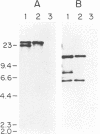
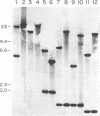
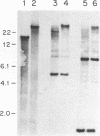
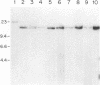
Abstract
Mucoid or highly encapsulated strains of group A streptococci have been associated both with unusually severe infections and with acute rheumatic fever. Previously, we described an acapsular mutant, TX4, derived from a mucoid M-type 18 strain of a group A streptococcus by transposon mutagenesis (M. R. Wessels, A. E. Moses, J. B. Goldberg, and T. J. DiCesare, Proc. Natl. Acad. Sci. USA 88:8317-8321, 1991). We now report studies further characterizing strain TX4 as well as an additional acapsular mutant, TX72. Strain TX4 was found to contain a 9.5-kb deletion of chromosomal DNA adjacent to the site of transposon Tn916 insertion. Cloned chromosomal DNA from TX4 flanking the transposon insertion site was used as a probe to demonstrate the presence of homologous regions in 11 of 11 wild-type group A streptococcal strains of various M protein types. A second acapsular mutant, TX72, had a single transposon insertion and had no apparent deletion of chromosomal DNA. The Tn916 insertion in TX72 was mapped to the hasA locus (encoding hyaluronate synthase), which lies within the chromosomal region deleted in TX4. Strain TX72 was avirulent in mice and sensitive to phagocytic killing in vitro. Transduction of either the insertion-deletion mutation from TX4 or the simple insertion mutation from TX72 to a type 24 group A streptococcus strain also resulted in loss of capsule expression, demonstrating that a homologous region of the chromosome controls capsule expression in another serotype of group A streptococci. We conclude that the hyaluronic acid capsule plays an important role in virulence and that a region of the chromosome essential for capsular polysaccharide expression is conserved among diverse group A streptococcal strains.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell. 1989 Dec 22;59(6):1027–1034. doi: 10.1016/0092-8674(89)90759-9. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Genetic manipulation of pathogenic streptococci. Methods Enzymol. 1991;204:556–586. doi: 10.1016/0076-6879(91)04028-m. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Flannagan S. E., Ike Y., Jones J. M., Gawron-Burke C. Sequence analysis of termini of conjugative transposon Tn916. J Bacteriol. 1988 Jul;170(7):3046–3052. doi: 10.1128/jb.170.7.3046-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis P. L., Papaconstantinou J., Weigel P. H. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. J Biol Chem. 1993 Jul 15;268(20):14568–14571. [PubMed] [Google Scholar]
- Dougherty B. A., van de Rijn I. Molecular characterization of a locus required for hyaluronic acid capsule production in group A streptococci. J Exp Med. 1992 May 1;175(5):1291–1299. doi: 10.1084/jem.175.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty B. A., van de Rijn I. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose dehydrogenase activity. J Biol Chem. 1993 Apr 5;268(10):7118–7124. [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. R., Stevens D. L., Kaplan E. L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992 Aug;166(2):374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Zamze S., Loynds B., Moxon E. R. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989 Jun;171(6):3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J. M., Heggen L. M., Rubens C. E. Molecular analysis of a region of the group B streptococcus chromosome involved in type III capsule expression. Infect Immun. 1989 Oct;57(10):3058–3065. doi: 10.1128/iai.57.10.3058-3065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J Exp Med. 1957 Oct 1;106(4):525–544. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. P., Cleary P. P. In vivo Streptococcus pyogenes C5a peptidase activity: analysis using transposon- and nitrosoguanidine-induced mutants. J Infect Dis. 1987 Sep;156(3):495–504. doi: 10.1093/infdis/156.3.495. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Perez-Casal J., Caparon M. G., Scott J. R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991 Apr;173(8):2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L., Tanner M. H., Winship J., Swarts R., Ries K. M., Schlievert P. M., Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989 Jul 6;321(1):1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- Vimr E. R., Aaronson W., Silver R. P. Genetic analysis of chromosomal mutations in the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1989 Feb;171(2):1106–1117. doi: 10.1128/jb.171.2.1106-1117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. T. The relative importance of the capsule and the M-antigen in determining colony form of group A streptococci. J Exp Med. 1959 Mar 1;109(3):257–270. doi: 10.1084/jem.109.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald E. R., Dashefsky B., Feidt C., Chiponis D., Byers C. Acute rheumatic fever in western Pennsylvania and the tristate area. Pediatrics. 1987 Sep;80(3):371–374. [PubMed] [Google Scholar]
- Wessels M. R., Moses A. E., Goldberg J. B., DiCesare T. J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake R. M., Graham T. P., Edwards K. M. An outbreak of acute rheumatic fever in Tennessee. Pediatr Infect Dis J. 1990 Feb;9(2):97–100. doi: 10.1097/00006454-199002000-00007. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]