovasc Surg 20017337–340. [PubMed] [Google Scholar]
- 16.McElhinney D B, Marianeschi S M, Reddy M. Additional pulmonary blood flow with the bidirectional Glenn anastomosis: does it make a difference? Ann Thorac Surg 199866668–672. [DOI] [PubMed] [Google Scholar]
- 17.Frommelt M A, Frommelt P C, Berger S.et al Does an additional source of pulmonary blood flow alter outcome after a bidirectional cavopulmonary shunt? Circulation 199592(suppl)II240–II244. [DOI] [PubMed] [Google Scholar]
- 18.Webber S A, Horvath P, LeBlanc J G.et al Influence of competitive pulmonary blood flow on the bidirectional superior cavopulmonary shunt: a multi‐institutional study. Circulation 199592(suppl)II279–II286. [DOI] [PubMed] [Google Scholar]
- 19.Mainwaring R D, Lamberti J J, Uzark K.et al Bidirectional Glenn: is accessory pulmonary blood flow good or bad? Circulation 199592(suppl)II294–II297. [DOI] [PubMed] [Google Scholar]
- 20.Uemura H, Yagihara T, Kawashima Y.et al Use of the bidirectional Glenn procedure in the presence of forward flow from the ventricles to the pulmonary arteries. Circulation 199592(suppl)II228–II232. [DOI] [PubMed] [Google Scholar]
- 21.Mainwaring R D, Lamberti J J, Uzark K.et al Effect of accessory pulmonary blood flow on survival after the bidirectional Glenn procedure. Circulation 1999100(suppl)II151–II156. [DOI] [PubMed] [Google Scholar]
- 22.Bartmus D A, Driscoll D J, Offord K P.et al The modified Fontan operation for children less than four years old. J Am Coll Cardiol 199015429–435. [DOI] [PubMed] [Google Scholar]
- 23.Kirklin J K, Blackstone E H, Kirklin J W.et al The Fontan operation: ventricular hypertrophy, age and date of operation as risk factors. J Thorac Cardiovasc Surg 1986921021–1028. [PubMed] [Google Scholar]
- 24.Weber H S, Gleason M M, Myers J L.et al The Fontan operation in infants less than two years of age. J Am Coll Cardiol 199219828–833. [DOI] [PubMed] [Google Scholar]
- 25.Pearl J M, Laks H, Drinkwater D C.et al Modified Fontan procedure in patients less than four years of age. Circulation 199286(suppl)II100–II105. [PubMed] [Google Scholar]
- 26.DeGroff C G, Carlton J D, Weinberg C E.et al Effect of vessel size on the flow efficiency of the total cavopulmonary connection: in vitro studies. Pediatr Cardiol 200223171–177. [DOI] [PubMed] [Google Scholar]
- 27.Lardo A C, Webber S A, Friehs I.et al Fluid dynamic comparison of intra‐atrial and extracardiac total cavopulmonary connections. J Thorac Cardiovasc Surg 1999117697–704. [DOI] [PubMed] [Google Scholar]