Abstract
Objective
To use quantitative myocardial contrast echocardiography (MCE) and strain rate imaging (SRI) to assess the role of microvascular disease in subclinical diabetic cardiomyopathy.
Methods
Stress MCE and SRI were performed in 48 patients (22 with type II diabetes mellitus (DM) and 26 controls), all with normal left ventricular systolic function and no obstructive coronary disease by quantitative coronary angiography. Real‐time MCE was acquired in three apical views at rest and after combined dipyridamole–exercise stress. Myocardial blood flow (MBF) was quantified in the 10 mid‐ and apical cardiac segments at rest and after stress. Resting peak systolic strain rate (SR) and peak systolic strain (ε) were calculated in the same 10 myocardial segments.
Results
The DM and control groups were matched for age, sex and other risk factors, including hypertension. The DM group had higher body mass index and left ventricular mass index. Quantitative SRI analysis was possible in all patients and quantitative MCE in 46 (96%). The mean ε, SR and MBF reserve were all significantly lower in the DM group than in controls, with diabetes the only independent predictor of each parameter. No correlation was seen between MBF and SR (r = −0.01, p = 0.54) or between MBF and ε (r = −0.20, p = 0.20).
Conclusions
Quantitative MCE shows that patients with diabetes but no evidence of obstructive coronary artery disease have impaired MBF reserve, but abnormal transmural flow and subclinical longitudinal myocardial dysfunction are not related.
Keywords: cardiomyopathy, diabetes mellitus, myocardial contrast echocardiography, strain rate imaging
In patients with diabetes mellitus (DM) with normal resting ejection fraction and normal stress echocardiograms, the presence of resting abnormalities of sensitive indices of myocardial function measured by tissue Doppler and strain rate imaging (SRI) has been attributed to a subclinical cardiomyopathy.1 These subclinical changes are considered to be the earliest manifestation of overt diabetic cardiomyopathy, a disease of heart muscle that is distinct from ischaemic injury due to significant epicardial coronary artery disease (CAD). The pathogenesis of this condition is unclear and probably heterogeneous,2 with metabolic derangements, myocardial fibrosis and coronary microangiopathy all being possible contributors.3
Patients with DM and no CAD show evidence of significantly impaired coronary flow reserve on non‐invasive4,5,6,7 and invasive testing.8 Coronary microangiopathy has therefore been proposed as a significant contributor to diabetic cardiomyopathy.2,3 However, patients with subclinical diabetic cardiomyopathy, manifest by impaired resting basal myocardial tissue Doppler velocities, have normally augmented systolic tissue velocity with incremental doses of dobutamine, suggesting that ischaemia from microvascular disease may not be a major determinant of subclinical dysfunction.9
Myocardial contrast stress echocardiography (MCE) has recently been validated as a technique for quantification of myocardial blood flow (MBF) reserve in patients with CAD,10,11 although this has not been explored in patients with type II DM and no CAD. In this study, we examined the use of quantitative stress MCE and resting SRI to assess the relationship between perfusion and function in patients with diabetes, without CAD and normal left ventricular (LV) systolic function (ejection fraction). In particular we sought a significant correlation between the degree of subclinical myocardial dysfunction and blood flow reserve.
METHODS
Study group
We prospectively studied 48 patients (22 with type II DM (mean duration 10 (SD 9) years) and 26 non‐diabetic controls) with no significant CAD at quantitative coronary angiography and normal resting regional and global LV systolic function. Patients were excluded if they had significant CAD (quantitative coronary angiographic stenosis diameter > 40%), regional wall motion abnormalities or impaired ejection fraction (< 55%) by resting echocardiography, previous surgical or percutaneous revascularisation, significant valvular regurgitation, or a contraindication to intravenous dipyridamole or the contrast agent. All other eligible patients were studied.
Clinical evaluation
All patients were assessed clinically, including evaluation of cardiac risk factor profile, height, weight and body mass index. Hypercholesterolaemia was defined by serum cholesterol > 4.5 mmol/l or lipid lowering treatment, and hypertension was defined by blood pressure > 140/90 mm Hg or ongoing treatment for hypertension.
Imaging procedure
Patients had all echocardiographic imaging performed on the same day. Patients underwent resting assessment of LV geometry and function, followed immediately by contrast stress echocardiography. Resting ventricular function and strain rate (SR) images were gathered with a commercial ultrasound machine (Vivid 7; GE Vingmed, Horten, Norway) equipped with a 2.5 MHz phased array probe.
Conventional Doppler echocardiography
Images were obtained in the standard tomographic views of the LV (parasternal long and short‐axis and apical four‐chamber, two‐chamber and long‐axis views). LV diameter and wall thickness were measured from the two‐dimensional targeted M mode echocardiographic tracings in the parasternal long axis, according to the criteria of the American Society of Echocardiography. LV mass was determined by Devereux's formula and indexed to body mass index. Relative wall thickness was calculated by the formula (2 × posterior wall thickness)/LV end diastolic diameter.
LV end diastolic and end systolic volumes and the LV ejection fraction at rest were computed from the apical two‐ and four‐chamber views by a modified Simpson's biplane method. Pulsed‐wave Doppler was used to measure transmitral peak early diastolic velocity (E), peak late diastolic velocity (A), E wave deceleration time and isovolumetric relaxation time. Pulmonary venous inflow was studied from the right upper pulmonary vein, with measures of peak systolic, diastolic and atrial reversal velocities. Each representative value was obtained from the average of three measurements.
Tissue Doppler imaging
Pulsed‐wave tissue Doppler of the septal and lateral mitral annulus was used for the measurement of S′, early peak diastolic (E′) and late annular diastolic myocardial velocities. The average of the septal and lateral values was used to calculate mean values. LV filling pressures were approximated from the relationship between E and E′ by using the mean E′ value from the medial and lateral annular measures.
Strain and SR data analysis
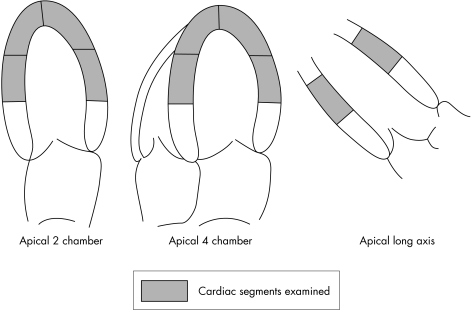
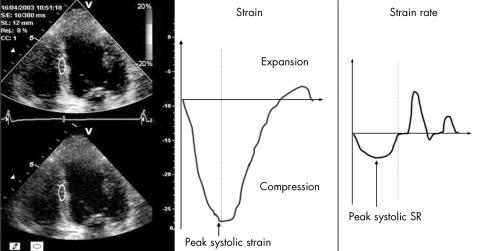
Strain and SR curves were extracted from colour tissue Doppler images by standard software (Echopac; GE Vingmed). Strain and SR were derived from strain and SR curves obtained by placing a sample bar (12 mm) in the mid‐ and apical segments from the three apical views (fig 1). The mid‐myocardial layer was sampled in each segment and maintained at the same position during the cardiac cycle by manually tracking wall motion (fig 2), but we excluded data if we were unable to obtain a smooth strain curve or the angle between the scan line and wall was > 20°. Peak systolic strain (ε) was defined as the greatest value on the strain curve, and peak systolic SR was measured from the strain curve as previously validated.12,13 Results are expressed as the average strain from the 10 mid‐ and apical segments for correlation with MCE. The difference between two observers' measurement of SR and strain in our laboratory is −0.1 (0.1)/s and 1.6 (1.7)%, with respective coefficients of variation of 8% and 10%.
Figure 1 Cardiac segments examined with strain rate imaging and quantitative myocardial contrast echocardiography.
Figure 2 Strain rate (SR) imaging from the mid‐inferoseptal myocardial segment from a study subject. Peak systolic strain is the peak percentage long‐axis shortening; peak systolic strain rate is the peak rate of shortening.
Stress imaging protocol
Patients were instructed to avoid methylxanthine derivatives for the day before the study. Intravenous access was secured. Perflutren‐filled microbubbles with a lipid shell (Definity; Bristol Myers Squibb Imaging, North Billerica, Massachusetts, USA) were prepared by diluting 1.8 ml agent in 18 ml of normal saline. The microbubbles were continuously infused to maintain adequate myocardial perfusion with attenuation at the mitral valve level. Low power (mechanical index 0.1–0.2), real‐time destruction replenishment images were acquired in the apical four‐chamber, two‐chamber and long‐axis views at rest by a commercially available system (Sonos 7500 with S3 transducer; Philips Ultrasound, Andover, Massachusetts, USA) and power modulation imaging. After a high mechanical index destruction pulse sequence (mechanical index 1.2), images were acquired at 20 frames/s for 10 s and stored digitally. All patients then underwent infusion of dipyridamole 0.56 mg/kg over 4 min followed immediately by symptom‐limited exercise with a standard Bruce protocol as previously described.14 A 12‐lead ECG was recorded and blood pressure was measured before exercise was started, at the end of each stage and during recovery. The ECG was monitored continuously for ST segment changes and arrhythmias. Tests were symptom limited, with standard end points. Immediately after completion of exercise, contrast was re‐administered in the same fashion and stress contrast images were obtained again in the three apical views.
Exercise assessment
The haemodynamic response to exercise, exercise capacity, symptom status and ECG changes with stress were noted in all patients. ST segments were assessed visually; for categorical analysis, significant ST displacement was defined as > 0.1 mV at 0.08 s after the J point in any lead. Additionally, the exercise capacity (minutes of the Bruce protocol), maximum ST segment deviation (in mm) and angina score (2 for limiting and 1 for non‐limiting angina) were used to calculate the Duke treadmill score, as previously described.15 Patients with scores above 5 and below −10 were considered to be at low and high risk, respectively.
Quantitative MCE
Quantitative MCE was performed offline by a single observer unaware of the angiographic or qualitative data. In large regions of interest and only end systolic frames,16 standard commercial software (Q‐lab; Philips Ultrasound) was used to quantify myocardial replenishment after bubble destruction16,17 by placing large regions of interest in each of the 10 mid‐ and apical cardiac segments; basal segments were not quantified because of concerns about attenuation. Plateau myocardial contrast intensity, representing myocardial blood volume, the rate of rise of intensity, representing mean myocardial red blood cell velocity, and their product, representing MBF,18 were assessed both at rest and after stress, enabling calculation of MBF reserves for each segment. The mean MBF reserves were then calculated in each patient.
Coronary angiography
Selective coronary angiography was performed in a standard fashion, by using local anaesthesia and a Judkins approach. Quantitative coronary angiography (Philips Medical Systems, Best, The Netherlands) was performed by an examiner blinded to all other data.19
Statistical analysis
Data are presented as mean (SD). Continuous variables were compared by Student's t test. Categorical variables were compared by the χ2 test for unpaired data. The Pearson correlation coefficient (r) was used to determine which clinical and echocardiographic variables were associated with MBF, ε and SR. Variables with a univariate association (p < 0.10) were introduced into a backward stepwise multiple linear regression to determine the independent predictors of MBF, ε and SR. Importantly, as diffuse disease may be difficult to identify from angiography, the analyses were repeated with exclusion of patients who had exercise‐induced ST changes. All data were analysed by standard software (SPSS V.12; SPSS, Chicago, Illinois, USA).
RESULTS
Clinical and exercise characteristics
Table 1 summarises the baseline clinical, echocardiographic and exercise characteristics of the groups. The groups were matched for age and sex, although the patients with DM had a larger body mass index and more of them were taking angiotensin‐converting enzyme (ACE) inhibitors. Although the rates of hypertension or recorded resting systolic, diastolic or pulse pressure did not differ significantly between the groups, patients with DM had a significantly higher relative wall thickness and overall LV mass index. Patients with DM also had significantly impaired exercise capacity compared with the non‐DM group, the rate of ST changes or chest pain did not differ.
Table 1 Clinical and exercise characteristics of the patients studied.
| DM (n = 22) | No DM (n = 26) | p Value | |
|---|---|---|---|
| Age (years) | 57 (9) | 54 (11) | NS |
| Women | 12 (58%) | 10 (38%) | NS |
| Height (cm) | 168 (9) | 172 (8) | NS |
| Weight (kg) | 87 (14) | 82 (14) | NS |
| SBP rest (mm Hg) | 124 (14) | 123 (19) | NS |
| Pulse pressure rest (mm Hg) | 48 (10) | 51 (18) | NS |
| Body mass index (kg/m2) | 30.6 (4.2) | 27.8 (4.8) | 0.04 |
| Risk factors | |||
| Diabetes | 22 (100%) | 0 (0%) | <0.001 |
| Hypertension | 16 (72%) | 12 (46%) | NS |
| Smoking | 2 (9%) | 7 (27%) | NS |
| Family history of CAD | 8 (36%) | 13 (50%) | NS |
| Hyperlipidaemia | 15 (68%) | 18 (69%) | NS |
| Drugs | |||
| ACE inhibitors | 14/19 (74%) | 9 (35%) | 0.01 |
| Calcium channel blockers | 8/19 (42%) | 5 (20%) | NS |
| β blockers | 13/19 (68%) | 13 (50%) | NS |
| Echocardiographic parameters | |||
| Ejection fraction (%) | 66 (4) | 65 (3) | NS |
| Relative wall thickness | 0.40 (0.07) | 0.35 (0.05) | 0.007 |
| LV mass index (g/m2)* | 78 (15) | 69 (11) | 0.02 |
| Exercise testing | |||
| Exercise duration (min) | 6 (2.5) | 8 (2.2) | 0.01 |
| ST changes | 5 (23%) | 5 (19%) | NS |
| Chest pain | 6 (27%) | 5 (19%) | NS |
| SBP stress (mm Hg) | 151 (21) | 155 (21) | NS |
| Pulse pressure stress (mm Hg) | 73 (19) | 79 (20) | NS |
Data are mean (SD) or number (%).
*Left ventricular (LV) mass/body surface area (square root[Ht (cm) × Wt (kg)/3600]).
ACE, angiotensin‐converting enzyme; CAD, coronary artery disease; DM, diabetes mellitus; SBP, systolic blood pressure.
Conventional measures of systolic and diastolic function
Table 2 summarises standard tests of cardiac function. LV ejection fraction did not differ between the DM and non‐DM groups (66 (4)% v 65 (3)%, NS). The standard indices of diastolic function, including transmitral E and A, deceleration time, isovolumetric relaxation time and annular pulsed‐wave tissue Doppler, also did not differ. The patients with DM had higher estimated filling pressures, evidenced by a significantly increased E:E′ ratio (9.6 (2.7) v 7.9 (2.3), p = 0.03), and the resting E:E′ was significantly correlated with exercise capacity (r = –0.43, p = 0.004).
Table 2 Measures of conventional, tissue Doppler, strain and myocardial contrast echocardiographic imaging.
| DM (n = 22) | No DM (n = 26) | p Value | |
|---|---|---|---|
| Ejection fraction (%) | 66 (4) | 65 (3) | NS |
| Transmitral E (cm/s) | 0.82 (0.20) | 0.76 (0.11) | NS |
| Transmitral A (cm/s) | 0.80 (0.23) | 0.66 (0.19) | NS |
| Transmitral EDT (ms) | 227 (38) | 225 (44) | NS |
| IVRT (ms) | 99 (16) | 100 (12) | NS |
| Pulmonary venous systolic velocity | 0.58 (0.10) | 0.58 (0.09) | NS |
| Pulmonary venous diastolic velocity | 0.48 (0.11) | 0.46 (0.13) | NS |
| Mean annular E′ (cm/s) | 9.0 (3.0) | 10.2 (2.2) | NS |
| Mean annular A′ (cm/s) | 9.6 (1.9) | 9.4 (1.6) | NS |
| Mean annular S′ (cm/s) | 8.0 (1.2) | 8.4 (1.3) | NS |
| Mean annular E/E′ | 9.6 (2.7) | 7.9 (2.3) | 0.03 |
| Strain rate (/s) | −0.80 (0.22) | −1.1 (0.28) | 0.006 |
| ε (%) | −14.4 (4.6) | −18.7 (3.1) | 0.006 |
| MBF reserve | 2.4 (1.0) | 3.8 (2.1) | 0.01 |
Data are mean (SD) or number (%).
A, peak late diastolic velocity; A′, late annular diastolic velocity; ε, peak systolic strain; E, peak early diastolic velocity; E′, early peak diastolic velocity; EDT, E wave deceleration time; IVRT, isovolumetric relaxation time; MBF, myocardial blood flow; S′, peak systolic velocity.
SRI parameters
Quantitative SRI analysis was feasible in all patients, with data available from 9 (1) of the 10 mid‐ and apical segments (fig 2). In keeping with the exclusion of resting wall motion abnormalities, there was no evidence of post‐systolic thickening on strain curves of any patient. Compared with controls, patients with DM had significantly impaired resting SR (−0.80 (0.22) v −1.1 (0.28), p = 0.006) and ε (−14.4 (4.6) v −18.7 (3.1), p = 0.006). This was also true if patients with stress‐induced ST changes were excluded (SR −0.80 (0.21)/s v −1.11 (0.30), p = 0.002; ε −14.1 (0.62)% v −18.9 (3.2)%, p < 0.001).
Quantitative MCE
Quantitative MCE was feasible in 46 patients (96%), with mean data from 7 (2) segments per patient. Mean resting MBF was not significantly different between patients with and without DM (5.1 (3.5) v 4.6 (3.2), p = 0.62). Patients with DM had significantly impaired MBF reserve compared with patients without DM (2.4 (1.0) v 3.8 (2.1), p = 0.01), and this was true when the patients with stress‐induced ST changes were excluded (2.13 (0.79) v 3.71 (2.0), p = 0.007).
Correlation between perfusion and dysfunction
Clinical or echocardiographic variables and MBF reserve were not significantly correlated. In a multivariate model that included age, DM, exercise duration, relative wall thickness and LV mass index, DM was the only independent predictor of MBF reserve (p = 0.01). Similarly, there were no clinical or echocardiographic correlates for ε, and the only correlate of SR was pulmonary vein systolic velocity. In multivariate models including age, DM, mass index, body mass index, lateral S′ and pulmonary systolic velocity, only DM was an independent predictor of ε (p = 0.004), and DM (p = 0.001) and lateral S′ (p = 0.03) were predictors of SR.
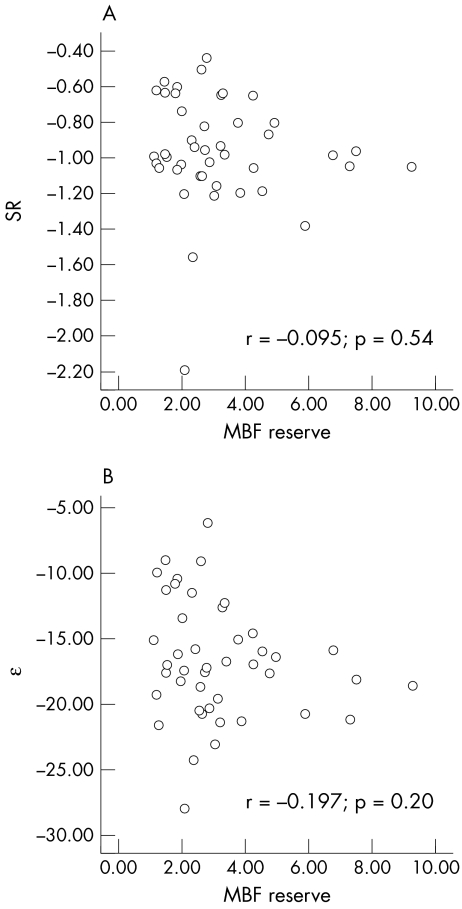
Importantly, the overall MBF reserve had no relationship to parameters of either SR (−0.095, p = 0.54) or ε (−0.197, p = 0.2) (fig 3). Similarly, flow and function were not correlated if patients with stress‐induced ECG changes were excluded (SR r = −0.12, p = 0.5; ε r = −0.19, p = 0.26) or when patients were analysed separately without (SR r = 0.15, p = 0.48; ε r = 0.05, p = 0.81) and with DM (SR r = −0.07, p = 0.77; ε r = −0.19, p = 0.42).
Figure 3 (A) Scatter plot of the relationship between myocardial blood flow (MBF) (the product of plateau myocardial contrast intensity and the rate of rise of intensity) reserve and strain rate (SR) and (B) relationship between MBF reserve and peak systolic strain (ε).
DISCUSSION
Patients with DM, normal LV systolic function and no CAD (defined by quantitative coronary angiography) have evidence of subclinical longitudinal myocardial dysfunction detected by SRI and evidence of impaired transmural MBF reserve, which can be shown by quantitative stress MCE. Although abnormal flow reserve and subclinical diabetic cardiomyopathy coexist, we found no significant relationship between them, raising the possibility that microvascular disease may not be the predominant causative factor in diabetic heart disease.
Subclinical cardiomyopathy in DM with no CAD
Heart failure is more likely to develop after a cardiac event in patients with DM than without DM. One of the reasons for this propensity to heart failure may be subclinical LV dysfunction, and several authors have shown disturbances in subtle indices of systolic and diastolic function including tissue Doppler, strain and SR.1,9,20,21 Myocardial ischaemia may provoke the same changes in these markers and, although some previous studies have excluded patients with an abnormal stress echocardiogram,1,9 our study is the first to document the presence of a subclinical diabetic cardiomyopathy in a group of patients with apparently normal systolic function, without evidence of CAD on quantitative coronary angiography.
Although these myocardial parameters may be influenced by other factors, including age, sex, risk factors and LV geometry,22 none of these variables was associated with SR in this study, in which the only independent predictor of SR or ε was DM. The lack of association of abnormal tissue Doppler and strain with abnormalities of conventional measurements is a pointer towards the sensitivity of these parameters for early‐stage disease. Nonetheless, the association of myocardial dysfunction with higher filling pressures (estimated by the E:E′ ratio) and reduced exercise capacity is consistent with previous reports that show myocardial disease associated with DM to be a clinically important entity.
Impaired MBF reserve in patients with DM
Animals with diabetes have an impaired vasodilator response of the coronary circulation to various stimuli, including increased myocardial oxygen demand and pharmacological vasodilators, indicating coronary microvascular disease. Invasive human studies with Doppler flow wire8 and argon gas chromatography5 have confirmed the presence of impaired maximal coronary flow reserve in response to pharmacological vasodilators in patients with DM and no CAD. Subsequent non‐invasive work with positron emission tomography has shown impaired MBF reserve in both type I and type II DM.6,23
MCE can quantify coronary flow reserve in humans.10 Our results confirm the finding of impaired MBF reserve in patients with DM and no CAD but no other relationship between MBF and clinical or echocardiographic parameters. In a multivariate model, including LV geometric parameters, DM was the only independent predictor of MBF reserve. This occurred despite a higher incidence of vasodilator usage (predominately ACE inhibitors) in the group with DM.
Relationship between myocardial flow and dysfunction in DM
The precise aetiology of diabetic myocardial disease remains controversial, with potential contributions from microvascular disease, direct metabolic effects on the myocyte and fibrosis. The similar pathology of diabetic renal disease often leads to an expectation that vascular disease may be a primary underlying feature of subclinical diabetic heart disease. As well as the aforementioned functional abnormalities of the coronary circulation, limited human studies have also shown altered cardiac microvascular structural changes (capillary basement membrane thickening) as well as surrounding interstitial fibrosis and myocyte atrophy.24,25 In a study of eight patients with type I diabetes and age‐matched controls, Hansen et al26 found impaired resting tissue Doppler parameters of diastolic function, abnormal resting blood flow by MCE, and augmentation of MBF and myocardial function after treatment with C peptide. Surprisingly, despite this response of the myocardium to improved flow (further suggesting microvascular disease as a contributor to the impaired function) these patients had no impairment of flow reserve after vasodilator stress with dipyridamole.26 Similarly, Fang et al9 showed that, while patients with DM had impaired basal myocardial Doppler tissue imaging parameters at rest when compared with controls, the augmentation of myocardial Doppler tissue imaging in response to incremental pharmacological stress with dobutamine was normal.9
Although our study confirms the clinical suspicion that coronary microangiopathy, manifest by impaired MBF reserve, is concomitantly present in patients with subclinical diabetic cardiomyopathy (manifest by impaired ε and SR), we found no correlation between MBF reserve and parameters of function. This suggests that, although microvascular disease may coexist with diabetic cardiomyopathy, it is not the predominant causative factor, at least early in the disease course when systolic function and traditional parameters of diastolic function are preserved.
Limitations
As with other studies examining flow in patients with DM, in our study coexistent hypertension, a common associated clinical problem, could have influenced the results. The rate of clinical hypertension did not differ significantly between the DM and control groups, and importantly strain or flow parameters were not correlated with any marker of LV geometry in univariate analysis. Nevertheless, LV mass index and relative wall thickness were forced into the multivariate models to prove the absence of a significant relationship. Additionally, although drug data were not available for all patients with diabetes, the only significant difference was the higher rates of ACE inhibitor and vasodilator use by patients with DM, despite which these patients still had impaired microvascular function.
Importantly, in this study we have compared longitudinal contraction from the mid‐myocardium (which probably largely reflects subendocardial function) with transmural myocardial flow. Ideally we would correlate subendocardial perfusion (but this has limited feasibility) with subendocardial strain parameters, which has limited feasibility because the lateral resolution of tissue Doppler‐based strain at the base of the heart is limited, as is the feasibility of measuring radial strain.
The coronary angiogram does not exclude the possibility of coronary disease in all patients, especially given the propensity of patients with DM to develop diffuse CAD. Nevertheless, the lack of difference in the incidence of chest pain and ST changes between the groups suggests that significant ischaemia from diffuse disease was not different between the groups. Additionally, results were not changed by exclusion of patients with stress‐induced ST changes. Lastly, the exclusion of CAD is not of pivotal significance to the main findings of this work, that reduced flow reserve (of whatever cause) is unrelated to functional changes in diabetic heart disease.
Conclusions
SRI can identify subclinical longitudinal myocardial dysfunction in patients with diabetes but no evidence of obstructive CAD. Quantitative MCE shows impaired transmural MBF reserve in these patients, but measured parameters of flow and function were not related. This raises the possibility that, although cardiac microangiopathy and subclinical diabetic cardiomyopathy may coexist, microvascular disease may not be the predominant causative factor in diabetic heart muscle disease.
Abbreviations
A - peak late diastolic velocity
ACE - angiotensin‐converting enzyme
CAD - coronary artery disease
DM - diabetes mellitus
ε - peak systolic strain
E - peak early diastolic velocity
E′ - early peak myocardial diastolic velocity
LV - left ventricular
MBF - myocardial blood flow
MCE - myocardial contrast echocardiography
S′ - peak myocardial systolic velocity
SR - strain rate
SRI - strain rate imaging
Footnotes
Supported in part by a Centres of Clinical Research Excellence award from the National Health and Medical Research Council, Canberra, Australia
References
- 1.Fang Z Y, Yuda S, Anderson V.et al Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol 200341611–617. [DOI] [PubMed] [Google Scholar]
- 2.Picano E, Lattanzi F, Masini M.et al Usefulness of the dipyridamole‐exercise echocardiography test for diagnosis of coronary artery disease. Am J Cardiol 19886267–70. [DOI] [PubMed] [Google Scholar]
- 3.Fang Z Y, Prins J B, Marwick T H. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 200425543–567. [DOI] [PubMed] [Google Scholar]
- 4.Strauer B E, Motz W, Vogt M.et al Impaired coronary flow reserve in NIDDM: a possible role for diabetic cardiopathy in humans. Diabetes 199746(Suppl 2)S119–S124. [DOI] [PubMed] [Google Scholar]
- 5.Strauer B E, Motz W, Vogt M.et al Evidence for reduced coronary flow reserve in patients with insulin‐dependent diabetes: a possible cause for diabetic heart disease in man. Exp Clin Endocrinol Diabetes 199710515–20. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama I, Momomura S, Ohtake T.et al Reduced myocardial flow reserve in non‐insulin‐dependent diabetes mellitus. J Am Coll Cardiol 1997301472–1477. [DOI] [PubMed] [Google Scholar]
- 7.Pitkanen O P, Nuutila P, Raitakari O T.et al Coronary flow reserve is reduced in young men with IDDM. Diabetes 199847248–254. [DOI] [PubMed] [Google Scholar]
- 8.Nahser P J, Jr, Brown R E, Oskarsson H.et al Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation 199591635–640. [DOI] [PubMed] [Google Scholar]
- 9.Fang Z Y, Najos‐Valencia O, Leano R.et al Patients with early diabetic heart disease demonstrate a normal myocardial response to dobutamine. J Am Coll Cardiol 200342446–453. [DOI] [PubMed] [Google Scholar]
- 10.Wei K, Ragosta M, Thorpe J.et al Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation 20011032560–2565. [DOI] [PubMed] [Google Scholar]
- 11.Peltier M, Vancraeynest D, Pasquet A.et al Assessment of the physiologic significance of coronary disease with dipyridamole real‐time myocardial contrast echocardiography: comparison with technetium‐99m sestamibi single‐photon emission computed tomography and quantitative coronary angiography. J Am Coll Cardiol 200443257–264. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg N L, Firstenberg M S, Castro P L.et al Doppler‐derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 200210599–105. [DOI] [PubMed] [Google Scholar]
- 13.Urheim S, Edvardsen T, Torp H.et al Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation 20001021158–1164. [DOI] [PubMed] [Google Scholar]
- 14.Moir S, Haluska B A, Jenkins C.et al Incremental benefit of myocardial contrast to combined dipyridamole‐exercise stress echocardiography for the assessment of coronary artery disease. Circulation 20041101108–1113. [DOI] [PubMed] [Google Scholar]
- 15.Mark D B, Hlatky M A, Harrell F E., Jret al Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987106793–800. [DOI] [PubMed] [Google Scholar]
- 16.Leong‐Poi H, Le E, Rim S J.et al Quantification of myocardial perfusion and determination of coronary stenosis severity during hyperemia using real‐time myocardial contrast echocardiography. J Am Soc Echocardiogr 2001141173–1182. [DOI] [PubMed] [Google Scholar]
- 17.Masugata H, Lafitte S, Peters B.et al Comparison of real‐time and intermittent triggered myocardial contrast echocardiography for quantification of coronary stenosis severity and transmural perfusion gradient. Circulation 20011041550–1556. [DOI] [PubMed] [Google Scholar]
- 18.Wei K, Jayaweera A R, Firoozan S.et al Quantification of myocardial blood flow with ultrasound‐induced destruction of microbubbles administered as a constant venous infusion. Circulation 199897473–483. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh K H, Bengtson J R, Helmy S.et al Relation of quantitative coronary lesion measurements to the development of exercise‐induced ischemia assessed by exercise echocardiography. J Am Coll Cardiol 1990151043–1051. [DOI] [PubMed] [Google Scholar]
- 20.Andersen N H, Poulsen S H, Eiskjaer H.et al Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with type II diabetes mellitus: a Doppler tissue tracking and strain rate echocardiography study. Clin Sci (Lond) 200310559–66. [DOI] [PubMed] [Google Scholar]
- 21.Vinereanu D, Nicolaides E, Tweddel A C.et al Subclinical left ventricular dysfunction in asymptomatic patients with type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond) 2003105591–599. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland G R, Di Salvo G, Claus P.et al Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr 200417788–802. [DOI] [PubMed] [Google Scholar]
- 23.Pitkanen O P, Nuutila P, Raitakari O T.et al Coronary flow reserve is reduced in young men with IDDM. Diabetes 199847248–254. [DOI] [PubMed] [Google Scholar]
- 24.Fischer V W, Barner H B, Leskiw M L. Capillary basal laminar thickness in diabetic human myocardium. Diabetes 197928713–719. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi M, Techigawara M, Ishihata T.et al A comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels 199712267–274. [DOI] [PubMed] [Google Scholar]
- 26.Hansen A, Johansson B L, Wahren J.et al C‐peptide exerts beneficial effects on myocardial blood flow and function in patients with type 1 diabetes. Diabetes 2002513077–3082. [DOI] [PubMed] [Google Scholar]