Abstract
Objectives
To compare the relationship between dobutamine myocardial blood flow (MBF), rate–pressure product (RPP) and stenosis severity in patients with coronary artery disease (CAD).
Methods
27 patients with single‐vessel CAD were allocated to three groups based on stenosis severity: group 1, 50–69% (n = 9); group 2, 70–89% (n = 9); and group 3, ⩾ 90% (n = 9). Nine normal volunteers served as controls. Resting and dobutamine MBF were measured by positron emission tomography in the territory subtended by the stenosis (Isc) and remote myocardium (Rem). Mean left ventricular MBF was used for controls.
Results
In group 1, mean dobutamine MBF‐Isc (2.48 (SD 0.48 ml/min/g)) and dobutamine MBF‐Rem (2.70 (0.50) ml/min/g, NS) were comparable. In groups 2 and 3, dobutamine MBF‐Isc (1.91 (0.44) and 1.22 (0.21) ml/min/g) was significantly lower than dobutamine MBF‐Rem (2.27 (0.28) and 1.98 (0.25) ml/min/g, p < 0.02 and p < 0.005, respectively). An inverse relation between dobutamine MBF and stenosis severity existed both in Isc (r = 0.79, p < 0.001) and in Rem territories (r = 0.71, p < 0.001). For any given RPP, dobutamine MBF was greater in controls than in Rem (p < 0.05), which in turn was greater than in Isc (p < 0.05).
Conclusion
Dobutamine MBF inversely correlated with stenosis severity and achieved significant flow heterogeneity for coronary stenoses > 70%. Dobutamine MBF and RPP were dissociated in both Isc and Rem segments in patients compared with controls.
Stress testing is widely used in the diagnosis and management of patients with coronary artery disease (CAD). Although physical exercise is the ideal stimulus to induce hyperaemia, pharmacological stressors such as adenosine and dobutamine offer a practical alternative particularly in patients with limited exercise capacity. Adenosine activates specific purinergic receptors on coronary resistive vessels, thereby increasing myocardial blood flow (MBF) by direct vasodilatation, and has a limited effect on cardiac work. In contrast, dobutamine is a sympathomimetic amine that acts through α and β adrenoceptors stimulating both positive inotropic and chronotropic effects and enhancing MBF, mainly through metabolic vasodilatation. In addition, dobutamine increases MBF through direct β2 adrenoceptor‐mediated vasodilatation of coronary resistive vessels. Although vasodilator agents such as adenosine may be more efficient stressors, dobutamine offers a more physiological approach to assess demand ischaemia.
The principle underlying myocardial perfusion imaging is the identification of differential regional flow reserve between normal myocardium and myocardium subtended by stenotic coronary arteries. Positron emission tomography (PET) provides accurate and reproducible non‐invasive measurements of absolute regional MBF and coronary flow reserve (CFR) in vivo.1 Previous PET studies have shown a good correlation between coronary artery stenosis severity and CFR by using vasodilator stress2,3 and between dobutamine‐induced MBF and regional wall motion abnormalities.4 However, limited information is available on the relationship between dobutamine hyperaemia and coronary artery stenosis severity.4,5 Furthermore, the effects on remote myocardium of dobutamine stress and its ability to highlight flow heterogeneity in CAD are poorly defined. Lastly, little data exist to compare the relationship between dobutamine‐induced hyperaemia and cardiac workload in normal subjects with that in patients with CAD.
The objective of this study was therefore to investigate the effect of intravenous dobutamine, at doses used in patients with CAD for conventional stress imaging, on the relation between MBF, rate–pressure product (RPP) and stenosis severity in territories subtended by a significant coronary lesion (Isc) and in remote non‐ischaemic myocardium (Rem). To allow direct comparison with normal myocardium we also assessed the effects of dobutamine stress on MBF in a group of normal subjects.
METHODS
Patient population
Twenty seven patients (25 men, mean age 63 (SD 6) years, range 51–77 years) were recruited from St Mary's and Hammersmith Hospitals, London, UK. All described a history of chronic stable angina of at least three months' duration and had ECG evidence of exercise‐induced myocardial ischaemia, defined as ⩾ 0.1 mV of horizontal or downsloping ST segment depression. Exclusion criteria were a recent history (< 3 months) of myocardial infarction or unstable angina, inability to perform exercise tolerance testing or resting ECG patterns that would interfere with interpretation of ST changes.
Nine normal male volunteers (aged 46 (7) years, range 40–57, p < 0.001 v patients) were recruited as controls. These subjects were asymptomatic and at low risk for CAD.6 Entry criteria were normal heart rate, blood pressure, ECG and transthoracic echocardiography. Subjects with a total cholesterol > 6.4 mmol/l were excluded from the study.7
The study protocol was approved by the research ethics committee of Hammersmith and St Mary's Hospitals. Radiation exposure was licensed by the UK Administration of Radioactive Substances Advisory Committee.
Coronary angiography
All patients underwent diagnostic cardiac catheterisation to confirm the presence of CAD and for quantification of stenosis severity. Two independent experienced operators analysed the coronary angiograms. The luminal diameter of the stenosed artery and adjacent reference lumen was measured at end diastole in the projection that showed the most severe stenosis. Quantitative analysis was performed by an automated edge contour detection system (Centricity quantitative coronary angiography, General Electric Medical Systems, Milwaukee, Wisconsin, USA). The degree of stenosis was expressed as percentage reduction of the internal luminal diameter in relation to the estimated diameter interpolated from the diameters at the proximal and distal boundaries of the stenosis. In patients left ventricular function was assessed by left ventriculography performed in a standard 30° right anterior oblique projection. An automated endocardial contour detector was used to measure left ventricular end systolic and end diastolic volumes according to Simpson's rule to permit calculation of ejection fraction. In controls left ventricular ejection fraction was measured by transthoracic echocardiography with similar application of Simpson's rule.
Positron emission tomography
Study protocol
Patients and control subjects underwent PET with oxygen‐15 labelled water (H215O) to measure MBF under resting conditions and during symptom‐limited dobutamine stress. All patients were instructed to omit angina drugs for 24 h except for β adrenoceptor blockers, which were withheld for > 72 h before the study.
Image acquisition
Images were acquired with an ECAT 931‐08/12 15‐slice tomograph giving a 10.5 cm axial field of view (CTI/Siemens, Knoxville, Tennessee, USA). Subjects lay supine in the scanner and the optimal imaging position was determined by a 5 min rectilinear scan after the exposure of an external germanium‐68 ring source. A 20 min transmission scan was then acquired to correct for attenuation of all subsequent emission scans. H215O (700–900 MBq) was injected as an intravenous bolus over 20 s at an infusion rate of 10 ml/min. MBF was measured at baseline and repeated 10 min later, to allow for decay of the 15O radioactivity, during dobutamine stress. Dobutamine was administered by a standard clinical protocol of incremental dosing, starting at 5 μg/kg/min and increasing every 3 min to 10, 15, 20, 30 and 40 μg/kg/min or until onset of chest pain. A single dose of intravenous atropine (600 μg) was given to those subjects whose heart rate did not increase > 100 beats/min at 40 μg/kg/min of dobutamine. PET acquisition was timed to start when a steady state had been achieved at the maximum dobutamine dose. Cardiac rhythm was monitored throughout the scanning protocol, blood pressure was recorded by automatic cuff sphygmomanometer (Dynamap) at 1 min intervals, and a 12‐lead ECG was recorded at baseline and every 2 min during stress.
Data analysis
A single operator analysed PET data before blinded angiographic findings were disclosed. The sinograms obtained were corrected for attenuation and reconstructed on a MicroVax II computer (Digital Equipment Corp, Marlboro, Massachusetts, USA) by dedicated array processors and standard reconstruction algorithms. Images were transferred to a SUN SPARC 2 workstation (Sun Microsystems, Mountain View, California, USA) and analysed with dedicated software (MATLAB; the MathWorks, Natick, Massachusetts, USA). Myocardial images, for the definition of regions of interest (ROIs), were generated directly from the dynamic H215O, as previously reported.8 Briefly, the creation of factor sinograms requires estimates of vascular (right and left heart) and myocardial tissue time–activity curves.9 Factor images describing tissue and blood distributions were generated by iterative reconstruction as previously described.10,11 Factor images were resliced into short‐axis images in an orientation perpendicular to the long axis of the left ventricle. Sixteen ROIs, corresponding to the territories of distribution of the three major coronary arteries, were drawn within the left ventricular myocardium on 12 consecutive image planes, according to the recommendations of the American Society of Echocardiography.12 The regions were drawn semiautomatically by using a centre line within the myocardium. For the present analysis, however, the original 16 left ventricular ROIs were regrouped into four larger segments in the septal, anterior, lateral and inferior walls. A separate set of ROIs was defined for the right ventricular cavity and the left atrium. Myocardial and blood time–activity curves were then generated from the dynamic images and fitted to a single tissue compartment tracer kinetic model to give values of MBF (ml/min/g).9 Then, in each patient the myocardial ROI subtended by the coronary stenosis was labelled Isc and the ROI subtended by an artery with minimal (< 50% diameter stenosis) or no disease was labelled Rem.
As resting MBF is determined by cardiac workload,13 we corrected resting MBF for the RPP, an index of myocardial oxygen consumption: MBFcorrected = (MBF/RPP) × 104.2 CFR was calculated as the ratio of MBF during dobutamine‐induced hyperaemia to MBF at rest corrected for RPP. In controls, minimum coronary resistance (mm Hg/min/g/ml) was calculated as mean arterial pressure divided by global left ventricular MBF. In patients, regional coronary resistance in the Isc and Rem territories was calculated by dividing mean arterial pressure by the respective regional MBF values.
Statistical analysis
All values are expressed as mean (SD). Two‐tailed paired and unpaired Student's t tests were used to compare group values. Conventional techniques of regression analysis were used to detect relationships and analysis of covariance was used to compare the steepness of regressions. Data were statistically analysed with StatView SE+ (Abacus Concepts, Berkeley California, USA) and p < 0.05 was considered to indicate significance.
RESULTS
All 27 patients had single‐vessel CAD at angiography and were allocated to three different groups based on stenosis severity: mild 50–69% (n = 9); moderate 70–89% (n = 9); severe ⩾ 90% (n = 9). Table 1 presents baseline characteristics of the patients.
Table 1 Characteristics of controls and patients with single‐vessel coronary artery disease grouped by stenosis severity.
| Controls | Stenosis severity | |||
|---|---|---|---|---|
| 50–69% | 70–89% | ⩾90% | ||
| Number | 9 | 9 | 9 | 9 |
| Diabetes | 0 | 3 (33%) | 4 (44%) | 4 (44%) |
| Hypertension | 0 | 6 (66%) | 6 (66%) | 7 (78%) |
| Age (years) | 46 (7) | 61 (6)* | 62 (8)* | 64 (4)* |
| LVEF (%) | 68 (3) | 51 (1)* | 53 (9)* | 44 (11)*† |
| Stenosis severity (%)‡ | NA | 64 (3) | 81 (5) | 94 (4) |
| CCS functional class | NA | 1.9 (0.6) | 2.1 (0.3) | 2.8 (0.4) |
*p<0.05 v control; †p<0.05 v 70–89%; ‡assessed by quantitative coronary angiography.
CCS, Canadian Cardiovascular Society; LVEF, left ventricular ejection fraction; NA, not applicable.
Resting RPP was comparable in the three patient groups but was lower in normal volunteers. However, peak RPP attained during dobutamine stress was comparable in all groups (table 2).
Table 2 Rate–pressure product (RPP) and myocardial blood flow at rest and during dobutamine stress.
| Controls | Stenosis severity | |||
|---|---|---|---|---|
| 50–69% | 70–89% | ⩾90% | ||
| RPP rest | 7112 (1621) | 8775 (3474) | 9979 (1364)* | 8447 (1393) |
| RPP dobutamine | 20511 (2703) | 21256 (2873) | 21785 (3622) | 19198 (4550) |
| Dobutamine dose (μg/kg/min) | 39 (3) | 33 (10) | 30 (9)* | 29 (9)* |
| Myocardial blood flow (ml/min/g) | ||||
| Isc at rest | 1.23 (0.36) | 1.10 (0.15) | 1.16 (0.33) | |
| Rem at rest | 1.22 (0.28) | 1.24 (0.28) | 1.08 (0.1) | 1.22 (0.17) |
| Ischaemic with dobutamine | 2.48 (0.48) | 1.91 (0.44)† | 1.22 (0.21)†‡ | |
| Rem with dobutamine | 3.18 (0.96) | 2.70 (0.5) | 2.27 (0.28)* | 1.98 (0.25)*† |
| Isc CFR | 2.08 (0.3) | 1.72 (0.24)† | 1.14 (0.38)†‡ | |
| Rem CFR | 2.62 (0.58) | 2.23 (0.34) | 2.12 (0.25)* | 1.64 (0.24)*†‡ |
| Isc dobutamineMBF−RPP | 1.20 (0.31) | 0.90 (0.27)† | 0.65 (0.12)†‡ | |
| Rem dobutamineMBF−RPP | 1.58 (0.52) | 1.31 (0.39)* | 1.07 (0.22)* | 1.08 (0.3)* |
Correction of dobutamine myocardial blood flow for RPP permits comparison of MBF independent of cardiac work. Coronary flow reserve (CFR) is the ratio of dobutamine MBF to rest MBF.
*p<0.05 v control; †p<0.05 v 50–69%; ‡p<0.05 v 70–89%.
Isc, myocardial territory subtended by significant coronary stenosis; Rem, myocardium subtended by coronary arteries with minimal or no disease.
MBF and CFR
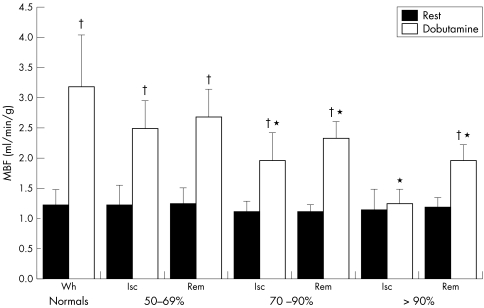
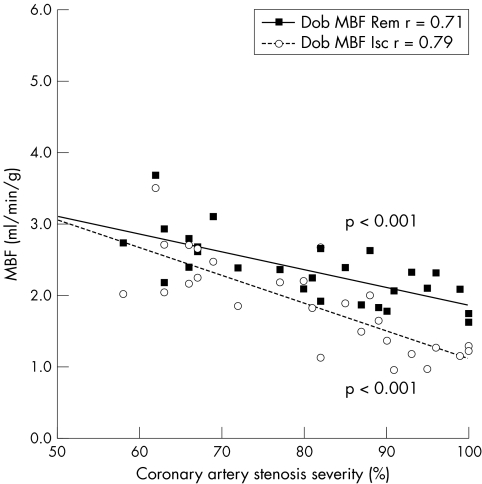
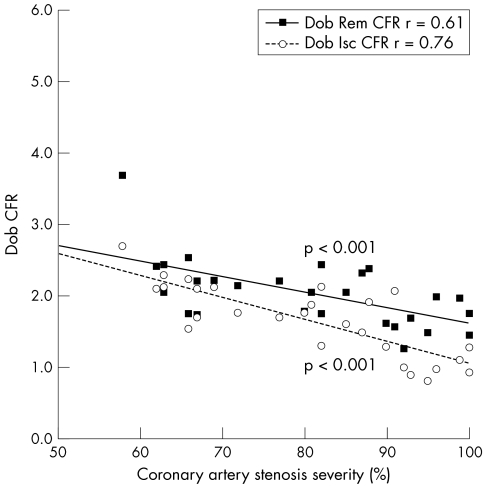
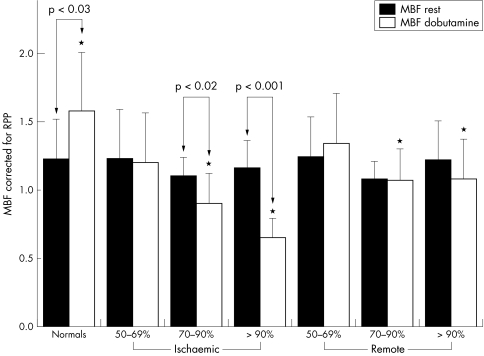
Table 2 presents MBF results. After dobutamine, MBF increased relative to baseline in all myocardial segments except when the segment was subtended by a stenosis ⩾ 90% (fig 1). Peak dobutamine MBF was progressively blunted with increasing coronary stenosis severity. A significant heterogeneity in MBF between ischaemic and remote territories became apparent for coronary stenoses > 70% (fig 1). The inverse relation between coronary artery stenosis severity and dobutamine MBF and CFR was significant in both ischaemic and remote myocardial territories (figs 2 and 3).
Figure 1 Myocardial blood flow (MBF) measured at rest and during dobutamine hyperaemia (Dob) in normal volunteers (minimum coronary resistance calculated as mean arterial pressure divided by global left ventricular MBF) and patients with coronary artery disease highlighting flow heterogeneity between ischaemic (Isc) and remote (Rem) myocardial territories in the presence of a coronary stenosis >70%. *p < 0.05 Isc v Rem Dob MBF; †p < 0.01 rest v Dob MBF.
Figure 2 Correlation between dobutamine myocardial blood flow (Dob MBF) and coronary artery stenosis severity in ischaemic (Isc) and remote (Rem) myocardial territories in patients with single‐vessel coronary artery disease.
Figure 3 Correlation between dobutamine coronary flow reserve (Dob CFR) and coronary artery stenosis severity in ischaemic (Isc ) and remote (Rem) myocardial territories in patients with single‐vessel coronary artery disease.
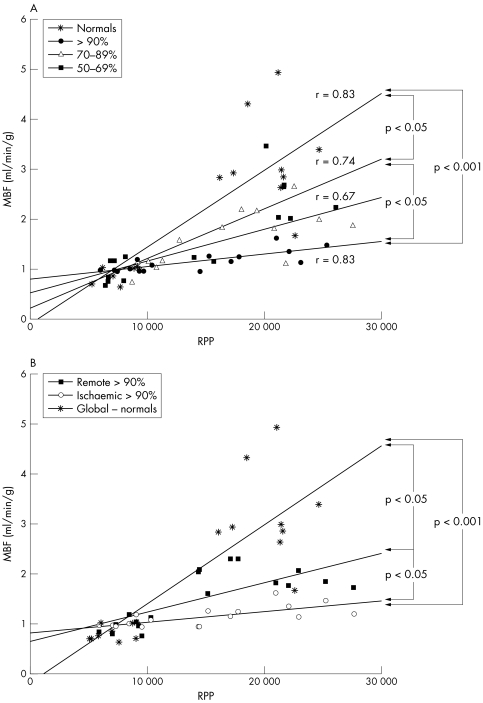
On average dobutamine stress resulted in a 2.9‐fold increase in RPP and a 3.8‐fold increase in global MBF in normal volunteers. In patients, the 2.3‐fold increase in RPP obtained after dobutamine was associated with a comparatively smaller increase in MBF (1.8‐fold in ischaemic territories and 2.3‐fold in remote myocardium) suggesting a dissociation between regional MBF and cardiac work. The severity of this mismatch progressively increased in the presence of increasing stenosis severity (fig 4A) and was also seen in remote myocardial territories subtended by angiographically normal coronary arteries (fig 4B).
Figure 4 Correlation between myocardial blood flow (MBF) and rate–pressure product (RPP) in normal volunteers and patients with coronary artery disease. The dissociation between MBF and cardiac work increases in ischaemic territories in the face of increasing stenosis severity. In patients with severe coronary artery stenosis (⩾ 90%), MBF and RPP are dissociated in both (A) ischaemic and (B) remote myocardial territories compared with normal volunteers.
To assess whether these findings were related to differences in cardiac workload among groups, we corrected dobutamine MBF for peak RPP (dobutamineMBF‐RPP) (table 2). In normal volunteers, dobutamineMBF‐RPP significantly increased from resting conditions (1.22 (0.28) v 1.58 (0.52) ml/min/g, p = 0.02) and was significantly higher than dobutamineMBF‐RPP in both ischaemic and remote segments in patients with > 70% stenosis severity. In the patients, dobutamineMBF‐RPP was significantly lower than rest MBFcorr in ischaemic territories and was similar to rest MBFcorr in remote myocardial territories (fig 5).
Figure 5 Myocardial blood flow (MBF) corrected for rate–pressure product (RPP) for resting conditions (Rest) and during peak dobutamine stress (Dob) in normal volunteers and patients with coronary artery disease showing that reduction of Dob MBF in both ischaemic and remote myocardium is independent of RPP.
Coronary resistance
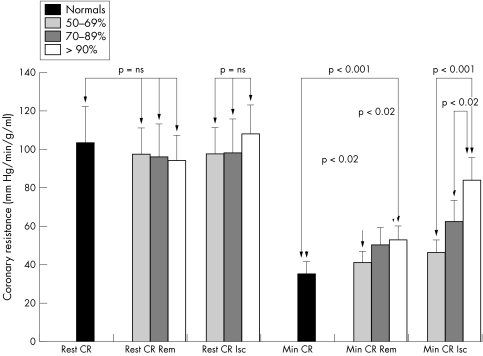
Resting coronary resistance was comparable in controls and patients and between ischaemic and remote territories in patients (table 3). Dobutamine stress resulted in consistently greater reductions in coronary resistance in remote than in ischaemic territories. Minimum coronary resistance during peak dobutamine stress was highest in myocardium subtended by severe coronary stenosis ⩾ 90% and lowest in normal volunteers. Minimum coronary resistance progressively increased with increasing stenosis severity in both ischaemic and remote myocardial regions (fig 6).
Table 3 Resting and minimum coronary resistance measured during peak dobutamine stress.
| Coronary resistance (mm Hg/min/g/ml) | Controls | Stenosis severity | ||
|---|---|---|---|---|
| 50–69% | 70–89% | ⩾90% | ||
| Resting, Isc | 97.5 (23.5) | 98.8 (19.6) | 108.2 (45.5) | |
| Resting, Rem | 103.4 (18.9) | 93.9 (14.1) | 95.8 (11.1) | 97.2 (15.8) |
| Minimum, Isc | 46.4 (12.6) | 63.9 (20.1)† | 84.6 (14.3)†‡ | |
| Minimum, Rem | 35.2 (13.2) | 41.2 (10.4) | 51.5 (10.7)* | 51.9 (9.3)*† |
*p<0.05 v controls; p<0.05 v 50–69%; ‡p<0.05 v 70–89%.
Isc, myocardial territory subtended by significant coronary stenosis; Rem, myocardium subtended by coronary arteries with minimal or no disease.
Figure 6 Assessment of resting coronary resistance (Rest CR) and minimum coronary resistance (Min CR) during dobutamine stress, measured in normal volunteers and in ischaemic (Isc) and remote (Rem) myocardial territories in patients with coronary artery disease.
DISCUSSION
To our knowledge, the present study is the first to compare the effects of dobutamine stress on regional absolute MBF between normal volunteers and patients with single‐vessel CAD across a wide range of coronary artery stenosis severity. Dobutamine‐induced hyperaemia and CFR were progressively blunted in the presence of increasing stenosis severity in ischaemic myocardial segments. Hyperaemic MBF was also attenuated in myocardial segments remote from significant coronary disease that was independent of cardiac work attained during stress.
A curvilinear inverse relationship between hyperaemic MBF and coronary artery stenosis severity with vasodilator stressors has been reported previously.2,3 Similarly, our data show an inverse correlation between MBF and stenosis severity with dobutamine stress. As in the study of Uren et al,2 although the relationship between angiographic and MBF data was significant, individual variability was substantial. The better correlation in our study (r = 0.79) between dobutamine hyperaemia and stenosis severity than in previous work (r = 0.494,5 and r = 0.624,5) probably reflects the higher numbers of patients and the higher grades of stenosis severity enrolled in the present study. Assessment of dobutamine CFR also showed similar correlation, thus supporting the validity of this measurement.
Attenuation of dobutamine MBF and CFR in regions subtended by stenotic coronary arteries is expected. However, hyperaemic flow in remote territories subtended by angiographically normal arteries was also increasingly blunted in relation to the severity of disease in the index artery. Abnormal CFR in myocardial segments subtended by angiographically normal coronary arteries has been previously reported with vasodilator stressors, but not dobutamine.14,15 Although reduction in dobutamine MBF and CFR in patients with CAD may reflect myocardial ischaemia limiting the workload attained, the consistency of our results after MBF correction for RPP suggests that this effect is rather independent of cardiac work. This effect is not intuitively logical, as the remote segment would be expected to contract more in compensation for the ischaemic segment leading to a relative increase in regional MBF.
Our study, however, supports the notion that the lower dobutamine CFR in remote myocardial segments reflects higher minimum coronary resistance. Several factors may explain this phenomenon. Previous experimental studies have shown enhanced contractile function in myocardial segments remote from acute coronary occlusion.16,17,18 This increase in contractile function in remote regions may compensate for the temporary dysfunction occurring in stenotic segments during ischaemia and is due to increased sympathetic activity, which affects both contractility and carbohydrate metabolism.14,19 The increased sympathetic activity may lead to α adrenoceptor‐mediated vasoconstriction20 resulting in an increase in minimum coronary resistance and reduction in CFR. Indeed, a previous study in patients after percutaneous transluminal coronary angioplasty showed that blockade of α adrenoceptors increased myocardial hyperaemia and improved vasodilator reserve.21 Alternatively, the impairment in CFR may reflect chronic adaptive remodelling in response to increased workload in remote segments and the development of hypertrophy,22 or the presence of early microvascular or endothelial dysfunction in the absence of angiographically detectable CAD.23 Ultimately, these different possible explanations are not mutually exclusive.
Under normal circumstances, a strong linear relationship exists between myocardial oxygen consumption, blood flow and cardiac workload.11 The excessive MBF response relative to RPP observed in normal subjects during dobutamine stress may be explained by the action of the drug on intrinsic contractility, its oxygen wasting effect and its direct vasodilator effect through β2 adrenoceptors.24,25,26 This disproportionate increase in MBF relative to RPP in normal volunteers in our study is consistent with previous work from other laboratories.24,27 In patients with CAD, dobutamine hyperaemia was reduced for any given RPP. In remote segments this flow–work mismatch was associated with higher minimum coronary resistance, increasing in parallel with increasing stenosis severity, possibly reflecting compensatory autoregulation of the coronary microcirculation during ischaemic stress. Alternatively, during inotropic stress, increased intramyocardial pressure (that is, extravascular resistance) and ischaemia‐induced isovolumic bulging with increased cardiac work may place remote territories at a haemodynamic and mechanical disadvantage.28 In ischaemic myocardial territories, this dissociation between MBF and RPP was greater in the face of increasing stenosis severity. Our data support the proposal that the primary determinant for hyperaemia during dobutamine stress is metabolic demand from increasing cardiac work and myocardial oxygen consumption. The degree of flow–work disparity in ischaemic segments therefore probably reflects a measure of metabolic derangement due to demand ischaemia.
Consistent with previous work by Krivokapich et al,5 our study shows the relationship between coronary artery stenosis severity and dobutamine hyperaemia and differential MBF for given RPP in ischaemic compared with remote territories. However, we studied more subjects, assessed flow heterogeneity in individual patients, and directly compared patients who had CAD with normal controls. Furthermore, we identified a disparity between MBF and RPP, a validated measure of myocardial oxygen consumption,29 in both the ischaemic and the remote myocardium in patients with CAD, the degree of which appears to correlate with stenosis severity in the index artery.
The high sensitivity of dobutamine stress echocardiography in detecting CAD by the identification of regional contractile dysfunction may be explained by the significant dissociation between MBF and cardiac work in ischaemic territories in our study. Conversely, the reduction in MBF and CFR for any given RPP in the remote territory may account for the false positive test in patients with microvascular dysfunction and the lower specificity seen with perfusion techniques and dobutamine stress than with vasodilators.30
Some important considerations may limit the conclusions that can be reached from the present study. Firstly, the control group studied by PET was significantly younger than the patients, and CFR is known to change with age.31,32 The wide heterogeneity of MBF in normal humans is well documented,33 however, and variability of CFR similar to that observed in the present study has been observed in controls ranging from 18 to 84 years of age.34 Secondly, MBF was measured only at rest and at maximum dobutamine dose. Although measuring flow at incremental doses in the same region in each patient was not possible, this relation was evaluated from the pooled data from all patients studied. Lastly, although 27 patients with CAD and nine normal volunteers would be considered a small study, this sample size is not unusual for studies using PET and did not appear to limit the clinical findings of our study.
Conclusions
Dobutamine MBF and CFR inversely correlated with stenosis severity and achieved significant flow heterogeneity for coronary stenosis > 70%. Dobutamine MBF and RPP was dissociated in patients with CAD compared with normal myocardial territories. In myocardial territories subtended by a coronary stenosis this disparity reflects a measure of metabolic derangement due to demand ischaemia, whereas in remote territories the mismatch was linked to an increase in minimum coronary resistance.
Abbreviations
CAD - coronary artery disease
CFR - coronary flow reserve
H215O - oxygen‐15 labelled water
Isc - myocardium subtended by significant coronary stenosis
MBF - myocardial blood flow
PET - positron emission tomography
Rem - myocardium subtended by coronary arteries with minimal or no disease
RPP - rate–pressure product
ROI - region of interest
References
- 1.De Silva R, Camici P G. Role of positron emission tomography in the investigation of human coronary circulatory function. Cardiovasc Res 1994281595–1612. [DOI] [PubMed] [Google Scholar]
- 2.Uren N G, Melin J A, de Bruyne B.et al Relation between myocardial blood flow and the severity of coronary‐artery stenosis. N Engl J Med 19943301782–1788. [DOI] [PubMed] [Google Scholar]
- 3.Di Carli M, Czernin J, Hoh C K.et al Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation 1995911944–1951. [DOI] [PubMed] [Google Scholar]
- 4.Severi S, Underwood R, Mohiaddin R H.et al Dobutamine stress: effects on regional myocardial blood flow and wall motion. J Am Coll Cardiol 1995261187–1195. [DOI] [PubMed] [Google Scholar]
- 5.Krivokapich J, Czernin J, Schelbert H R. Dobutamine positron emission tomography: absolute quantitation of rest and dobutamine myocardial blood flow and correlation with cardiac work and percent diameter stenosis in patients with and without coronary artery disease. J Am Coll Cardiol 199628565–572. [DOI] [PubMed] [Google Scholar]
- 6.Diamond G A, Forrester J S. Analysis of probability as an aid in the clinical diagnosis of coronary‐artery disease. N Engl J Med 19793001350–1358. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd J, Cobbe S M, Ford I.et al Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 19953331301–1307. [DOI] [PubMed] [Google Scholar]
- 8.Hermansen F, Ashburner J, Spinks T J.et al Generation of myocardial factor images directly from the dynamic oxygen‐15‐water scan without use of an oxygen‐15‐carbon monoxide blood‐pool scan. J Nucl Med 1998391696–1702. [PubMed] [Google Scholar]
- 9.Hermansen F, Rosen S D, Fath‐Ordoubadi F.et al Measurement of myocardial blood flow with oxygen‐15 labelled water: comparison of different administration protocols. Eur J Nucl Med 199825751–759. [DOI] [PubMed] [Google Scholar]
- 10.Hermansen F, Bloomfield P M, Ashburner J.et al Linear dimension reduction of sequences of medical images: II. Direct sum decomposition. Phys Med Biol 1995401921–1941. [DOI] [PubMed] [Google Scholar]
- 11.Hermansen F, Lammertsma A A. Linear dimension reduction of sequences of medical images: III. Factor analysis in signal space. Phys Med Biol 1996411469–1481. [DOI] [PubMed] [Google Scholar]
- 12.Schiller N B, Foster E. Analysis of left ventricular systolic function. Heart 199675(6 Suppl 2)17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camici P, Marraccini P, Marzilli M.et al Coronary hemodynamics and myocardial metabolism during and after pacing stress in normal humans. Am J Physiol 1989257E309–E317. [DOI] [PubMed] [Google Scholar]
- 14.Uren N G, Marraccini P, Gistri R.et al Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease. J Am Coll Cardiol 199322650–658. [DOI] [PubMed] [Google Scholar]
- 15.Sambuceti G, Parodi O, Marcassa C.et al Alteration in regulation of myocardial blood flow in one‐vessel coronary artery disease determined by positron emission tomography. Am J Cardiol 199372538–543. [DOI] [PubMed] [Google Scholar]
- 16.Buda A J, Lefkowitz C A, Gallagher K P. Augmentation of regional function in nonischemic myocardium during coronary occlusion measured with two‐dimensional echocardiography. J Am Coll Cardiol 199016175–180. [DOI] [PubMed] [Google Scholar]
- 17.Naccarella F F, Weintraub W S, Agarwal J B.et al Evaluation of “ischemia at a distance”: effects of coronary occlusion on a remote area of left ventricle. Am J Cardiol 198454869–874. [DOI] [PubMed] [Google Scholar]
- 18.Smalling R W, Ekas R D, Felli P R.et al Reciprocal functional interaction of adjacent myocardial segments during regional ischemia: an intraventricular loading phenomenon affecting apparent regional contractile function in the intact heart. J Am Coll Cardiol 198671335–1346. [DOI] [PubMed] [Google Scholar]
- 19.Camici P, Ferrannini E, Opie L H. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis 198932217–238. [DOI] [PubMed] [Google Scholar]
- 20.Heusch G. Alpha‐adrenergic mechanisms in myocardial ischemia. Circulation 1990811–13. [DOI] [PubMed] [Google Scholar]
- 21.Rimoldi O, Spyrou N, Foale R.et al Limitation of coronary reserve after successful angioplasty is prevented by oral pretreatment with an alpha1‐adrenergic antagonist. J Cardiovasc Pharmacol 200036310–315. [DOI] [PubMed] [Google Scholar]
- 22.Marcus M L, Koyanagi S, Harrison D G.et al Abnormalities in the coronary circulation that occur as a consequence of cardiac hypertrophy. Am J Med 19837562–66. [DOI] [PubMed] [Google Scholar]
- 23.Zeiher A M, Drexler H, Wollschlager H.et al Modulation of coronary vasomotor tone in humans: progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 199183391–401. [DOI] [PubMed] [Google Scholar]
- 24.Vanoverschelde J L, Wijns W, Essamri B.et al Hemodynamic and mechanical determinants of myocardial O2 consumption in normal human heart: effects of dobutamine. Am J Physiol 1993265H1884–H1892. [DOI] [PubMed] [Google Scholar]
- 25.Vatner S F, McRitchie R J, Braunwald E. Effects of dobutamine on left ventricular performance, coronary dynamics, and distribution of cardiac output in conscious dogs. J Clin Invest 1974531265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willerson J T, Hutton I, Watson J T.et al Influence of dobutamine on regional myocardial blood flow and ventricular performance during acute and chronic myocardial ischemia in dogs. Circulation 197653828–833. [DOI] [PubMed] [Google Scholar]
- 27.Skopicki H A, Abraham S A, Picard M H.et al Effects of dobutamine at maximally tolerated dose on myocardial blood flow in humans with ischemic heart disease. Circulation 1997963346–3352. [DOI] [PubMed] [Google Scholar]
- 28.Daher E, Dione D P, Heller E N.et al Acute ischemic dysfunction alters coronary flow reserve in remote nonischemic regions: potential mechanical etiology identified in an acute canine model. J Nucl Cardiol 20007112–122. [DOI] [PubMed] [Google Scholar]
- 29.Gobel F L, Norstrom L A, Nelson R R.et al The rate‐pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 197857549–556. [DOI] [PubMed] [Google Scholar]
- 30.Kim C, Kwok Y S, Heagerty P.et al Pharmacologic stress testing for coronary disease diagnosis: a meta‐analysis. Am Heart J 2001142934–944. [DOI] [PubMed] [Google Scholar]
- 31.Uren N G, Camici P G, Melin J A.et al Effect of aging on myocardial perfusion reserve. J Nucl Med 1995362032–2036. [PubMed] [Google Scholar]
- 32.Czernin J, Muller P, Chan S.et al Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 19938862–69. [DOI] [PubMed] [Google Scholar]
- 33.Chareonthaitawee P, Kaufmann P A, Rimoldi O.et al Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res 200150151–161. [DOI] [PubMed] [Google Scholar]
- 34.Chan S Y, Brunken R C, Czernin J.et al Comparison of maximal myocardial blood flow during adenosine infusion with that of intravenous dipyridamole in normal men. J Am Coll Cardiol 199220979–985. [DOI] [PubMed] [Google Scholar]