Abstract
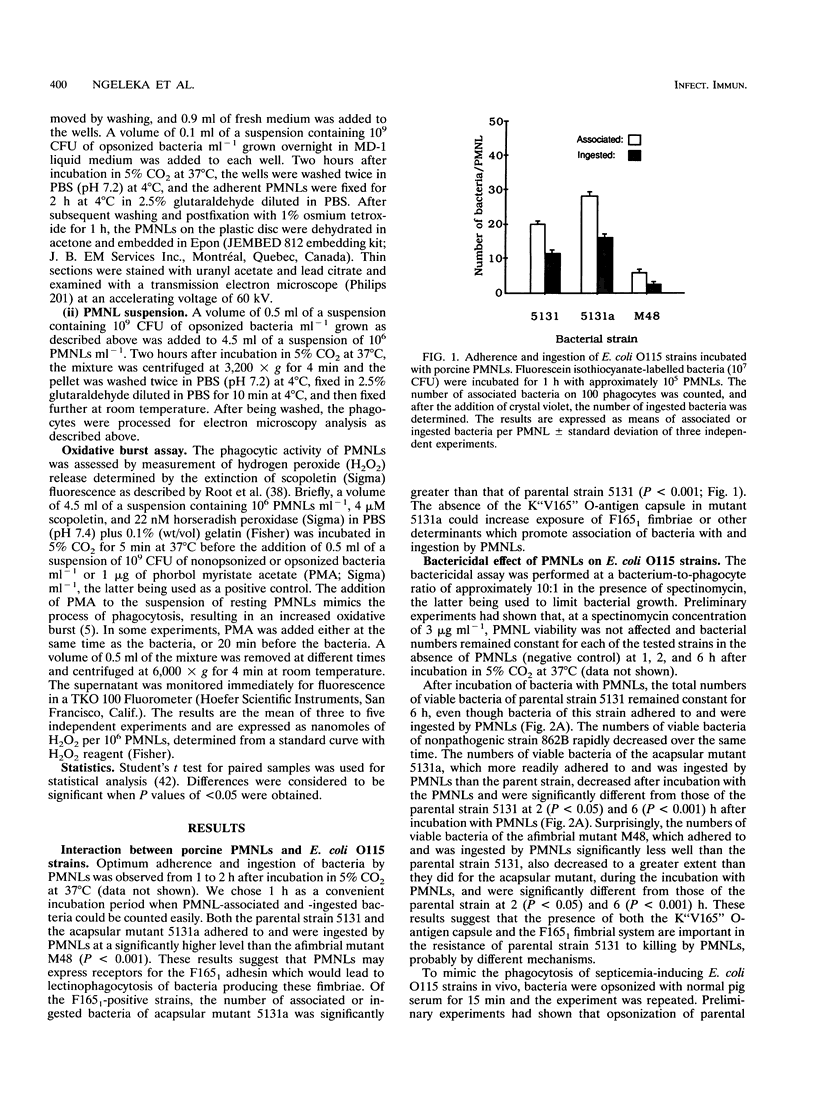
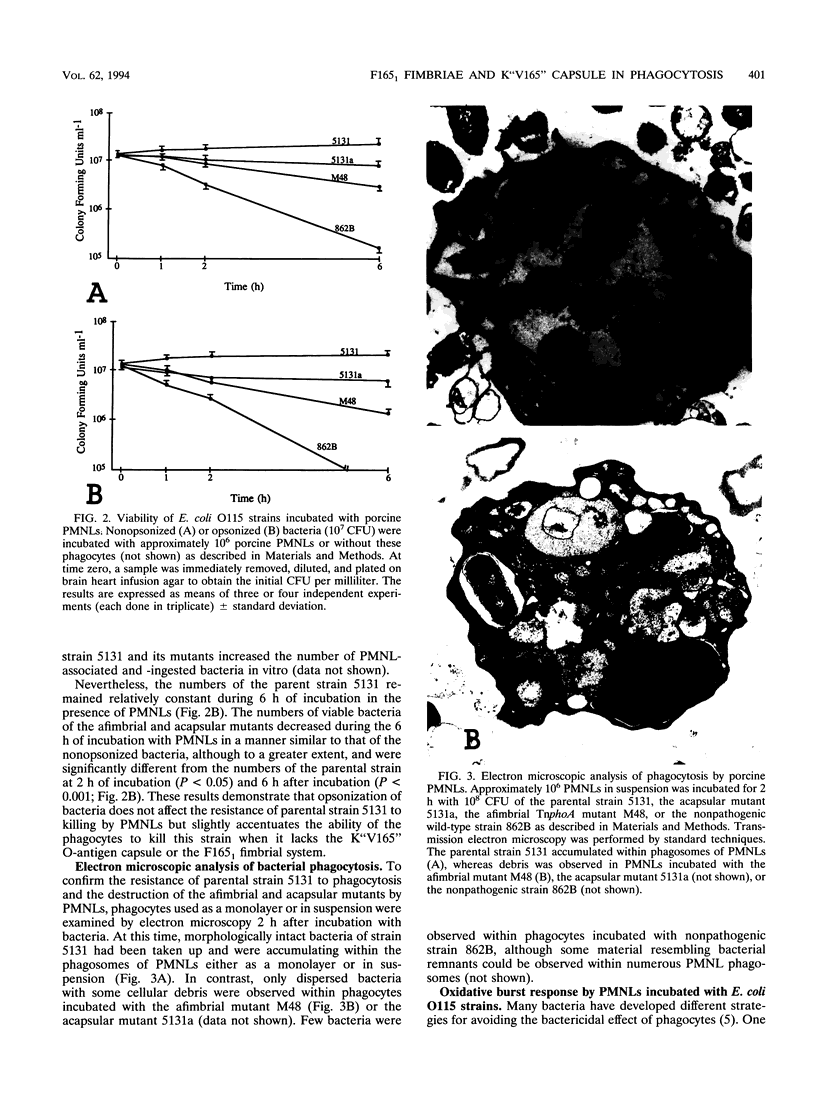
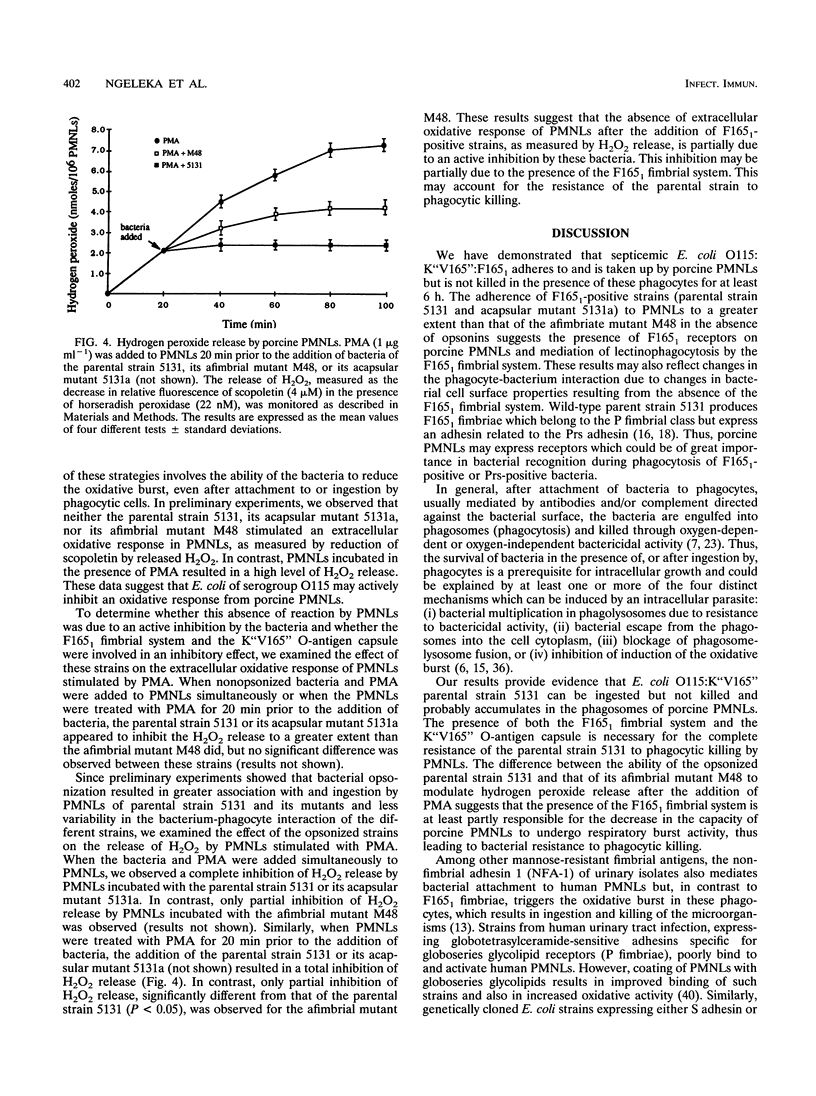
Escherichia coli O115:K"V165":F165(1) wild-type strain 5131 survives in the bloodstream of experimentally inoculated gnotobiotic pigs and induces septicemia, whereas its afimbriate (F165(1)-negative) TnphoA mutant M48 and its acapsular (K"V165"-negative) spontaneous mutant 5131a are both nonpathogenic. We evaluated the role of the mannose-resistant F165(1) fimbrial system and of the O-antigen K"V165" capsule in resistance to phagocytosis by porcine polymorphonuclear leucocytes (PMNLs) in vitro. F165(1)-positive strains (5131 and 5131a) attached to and were ingested by PMNLs at a significantly higher level than afimbrial mutant M48 (P < 0.001) after 1 h of incubation. During incubation of these strains with PMNLs for up to 6 h, parental strain 5131 resisted killing whereas afimbriate mutant M48 and acapsular mutant 5131a were gradually killed and were found at significantly lower numbers than the parental strain 5131 at 2 (P < 0.05) and 6 (P < 0.001) h. When bacteria were opsonized with normal pig serum, the afimbriate and acapsular mutants survived less well than when the bacteria were nonopsonized. Upon examination by electron microscopy of PMNLs after 2 h of incubation with bacteria, structurally normal bacteria were observed more often within phagosomes of PMNLs incubated with the parental strain than within phagosomes of PMNLs incubated with the afimbriate or the acapsular mutant. The extracellular oxidative response (as tested by release of hydrogen peroxide) of PMNLs stimulated by phorbol myristate acetate was completely inhibited by F165(1)-positive strains but only partially inhibited by the afimbriate mutant. These results suggest that the F165(1) fimbrial system may mediate adherence of E. coli O115 to PMNLs. Survival of the parental strain in the presence of PMNLs, which may be intracellular, is at least partially due to the presence of the F165(1) fimbrial system and of the O-antigen capsule K"V165". Furthermore, the presence of the F165(1) fimbrial system may contribute to the bacterial inhibition of the oxidative response of porcine PMNLs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkstén B., Wadström T. Interaction of Escherichia coli with different fimbriae and polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):298–305. doi: 10.1128/iai.38.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Cross A. S. The biologic significance of bacterial encapsulation. Curr Top Microbiol Immunol. 1990;150:87–95. doi: 10.1007/978-3-642-74694-9_5. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. Oxygen-dependent and oxygen-independent mechanisms of microbicidal activity of neutrophils. Immunol Lett. 1985;11(3-4):159–163. doi: 10.1016/0165-2478(85)90163-4. [DOI] [PubMed] [Google Scholar]
- Fairbrother J. M., Broes A., Jacques M., Larivière S. Pathogenicity of Escherichia coli O115:K"V165" strains isolated from pigs with diarrhea. Am J Vet Res. 1989 Jul;50(7):1029–1036. [PubMed] [Google Scholar]
- Fairbrother J., Harel J., Forget C., Desautels C., Moore J. Receptor binding specificity and pathogenicity of Escherichia coli F165-positive strains isolated from piglets and calves and possessing pap related sequences. Can J Vet Res. 1993 Jan;57(1):53–55. [PMC free article] [PubMed] [Google Scholar]
- Goetz M. B., Kuriyama S. M., Silverblatt F. J. Phagolysosome formation by polymorphonuclear neutrophilic leukocytes after ingestion of Escherichia coli that express type 1 pili. J Infect Dis. 1987 Jul;156(1):229–233. doi: 10.1093/infdis/156.1.229. [DOI] [PubMed] [Google Scholar]
- Goetz M. B., Silverblatt F. J. Stimulation of human polymorphonuclear leukocyte oxidative metabolism by type 1 pili from Escherichia coli. Infect Immun. 1987 Mar;55(3):534–540. doi: 10.1128/iai.55.3.534-540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhar J., Yavzori M., Keisari Y., Ofek I. Phagocytosis of Escherichia coli mediated by mannose resistant non-fimbrial haemagglutinin (NFA-1). Microb Pathog. 1991 Sep;11(3):171–178. doi: 10.1016/0882-4010(91)90047-e. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Harel J., Daigle F., Maiti S., Désautels C., Labigne A., Fairbrother J. M. Occurrence of pap-, sfa-, and afa-related sequences among F165-positive Escherichia coli from diseased animals. FEMS Microbiol Lett. 1991 Aug 1;66(2):177–182. doi: 10.1016/0378-1097(91)90329-9. [DOI] [PubMed] [Google Scholar]
- Harel J., Forget C., Ngeleka M., Jacques M., Fairbrother J. M. Isolation and characterization of adhesin-defective TnphoA mutants of septicaemic porcine Escherichia coli of serotype O115:K-:F165. J Gen Microbiol. 1992 Nov;138(11):2337–2345. doi: 10.1099/00221287-138-11-2337. [DOI] [PubMed] [Google Scholar]
- Harel J., Forget C., Saint-Amand J., Daigle F., Dubreuil D., Jacques M., Fairbrother J. Molecular cloning of a determinant coding for fimbrial antigen F165(1), a Prs-like fimbrial antigen from porcine septicaemic Escherichia coli. J Gen Microbiol. 1992 Jul;138(7):1495–1502. doi: 10.1099/00221287-138-7-1495. [DOI] [PubMed] [Google Scholar]
- Hurst N. P. Molecular basis of activation and regulation of the phagocyte respiratory burst. Ann Rheum Dis. 1987 Apr;46(4):265–272. doi: 10.1136/ard.46.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B. R., Harris S. L., Russell P. W., Orndorff P. E. Effect of type 1 piliation on in vitro killing of Escherichia coli by mouse peritoneal macrophages. Infect Immun. 1990 Oct;58(10):3448–3454. doi: 10.1128/iai.58.10.3448-3454.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Virkola R., Westurlund B., Holthöfer H., Parkkinen J. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr Top Microbiol Immunol. 1990;151:115–127. doi: 10.1007/978-3-642-74703-8_6. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Oxygen-independent bactericidal systems. Mechanisms and disorders. Hematol Oncol Clin North Am. 1988 Mar;2(1):159–169. [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Participation of immunoglobulins and complement components in the intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Infect Immun. 1981 Sep;33(3):714–724. doi: 10.1128/iai.33.3.714-724.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Pai C. H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985 Jul;49(1):145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt R., Baker N., Falk P., Hull R., Hull S., Karr J., Leffler H., Svanborg Edén C., Larson G. Binding specificities of wild-type and cloned Escherichia coli strains that recognize globo-A. Infect Immun. 1989 Nov;57(11):3389–3394. doi: 10.1128/iai.57.11.3389-3394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt R., Larson G., Falk P., Jodal U., Leffler H., Svanborg C. The receptor repertoire defines the host range for attaching Escherichia coli strains that recognize globo-A. Infect Immun. 1991 Mar;59(3):1086–1092. doi: 10.1128/iai.59.3.1086-1092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R., Dahlgren C., Lindén M., Stendahl O., Svensbergh A., Ohman L. Neutrophil killing of two type 1 fimbria-bearing Escherichia coli strains: dependence on respiratory burst activation. Infect Immun. 1990 Jan;58(1):37–42. doi: 10.1128/iai.58.1.37-42.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Kroll J. S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- Ngeleka M., Harel J., Jacques M., Fairbrother J. M. Characterization of a polysaccharide capsular antigen of septicemic Escherichia coli O115:K "V165" :F165 and evaluation of its role in pathogenicity. Infect Immun. 1992 Dec;60(12):5048–5056. doi: 10.1128/iai.60.12.5048-5056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngeleka M., Jacques M., Martineau-Doizé B., Daigle F., Harel J., Fairbrother J. M. Pathogenicity of an Escherichia coli O115:K"V165" mutant negative for F165(1) fimbriae in septicemia of gnotobiotic pigs. Infect Immun. 1993 Mar;61(3):836–843. doi: 10.1128/iai.61.3.836-843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988 Mar;56(3):539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G. Perturbation of the normal mechanisms of intraleukocytic killing of bacteria. J Infect Dis. 1983 Aug;148(2):189–193. doi: 10.1093/infdis/148.2.189. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Bjursten L. M., Hull R., Hull S., Magnusson K. E., Moldovano Z., Leffler H. Influence of adhesins on the interaction of Escherichia coli with human phagocytes. Infect Immun. 1984 Jun;44(3):672–680. doi: 10.1128/iai.44.3.672-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventur Y., Scheffer J., Hacker J., Goebel W., König W. Effects of adhesins from mannose-resistant Escherichia coli on mediator release from human lymphocytes, monocytes, and basophils and from polymorphonuclear granulocytes. Infect Immun. 1990 Jun;58(6):1500–1508. doi: 10.1128/iai.58.6.1500-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]