Abstract
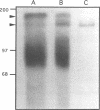
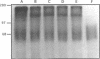
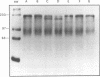
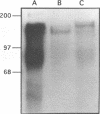
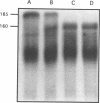
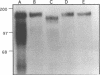
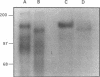
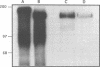
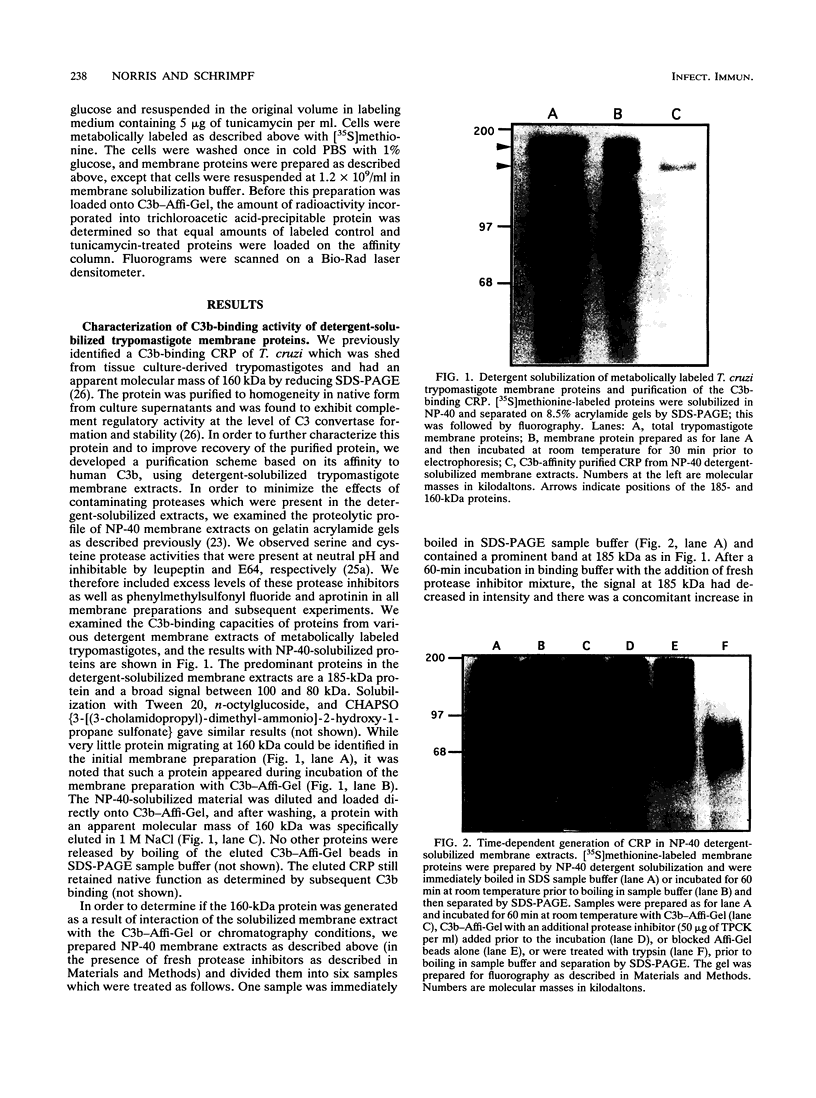
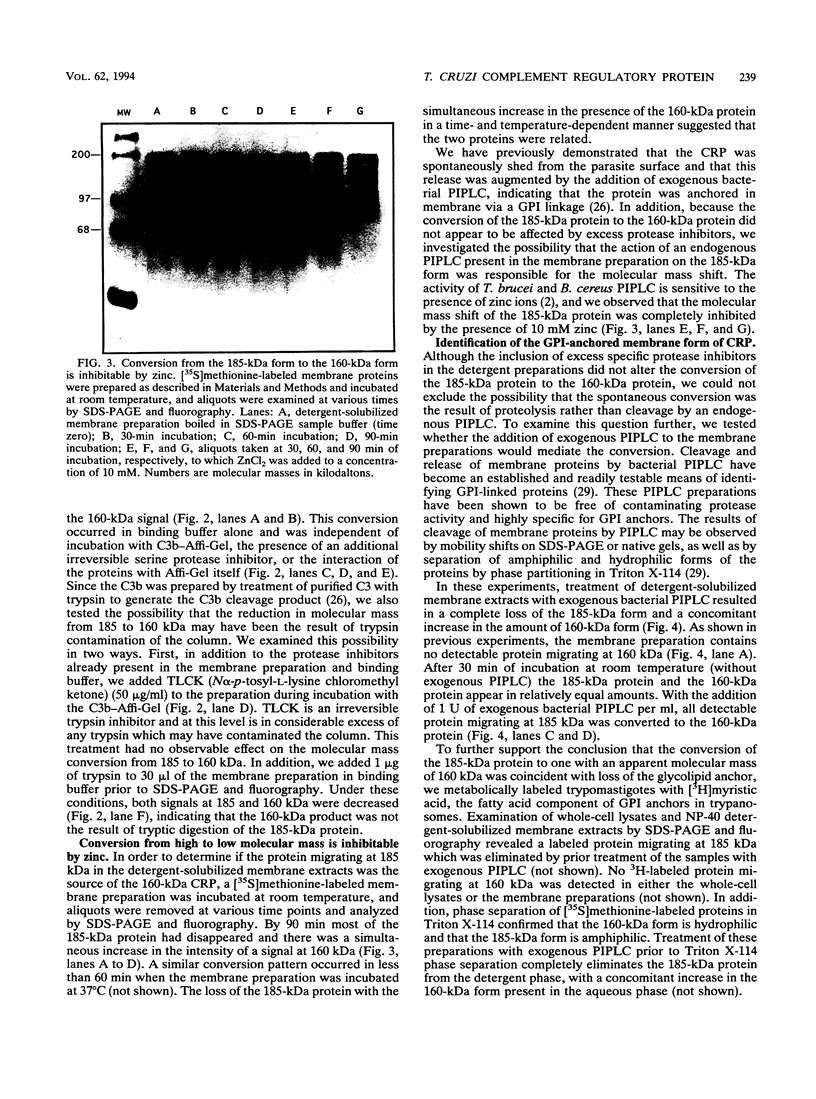
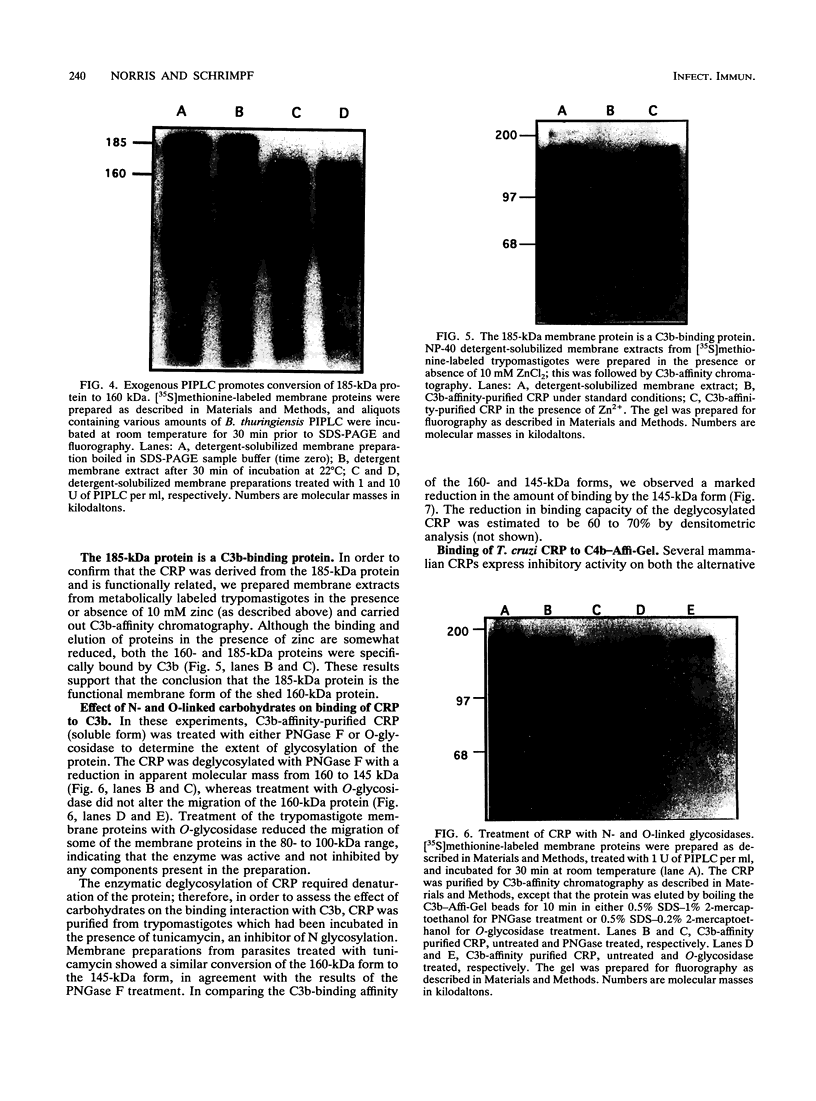
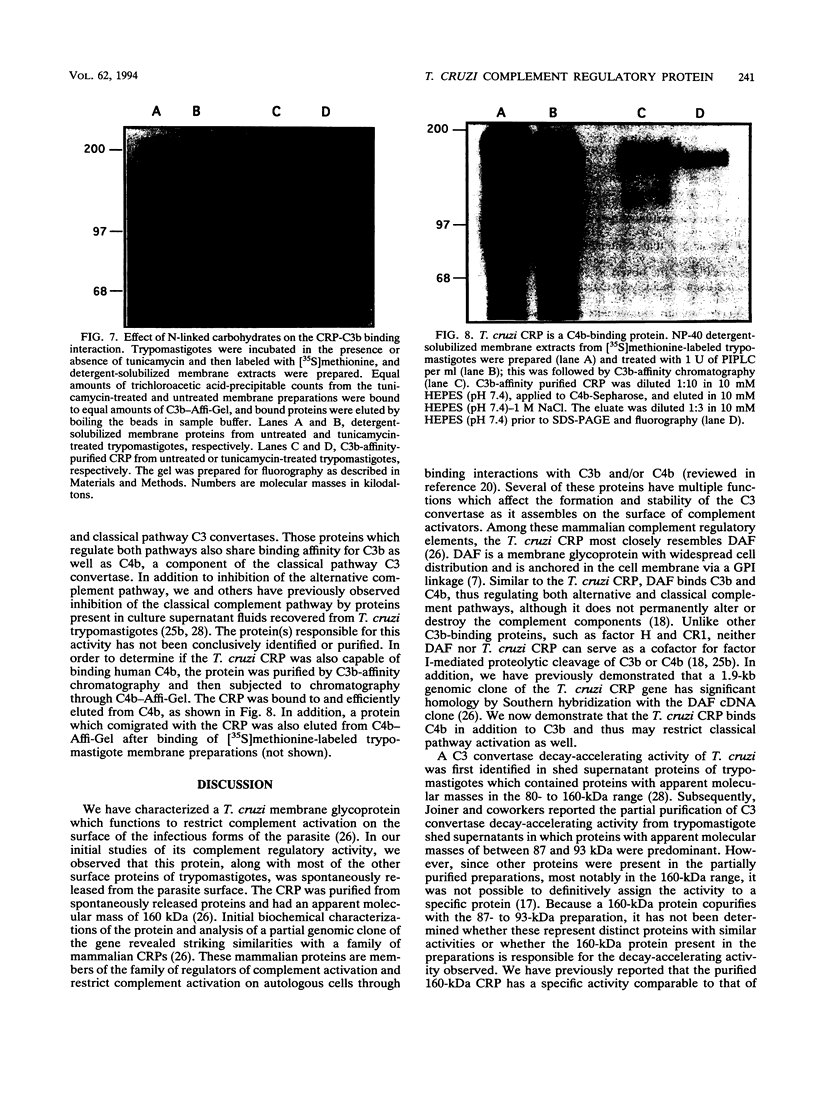
A developmentally regulated, 160-kDa trypomastigote surface glycoprotein was previously shown to bind the third component of complement and to inhibit activation of the alternative complement pathway, thus providing the parasites a means of avoiding the lytic effects of complement. We now show that this complement regulatory protein (CRP) binds human C4b, a component of the classical pathway C3 convertase, and may therefore also act to restrict classical complement activation. Characterization of the extent of carbohydrate modification of the protein revealed extensive N-linked glycosylation and no apparent O-linked sugars. The CRP purified from parasites treated with an inhibitor of N-linked glycosylation exhibited a decreased binding affinity for C3b compared with that of the fully glycosylated protein. We have previously shown that the protein was anchored to the membrane via a glycosyl phosphatidylinositol linkage and was spontaneously shed from the parasite surface. The spontaneous release of CRP from the parasite surface may augment the protection of the parasites from complement-mediated lysis by the removal of complement-CRP complexes. The majority of the shed CRP had an apparent molecular mass of 160 kDa and lacked the glycolipid anchor, whereas the membrane form was recovered with the glycolipid anchor attached and had an apparent molecular mass of 185 kDa. Both the membrane form (185 kDa) and the soluble form (160 kDa) retained binding affinity for C3b. Evidence is presented to indicate that the conversion of the 185-kDa membrane form to the 160-kDa form is the result of cleavage by an endogenous phospholipase C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J Virol. 1992 Jun;66(6):3937–3940. doi: 10.1128/jvi.66.6.3937-3940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Etges R. J., Ward J., Turner M. J., Cardoso de Almeida M. L. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5988–5991. doi: 10.1073/pnas.83.16.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R. Complement evasion strategies of microorganisms. Immunol Today. 1991 Sep;12(9):327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Gurnett A. M., Low M. G., Turner M. J., Nussenzweig V. Decay-accelerating factor (DAF) shares a common carbohydrate determinant with the variant surface glycoprotein (VSG) of the African Trypanosoma brucei. J Immunol. 1987 Jan 15;138(2):520–523. [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Hastings J. C., Cohen G. H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987 Dec;3(6):423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Fischer E., Ouaissi M. A., Velge P., Cornette J., Kazatchkine M. D. gp 58/68, a parasite component that contributes to the escape of the trypomastigote form of T. cruzi from damage by the human alternative complement pathway. Immunology. 1988 Oct;65(2):299–303. [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fries L. F., Friedman H. M., Cohen G. H., Eisenberg R. J., Hammer C. H., Frank M. M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986 Sep 1;137(5):1636–1641. [PubMed] [Google Scholar]
- Gonçalves M. F., Umezawa E. S., Katzin A. M., de Souza W., Alves M. J., Zingales B., Colli W. Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol. 1991 Jan;72(1):43–53. doi: 10.1016/0014-4894(91)90119-h. [DOI] [PubMed] [Google Scholar]
- Güther M. L., de Almeida M. L., Yoshida N., Ferguson M. A. Structural studies on the glycosylphosphatidylinositol membrane anchor of Trypanosoma cruzi 1G7-antigen. The structure of the glycan core. J Biol Chem. 1992 Apr 5;267(10):6820–6828. [PubMed] [Google Scholar]
- Holder A. A. Characterisation of the cross-reacting carbohydrate groups on two variant surface glycoproteins of Trypanosoma brucei. Mol Biochem Parasitol. 1983 Apr;7(4):331–338. doi: 10.1016/0166-6851(83)90015-4. [DOI] [PubMed] [Google Scholar]
- Hung S. L., Srinivasan S., Friedman H. M., Eisenberg R. J., Cohen G. H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J Virol. 1992 Jul;66(7):4013–4027. doi: 10.1128/jvi.66.7.4013-4027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., daSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J Biol Chem. 1988 Aug 15;263(23):11327–11335. [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Nussenzweig V. Endogenous association of decay-accelerating factor (DAF) with C4b and C3b on cell membranes. J Immunol. 1986 May 1;136(9):3390–3395. [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Kristensen T., D'Eustachio P., Ogata R. T., Chung L. P., Reid K. B., Tack B. F. The superfamily of C3b/C4b-binding proteins. Fed Proc. 1987 May 15;46(7):2463–2469. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H., Pino-Heiss S., Lindquist R., Werb Z. Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem. 1985 Mar 25;260(6):3703–3707. [PubMed] [Google Scholar]
- McNearney T. A., Odell C., Holers V. M., Spear P. G., Atkinson J. P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987 Nov 1;166(5):1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold C., Bradt B. M., Nemerow G. R., Cooper N. R. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med. 1988 Sep 1;168(3):949–969. doi: 10.1084/jem.168.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Bradt B., Cooper N. R., So M. Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the human complement regulatory protein, decay-accelerating factor. J Immunol. 1991 Oct 1;147(7):2240–2247. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):193–197. doi: 10.1073/pnas.85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L., Toutant J. P., Haas R., Roberts W. L. Identification and analysis of glycoinositol phospholipid anchors in membrane proteins. Methods Cell Biol. 1989;32:231–255. doi: 10.1016/s0091-679x(08)61173-5. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A., Twomey C. E. The growth of Trypanosoma cruzi in human diploid cells for the production of trypomastigotes. Parasitology. 1980 Feb;80(1):153–162. doi: 10.1017/s0031182000000615. [DOI] [PubMed] [Google Scholar]
- Tambourgi D. V., Kipnis T. L., da Silva W. D., Joiner K. A., Sher A., Heath S., Hall B. F., Ogden G. B. A partial cDNA clone of trypomastigote decay-accelerating factor (T-DAF), a developmentally regulated complement inhibitor of Trypanosoma cruzi, has genetic and functional similarities to the human complement inhibitor DAF. Infect Immun. 1993 Sep;61(9):3656–3663. doi: 10.1128/iai.61.9.3656-3663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausk F., Fey M., Gigli I. Endocytosis and shedding of the decay accelerating factor on human polymorphonuclear cells. J Immunol. 1989 Nov 15;143(10):3295–3302. [PubMed] [Google Scholar]