Abstract
Objective
To examine the long‐term cardiovascular consequences of angina in a large epidemiological study.
Design
Prospective cohort study conducted between 1972 and 1976 with 20 years of follow‐up (the Renfrew–Paisley Study).
Setting
Renfrew and Paisley, West Scotland, UK.
Participants
7048 men and 8354 women aged 45–64 years who underwent comprehensive cardiovascular screening at baseline, including the Rose Angina Questionnaire and electrocardiography (ECG).
Main outcome measures
All deaths and hospitalisations for cardiovascular reasons occurring over the subsequent 20 years, according to the baseline Rose angina score and baseline ECG.
Results
At baseline, 669 (9.5%) men and 799 (9.6%) women had angina on Rose Angina Questionnaire. All‐cause mortality for those with Rose angina was 67.7% in men and 43.3% in women at 20 years compared with 45.4% and 30.4%, respectively, in those without angina (p<0.001). Values are expressed as hazards ratio (HR) (95% confidence interval (CI). In a multivariate analysis, men with Rose angina had an increased risk of cardiovascular death or hospitalisation (1.49 (1.33 to 1.66), myocardial infarction (1.63 (1.41 to 1.85)) or heart failure (1.54 (1.13 to 2.10)) compared with men without angina. The corresponding HR (95% CI) for women were 1.38 (1.23 to 1.55), 1.56 (1.31 to 1.85) and 1.92 (1.44 to 2.56). An abnormality on the electrocardiogram (ECG) increased risk further, and both angina and an abnormality on the ECG increased risk most of all compared with those with neither angina nor ischaemic changes on the ECG. Compared with men, women with Rose angina were less likely to have a cardiovascular event (0.54 (0.46 to 0.64)) or myocardial infarction (0.44 (0.35 to 0.56)), although there was no sex difference in the risk of stroke (1.11 (0.75 to 1.65)), atrial fibrillation (0.84 (0.38 to 1.87)) or heart failure (0.79 (0.51 to 1.21)).
Conclusions
Angina in middle age substantially increases the risk of death, myocardial infarction, heart failure and other cardiovascular events.
Although the incidence of myocardial infarction is decreasing, the incidence of angina pectoris is increasing.1 Despite angina being the most common manifestation of coronary heart disease and the most common symptomatic cardiac condition, relatively little is known about the long‐term natural history of angina at a population level, especially in women.2,3,4,5 Most existing population‐based studies have mainly or exclusively reported outcomes in men.6,7,8,9 Only a few described prognosis in women, but have not usually compared it with men in the same population.10,11 Most existing studies that have reported clinical outcomes have focused on death, myocardial infarction or both.2,3,4,6,7,8 Patients with angina are likely to be at increased risk of other complications of coronary heart disease (eg, heart failure) or non‐cardiac vascular events (eg, stroke).9 Similarly, the true lifetime burden of angina is reflected not only in fatal but also in non‐fatal events, especially those leading to hospital admission. Consequently, the aim of this study was to examine the long‐term population risk of fatal and non‐fatal cardiovascular events in both men and women with angina, during 20‐year follow‐up of 15 402 initially middle‐aged people (8354 women) who were first screened between 1972 and 1976.12,13,14
Methods
Sample and baseline data
Details of the Renfrew–Paisley study have been published previously.15 In brief, between 1972 and 1976, 7048 men and 8354 women aged 45–64 years, living in the industrialised towns of Renfrew and Paisley, western Scotland, UK, participated in this epidemiological study.12 These people represented 80% of the target population residing in these two towns. Each person's demographic profile and cardiorespiratory health status were documented.12
Rose angina criteria
The standard Rose angina classification was used.16 The validity of the Rose Angina Questionnaire has been tested in studies comparing it to a clinical diagnosis of angina, abnormality on the ECG, thallium scanning and as a predictor of mortality due to coronary artery disease.17,18,19,20,21 In this classification, grade I angina is defined as pain or discomfort when walking uphill or hurrying. Angina is classified as grade II when the person reports chest pain or discomfort also when walking at an ordinary pace on level ground. Angina is further classified as “definite” if, in addition, the pain is sited in the sternum or the left chest and arm, causes the person to stop or slow down, and resolves within 10 min of the person stopping or slowing down. If these additional criteria are not satisfied, angina is classified as “possible”. For the purpose of this study, angina was defined as Rose grade I and II “definite” angina and was not confirmed by further investigation or evaluation. Possible myocardial infarction (identified by a separate question on the Rose Questionnaire, as having ever experienced a severe pain across the front of the chest, lasting for ⩾30 min) was noted.
Criteria for ischaemic changes on the ECG
A six‐lead ECG was also obtained and coded.12 The following Minnesota codes were considered to represent ECG evidence of ischaemia: 1.1–1.3, Q waves; 4.1–4.3, ST junction and segment depression; 5.1–5.3, T wave abnormalities; and 7.1, left bundle branch block.22
Study follow‐up
Electronic linkage to hospital and death records is possible for all residents of Scotland, as previously reported.13,14,15 We followed up all people until date of death or censorship. Date of censorship was 20 years from the date of each person's initial screening visit. The Scottish Morbidity Record Scheme was used to retrieve details of all discharges from hospital (according to the eighth (a small number of initial episodes) and ninth revisions of the World Health Organization International classification of diseases (ICD9)) during the 20 years after initial screening.23 We noted the occurrence and timing of admissions for acute myocardial infarction (410), coronary heart disease (410–414), heart failure (425.4, 425.5, 428 and 402), thromboembolic disease (415 and 451.1), aortic aneurysm (441), atrial fibrillation (427.3) and cerebrovascular incidents (430–438). These diagnoses, in combination with several other less commonly recorded diagnoses, are collectively called cardiovascular hospitalisations. Audits have shown that these data are about 90% accurate in identifying the correct discharge diagnosis.24 Deaths and their certified cause were obtained from the National Health Service Central Register for the same period. We excluded 17 (0.1%) patients who emigrated, as their vital status was unknown.
Statistical analysis
Data on men and women were analysed separately and compared. As an abnormality on the ECG is recognised to be of prognostic importance, people with ischaemic changes on ECGs were analysed separately from those without ischaemic changes on ECGs, and the groups were compared.25 Categorical variables were compared using the χ2 test and continuous variables using Student's t test. We used Cox's proportional hazards regression models to examine the relationship between Rose‐positive angina (with and without ischaemic changes on an ECG) and outcome. We adjusted for age, blood pressure, cholesterol, history of diabetes, number of cigarettes smoked per day (with an additional 0/1 variable for ex‐smoker), Rose myocardial infarction, adjusted forced expiratory volume in one second and social class. All data were analysed using SPSS V.11.5.
Results
Prevalence of angina
At baseline screening, 669 (9.5%) men and 799 (9.6%) women had Rose‐positive angina. Of these, 22.7% of men and 14.8% of women had an ischaemic change on the ECG. Conversely, 697 (9.9%) men and 712 (8.5%) women had an ischaemic change on the ECG. Of these, 21.8% of men and 16.6% of women had angina.
Baseline characteristics according to the presence of angina at baseline
Compared with people with no angina and no ischaemic changes on the ECG, those with angina or ischaemic changes on the ECG were older and more likely to have cardiovascular risk factors or prior cardiovascular disease (these findings were generally more marked for people with an abnormality on the ECG; table 1). This gradient was also seen for pulmonary disease.
Table 1 Baseline characteristics of the Renfrew–Paisley cohort, according to the presence of angina and ischaemic changes on the electrocardiogram at baseline.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| No angina, no ischaemic changes on ECG (n = 5834) | No angina, ischaemic changes on ECG (n = 545) | Angina, no ischaemic changes on ECG (n = 517) | Angina, ischaemic changes on ECG (n = 152) | No angina, no ischaemic changes on ECG (n = 6961) | No angina, ischaemic changes on ECG (n = 594) | Angina, no ischaemic changes on ECG (n = 681) | Angina, ischaemic changes on ECG (n = 118) | |
| Age (years)† | 54 (6) | 56 (6)*** | 56 (5)*** | 57 (5)*** | 54 (6) | 56 (5)*** | 55 (6)*** | 58 (5)*** |
| History of stroke‡ | 55 (0.9) | 19 (3.5)*** | 13 (2.5)** | 6 (4.0)** | 53 (0.8) | 30 (5.1)*** | 12 (1.8)** | 9 (7.6)*** |
| Rose myocardial infarction‡ | 380 (6.5) | 98 (18.0)*** | 136 (26.3)*** | 74 (48.7)*** | 296 (4.3) | 58 (9.8)*** | 121 (17.8)*** | 29 (24.6)*** |
| Current or ex‐smoker‡ | 4811 (82.5) | 462 (84.8) | 463 (89.6)*** | 131 (86.2) | 3786 (54.4) | 284 (47.8)** | 387 (56.8) | 69 (58.5) |
| Diabetes‡ | 67 (1.1) | 10 (1.6) | 9 (1.7) | 4 (2.6) | 70 (1.0) | 17 (2.9)*** | 8 (1.2) | 5 (4.2)** |
| Blood glucose (mmol/l)† | 5.1 (1.6) | 5.5 (2.1)*** | 5.1 (1.6) | 5.2 (1.3) | 5.0 (1.3) | 5.4 (2.2)** | 5.1 (1.3) | 5.6 (2.3)* |
| Plasma cholesterol (mmol/l)† | 5.9 (1.0) | 5.9 (1.0)* | 5.9 (1.0) | 6.0 (1.0)* | 6.4 (1.1) | 6.5 (1.1) | 6.4 (1.1) | 6.6 (1.2)* |
| Body mass index (kg/m2)† | 25.8 (3.4) | 26.5 (3.4)*** | 25.9 (3.6) | 26.3 (3.6) | 25.6 (4.4) | 26.7 (4.9)*** | 26.9 (4.9)*** | 27.0 (5.5)** |
| Systolic BP (mm Hg)† | 147 (22) | 159 (26)*** | 150 (24)* | 154 (±25)** | 149 (24) | 163 (29)*** | 153 (27)*** | 165 (31)*** |
| Diastolic BP (mm Hg)† | 85 (13) | 91 (15)*** | 86 (14) | 89 (16)** | 84 (13) | 90 (15)*** | 87 (14)*** | 92 (17)*** |
| Cardiothoracic ratio† | 0.46 (0.05) | 0.49 (0.05)*** | 0.47 (0.05)* | 0.50 (0.05)*** | 0.48 (0.05) | 0.50 (0.06)*** | 0.49 (0.05)*** | 0.52 (0.06)*** |
| Adjusted FEV1 (%)† | 90.2 (21.6) | 85.4 (21.8)*** | 78.7 (25.7)*** | 79.9 (25.1)*** | 93.7 (22.7) | 88.8 (23.6)*** | 84.8 (25.6)*** | 83.5 (26.2)*** |
| Chronic bronchitis‡ | 247 (4.2) | 38 (7.0)** | 100 (19.3)*** | 27 (17.8)*** | 207 (3.0) | 32 (5.4)** | 96 (14.1)*** | 9 (7.6)* |
| Atrial fibrillation on ECG‡ | 35 (0.6) | 10 (1.8)** | 5 (1.0) | 3 (2.0) | 20 (0.3) | 14 (2.4)*** | 6 (0.9)* | 7 (5.9)*** |
BP, blood pressure; ECG, electrocardiogram; FEV1, forced expiratory volume in one second.
*p<0.05; **p<0.01; ***p<0.001 for each group compared with the group having no angina or no ischaemic changes on ECG.
†Values are mean (SD).
‡Values are n (%).
Compared with women, men with angina were significantly more likely to be current or ex‐smokers (p<0.001), and to have a lower forced expiratory volume in one second (p<0.001) and more chronic bronchitis (p = 0.002). Men were also more likely to have a history of Rose‐myocardial infarction (p<0.001). Conversely, women had a higher total cholesterol (p<0.001), systolic blood pressure (p = 0.005) and cardiothoracic ratio (p<0.001).
Survival over 20 years follow‐up
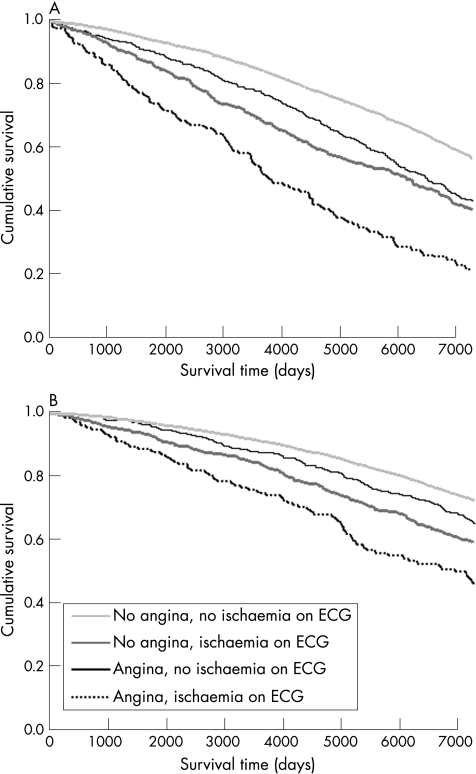
A larger proportion of men than women died over the 20‐year follow‐up period, irrespective of whether or not they had angina (fig 1; table 2). Men and women with angina were more likely to die than those without angina. In all, 67.7% of men with angina died over the 20 years of follow‐up compared with 45.4% of those without angina (p<0.001). The corresponding figures for women were 43.3% and 30.4% (p<0.001). Both angina and ischaemic changes on ECG were associated with increased risk. An ischaemic change on ECG was associated with a greater risk than that seen with angina alone (ie, when there were no ischaemic changes on ECG).
Figure 1 Age‐adjusted Cox's survival curves for all‐cause death, according to the presence of angina or ischaemic changes on an electrocardiogram (ECG) in (A) men and (B) women.
Table 2 Cause of death over 20 years according to the presence of angina and ischaemic change on electrocardiogram at baseline.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| No angina, no ischaemic changes on ECG (n = 5834) | No angina, ischaemic changes on ECG (n = 545) | Angina, no ischaemic changes on ECG (n = 517) | Angina, ischaemic changes on ECG (n = 152) | No angina, no ischaemic changes on ECG (n = 6961) | No angina, ischaemic changes on ECG (n = 594) | Angina, no ischaemic changes on ECG (n = 681) | Angina, ischaemic changes on ECG (n = 118) | |
| Any cause | 2537 (43.5) | 358 (65.7)*** | 325 (62.9)*** | 128 (84.2)*** | 2012 (28.9) | 285 (48.0)*** | 268 (39.4)*** | 78 (66.1)*** |
| 1.00 | 1.53 (1.37 to 1.71) | 1.18 (1.04 to 1.33) | 2.47 (2.06 to 2.97) | 1.00 | 1.48 (1.30 to 1.68) | 1.15 (1.01 to 1.31) | 1.99 (1.58 to 2.51) | |
| Any cardiovascular cause | 1249 (21.4) | 246 (45.1)*** | 179 (34.6)*** | 96 (63.2)*** | 916 (13.2) | 190 (32.0)*** | 141 (20.7)*** | 58 (49.2)*** |
| 1.00 | 2.02 (1.75 to 2.32) | 1.35 (1.14 to 1.59) | 3.50 (2.81 to 4.34) | 1.00 | 1.90 (1.61 to 2.23) | 1.25 (1.04 to 1.50) | 2.71 (2.06 to 3.58) | |
| Coronary heart disease | 889 (15.2) | 170 (31.2)*** | 145 (28.0)*** | 77 (50.7)*** | 543 (7.8) | 104 (17.5)*** | 83 (12.2)*** | 38 (32.2)*** |
| 1.00 | 1.97 (1.67 to 2.34) | 1.54 (1.28 to 1.84) | 3.82 (2.99 to 4.88) | 1.00 | 1.78 (1.43 to 2.21) | 1.23 (0.97 to 1.57) | 2.92 (2.07 to 4.18) | |
| Myocardial infarction | 730 (12.5) | 143 (26.2)*** | 112 (21.7)*** | 65 (42.8)*** | 462 (6.6) | 82 (13.8)*** | 73 (10.7)*** | 33 (28.0)*** |
| 1.00 | 1.99 (1.65 to 2.39) | 1.44 (1.17 to 1.77) | 3.84 (2.94 to 5.02) | 1.00 | 1.64 (1.29 to 2.10) | 1.28 (0.99 to 1.65) | 2.98 (2.05 to 4.32) | |
| Heart failure | 34 (0.6) | 7 (1.3) | 5 (1.0) | 2 (1.3) | 26 (0.4) | 10 (1.7)*** | 6 (0.9) | 3 (2.5)* |
| 1.00 | 2.56 (1.11 to 5.91) | 1.67 (0.63 to 4.42) | 3.72 (0.86 to 16.13) | 1.00 | 3.55 (1.66 to 7.58) | 1.82 (0.73 to 4.52) | 4.68 (1.32 to 16.52) | |
| Atrial fibrillation | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) | 3 (0.4)** | 0 (0) |
| 1.00 | — | — | — | 1.00 | — | — | — | |
| Stroke | 232 (4.0) | 48 (8.8)*** | 14 (2.5) | 8 (5.3) | 277 (4.0) | 58 (9.8)*** | 38 (5.6)* | 14 (11.9)*** |
| 1.00 | 1.99 (1.45 to 2.75) | 0.56 (0.32 to 0.97) | 1.60 (0.78 to 3.29) | 1.00 | 1.86 (1.39 to 2.50) | 1.17 (0.83 to 1.65) | 2.25 (1.29 to 3.92) | |
| Aortic aneurysm | 26 (0.4) | 5 (0.9) | 2 (0.4) | 1 (0.7) | 7 (0.1) | 0 (0) | 1 (0.1) | 0 (0) |
| 1.00 | 1.89 (0.71 to 5.04) | 0.69 (0.16 to 3.02) | 2.12 (0.27 to 16.40) | 1.00 | — | 1.19 (0.14 to 10.03) | — | |
| Venous thromboembolism | 17 (0.3) | 2 (0.4) | 2 (0.4) | 1 (0.7) | 12 (0.2) | 4 (0.7)* | 1 (0.1) | 0 (0) |
| 1.00 | 1.47 (0.33 to 6.51) | 1.34 (0.30 to 6.06) | 3.51 (0.43 to 28.88) | 1.00 | 2.49 (0.76 to 8.19) | — | — | |
ECG, electrocardiogram.
Values are n (%) or adjusted HR (95% CI).
*p<0.05; **p<0.01; ***p<0.001 for the proportion of deaths in each group compared with the group with no angina and no ischaemic changes on ECG.
Patients with angina and an abnormality on the ECG had the highest risk for death. Angina was associated with an increase in the adjusted risk for death from all causes, cardiovascular causes and, especially, coronary causes. The adjusted hazard ratio (HR; 95% (CI)) confidence interval (CI) for death from any cause was 1.30 (1.17 to 1.44) in men and 1.20 (1.07 to 1.35) in women (compared with those without angina), whereas the HR for death from a coronary cause was 1.71 (1.47 to 2.00) in men and 1.36 (1.11 to 1.67) in women.
Both angina and an ischaemic change on ECG were separately associated with increased risk, although the risk associated with an abnormality on the ECG was greater. Men with angina but no ECG changes had a 54% increase in the adjusted RR for dying from coronary heart disease (compared with people with neither angina nor ECG changes), whereas women had a 23% increased risk. The corresponding increase in people with an abnormality on the ECG but no angina was 97% in men and 78% in women.
The increase in RR was greatest in patients with angina and an abnormality on the ECG, where men had a nearly fourfold and women had a nearly threefold increased risk of death from coronary heart disease compared with that in people without angina or an ischaemic change on ECG.
Hospitalisation from cardiovascular causes
Men and women with angina were more likely than those without angina to experience a cardiovascular hospitalisation (table 3). In all, 43.5% of men with angina experienced such an event over the 20 years of follow‐up compared with 32.7% of those without angina (p<0.001). The corresponding figures for women were 37.4% and 24.6% (p<0.001). The adjusted RR for admission to hospital for a cardiovascular reason was increased to a similar extent as the risk for death from a cardiovascular cause. As with death, the increased risk of a major coronary event was even greater.
Table 3 Proportion of patients admitted to hospital for a cardiovascular cause (principal discharge diagnosis) over 20 years, according to the presence of angina and ischaemic change on electrocardiogram at baseline.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| No angina, no ischaemic changes on ECG (n = 5834) | No angina, ischaemic changes on ECG (n = 545) | Angina, no ischaemic changes on ECG (n = 517) | Angina, ischaemic changes on ECG (n = 152) | No angina, no ischaemic changes on ECG (n = 6961) | No angina, ischaemic changes on ECG (n = 594) | Angina, no ischaemic changes on ECG (n = 681) | Angina, ischaemic changes on ECG (n = 118) | |
| Any cardiovascular cause | 1859 (31.9) | 230 (42.2)*** | 219 (42.4)*** | 72 (47.4)*** | 1647 (23.7) | 211 (35.5)*** | 237 (34.8)*** | 62 (52.5)*** |
| 1.00 | 1.57 (1.37 to 1.81) | 1.40 (1.21 to 1.62) | 2.40 (1.88 to 3.07) | 1.00 | 1.45 (1.25 to 1.68) | 1.38 (1.20 to 1.59) | 2.54 (1.96 to 3.29) | |
| Coronary heart disease | 821 (14.1) | 113 (20.7)*** | 117 (22.6)*** | 37 (24.3)*** | 557 (8.0) | 83 (14.0)*** | 110 (16.2)*** | 24 (20.3)*** |
| 1.00 | 1.78 (1.46 to 2.18) | 1.72 (1.40 to 2.10) | 2.51 (1.77 to 3.54) | 1.00 | 1.65 (1.30 to 2.09) | 1.76 (1.42 to 2.18) | 2.13 (1.40 to 3.26) | |
| Myocardial infarction | 652 (11.2) | 79 (14.5)* | 86 (16.6)*** | 32 (21.1)*** | 393 (5.6) | 61 (10.3)*** | 78 (11.5)*** | 18 (15.4)*** |
| 1.00 | 1.50 (1.18 to 1.91) | 1.57 (1.24 to 1.98) | 2.79 (1.92 to 4.06) | 1.00 | 1.66 (1.67 to 2.19) | 1.78 (1.89 to 2.29) | 2.36 (1.45 to 3.85) | |
| Heart failure | 227 (3.9) | 40 (7.3)*** | 33 (6.4)** | 15 (9.9)*** | 177 (2.5) | 51 (8.6)*** | 47 (6.9)*** | 13 (11.0)*** |
| 1.00 | 2.01 (1.42 to 2.83) | 1.34 (0.92 to 1.96) | 3.48 (2.03 to 5.98) | 1.00 | 2.80 (2.03 to 3.87) | 2.11 (1.51 to 3.00) | 3.89 (2.18 to 6.94) | |
| Atrial fibrillation | 60 (1.0) | 7 (1.3) | 12 (2.3)** | 0 (0) | 65 (0.9) | 11 (1.9)* | 20 (2.9)*** | 1 (0.8) |
| 1.00 | 1.51 (0.68 to 3.34) | 2.24 (1.15 to 4.34) | — | 1.00 | 1.92 (0.99 to 3.69) | 2.92 (1.73 to 4.92) | 1.00 (0.14 to 7.33) | |
| Stroke | 371 (6.4) | 54 (9.9)** | 35 (6.8) | 12 (7.9) | 423 (6.1) | 59 (9.9)*** | 61 (9.0)** | 14 (11.9)* |
| 1.00 | 1.57 (1.17 to 2.10) | 0.93 (0.65 to 1.33) | 1.75 (0.97 to 3.14) | 1.00 | 1.27 (0.95 to 1.69) | 1.30 (0.98 to 1.71) | 1.87 (1.08 to 3.22) | |
| Aortic aneurysm | 54 (0.9) | 6 (1.1) | 4 (0.8) | 4 (2.6) | 13 (0.2) | 0 (0) | 1 (0.1) | 1 (0.8) |
| 1.00 | 1.33 (0.56 to 3.14) | 0.76 (0.27 to 2.17) | 4.67 (1.60 to 13.57) | 1.00 | — | 0.71 (0.09 to 5.47) | 5.45 (0.67 to 44.30) | |
| Venous thromboembolism | 102 (1.7) | 10 (1.8) | 9 (1.7) | 2 (1.3) | 72 (1.0) | 6 (1.0) | 10 (1.5) | 1 (0.8) |
| 1.00 | 1.33 (0.69 to 2.58) | 1.08 (0.54 to 2.18) | 1.40 (0.34 to 5.78) | 1.00 | 0.98 (0.42 to 2.27) | 1.12(0.56 to 2.21) | 0.76 (0.10 to 5.59) | |
ECG, electrocardiogram.
Values are n (%) or adjusted HR (95% CI).
*p<0.05; **p<0.01; ***p<0.001 for the proportion of hospitalisations in each group compared with the group with no angina and no ischaemic change on ECG.
As with death alone, the risk for hospital admission for a cardiovascular reason was increased by both angina and an ischaemic change on ECG, separately. Unlike death, the increase in risk was not substantially greater with an abnormality on the ECG than with angina. However, as with death, the greatest risk was seen in patients with angina and an abnormality on the ECG. In these patients, the relative risk of a cardiovascular hospitalisation was increased 2.4‐fold in men and 2.5‐fold in women (compared with people with neither angina nor an ischaemic change on ECG).
Angina, an ischaemic change on ECG and, especially, both together, were associated with an increased risk for hospitalisation due to heart failure and stroke (angina alone did not increase the risk for stroke in men).
Death or hospitalisation from cardiovascular causes
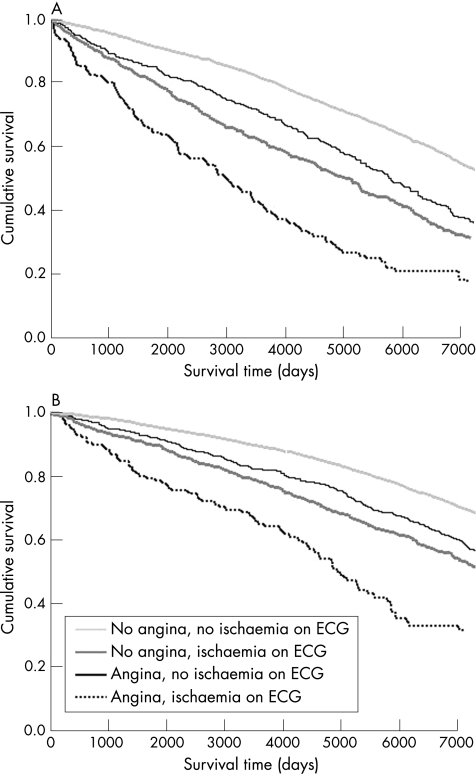
Patients with angina were more likely to experience a major non‐fatal or fatal cardiovascular event than those without angina (fig 2; table 4). In all, 61.6% of men with angina experienced such an event over the 20 years of follow‐up compared with 43.4% of those without angina (p<0.001). The corresponding figures for women were 45.9% and 30.9% (p<0.001). The adjusted RR for a major fatal or non‐fatal cardiovascular event was increased to the same extent as the risk for death from a cardiovascular cause. As with death, the increased risk of a major coronary event was even greater. Men with angina had an increased risk of cardiovascular death or hospitalisation (HR 1.49, 95% CI 1.33 to 1.66), myocardial infarction (HR 1.63, 95% CI 1.41 to 1.89) and heart failure (HR 1.54, 95% CI 1.13 to 2.10) compared with men without angina. The corresponding HRs (95% CI) for women were 1.38 (1.23 to 1.55), 1.56 (1.31 to 1.85) and 1.92 (1.44 to 2.56).
Figure 2 Age‐adjusted Cox's survival curves for cardiovascular death or hospital admission, according to the presence of angina or ischaemic changes on electrocardiogram (ECG) in (A) men and (B) women.
Table 4 Cardiovascular events (death or hospital admission) over 20 years, according to the presence of angina and ischaemic changes on electrocardiogram at baseline.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| No angina, no ischaemic changes on ECG (n = 5834) | No angina, ischaemic changes on ECG (n = 545) | Angina, no ischaemic changes on ECG (n = 517) | Angina, ischaemic changes on ECG (n = 152) | No angina, no ischaemic changes on ECG (n = 6961) | No angina, ischaemic changes on ECG (n = 594) | Angina, no ischaemic changes on ECG (n = 681) | Angina, ischaemic changes on ECG (n = 118) | |
| Any cardiovascular cause | 2421 (41.5) | 345 (63.3)*** | 294 (56.9)*** | 118 (77.6)*** | 2046 (29.4) | 292 (49.2)*** | 285 (41.9)*** | 82 (69.5)*** |
| 1.00 | 1.79 (1.55 to 1.95) | 1.37 (1.21 to 1.56) | 2.88 (2.34 to 3.49) | 1.00 | 1.57 (1.38 to 1.78) | 1.30 (1.14 to 1.48) | 2.56 (2.04 to 3.21) | |
| Coronary heart disease | 1392 (23.9) | 224 (41.1)*** | 205 (39.7)*** | 91 (59.9)*** | 913 (13.1) | 155 (26.1)*** | 158 (23.2)*** | 48 (40.7)*** |
| 1.00 | 1.89 (1.64 to 2.19) | 1.59 (1.37 to 1.86) | 3.33 (2.66 to 4.16) | 1.00 | 1.79 (1.50 to 2.13) | 1.52 (1.28 to 1.81) | 2.46 (1.82 to 3.33) | |
| Myocardial infarction | 1128 (19.3) | 186 (34.1)*** | 154 (29.8)*** | 80 (52.6)*** | 710 (10.2) | 117 (19.7)*** | 124 (18.2)*** | 39 (33.1)*** |
| 1.00 | 1.85 (1.56 to 2.17) | 1.44 (1.21 to 1.72) | 3.60 (2.83 to 4.57) | 1.00 | 1.68 (1.37 to 2.06) | 1.52 (1.24 to 1.85) | 2.57 (1.84 to 3.59) | |
| Heart failure | 243 (4.2) | 42 (7.7)*** | 36 (7.0)** | 16 (10.5)*** | 192 (2.8) | 57 (9.6)*** | 50 (7.2)*** | 15 (12.7)*** |
| 1.00 | 1.98 (1.42 to 2.77) | 1.39 (0.96 to 2.00) | 3.52 (2.08 to 5.93) | 1.00 | 2.90 (2.13 to 3.94) | 2.06 (1.49 to 2.85) | 4.02 (2.34 to 6.92) | |
| Atrial fibrillation | 61 (1.0) | 7 (1.3) | 12 (2.3)** | 0 (0) | 65 (0.9) | 12 (2.0)* | 23 (3.4)*** | 1 (0.8) |
| 1.00 | 1.48 (0.69 to 3.28) | 2.21 (1.14 to 4.28) | — | 1.00 | 2.04 (1.08 to 3.84) | 3.39 (2.07 to 5.57) | 0.99 (0.14 to 7.29) | |
| Stroke | 473 (8.1) | 74 (13.6)*** | 40 (7.7) | 15 (9.9) | 523 (7.5) | 85 (14.3)*** | 75 (11.0)** | 22 (18.6)*** |
| 1.00 | 1.67 (1.30 to 2.15) | 0.83 (0.59 to 1.16) | 1.72 (1.02 to 2.91) | 1.00 | 1.52 (1.19 to 1.93) | 1.27 (0.99 to 1.63) | 2.25 (1.45 to 3.49) | |
| Aortic aneurysm | 67 (1.1) | 9 (1.7) | 5 (1.0) | 4 (2.6) | 19 (0.3) | 0 (0) | 2 (0.3) | 1 (0.8) |
| 1.00 | 1.55 (0.76 to 3.16) | 0.77 (0.30 to 1.97) | 3.76 (1.32 to 10.75) | 1.00 | — | 0.97 (0.22 to 4.30) | 3.66 (0.47 to 28.42) | |
| Venous thromboembolism | 115 (2.0) | 12 (2.2) | 10 (1.9) | 3 (2.0) | 77 (1.1) | 9 (1.5) | 11 (1.6) | 1 (0.8) |
| 1.00 | 1.40 (0.76 to 2.56) | 1.05 (0.54 to 2.04) | 1.81 (0.56 to 5.82) | 1.00 | 1.32 (0.65 to 2.66) | 1.02 (0.52 to 2.00) | 0.69 (0.09 to 5.03) | |
ECG, electrocardiogram.
Values are n (%) or adjusted HR (95% CI).
*p<0.05; **p<0.01; ***p<0.001 for the proportion of events in each group compared with the group with no angina and no ischaemic changes on ECG.
As with death alone, the risk for either a major fatal or non‐fatal event was increased by both angina and an ischaemic change on ECG separately. The increase in risk was greater with an abnormality on the ECG. However, even those with angina, but without an abnormality on the ECG, had substantial and significant increases in risk of cardiovascular and coronary events compared with those with neither angina nor ischaemic changes on ECG.
All of these risks were increased in patients with angina and an abnormality on the ECG, in whom the RR of a major fatal or non‐fatal coronary event was increased fourfold in men and threefold in women (compared with people with neither angina nor an ischaemic change on ECG).
Examination of composite fatal and non‐fatal outcomes also showed that angina, an ischaemic change on ECG and, especially, both, were associated with an increased risk of death or hospitalisation due to heart failure and stroke (angina alone did not increase the risk for stroke in men).
Outcomes in men compared with those in women
The adjusted HR (95% CI) for death from any cause in women compared with that for men was 0.52 (0.44 to 0.61; p<0.001; table 5). Even after adjusting for the powerful effect of an ischaemic change on ECG (which was more commonly found in men), women had less than half the risk in men of any adverse cardiovascular outcome. However, this difference was mainly explained by a lower risk of coronary events; the risk of stroke and heart failure was not lower in women.
Table 5 Adjusted risk* of fatal and non‐fatal outcomes over 20 years in women compared with outcomes in men with angina.
| Death | Hospital admission | Death or admission | |
|---|---|---|---|
| Any cardiovascular cause | 0.44 (0.36 to 0.55) | 0.61 (0.51 to 0.74) | 0.54 (0.46 to 0.64) |
| Coronary heart disease | 0.33 (0.25 to 0.42) | 0.49 (0.38 to 0.65) | 0.42 (0.34 to 0.51) |
| Myocardial infarction | 0.38 (0.29 to 0.51) | 0.50 (0.36 to 0.68) | 0.44 (0.35 to 0.56) |
| Heart failure | 1.05 (0.32 to 3.45) | 0.77 (0.49 to 1.20) | 0.79 (0.51 to 1.21) |
| Atrial fibrillation | — | 0.77 (0.34 to 1.74) | 0.84 (0.38 to 1.87) |
| Cerebrovascular incident | 1.50 (0.83 to 2.70) | 0.95 (0.61 to 1.47) | 1.11 (0.75 to 1.65) |
| Aortic aneurysm | 0.30 (0.02 to 5.02) | 0.19 (0.03 to 1.07) | 0.24 (0.05 to 1.08) |
| Venous thromboembolism | — | 0.52 (0.20 to 1.36) | 0.48 (0.19 to 1.20) |
Values are adjusted HR (95% CI).
ECG, electrocardiogram; FEV1, forced expiratory volume in one second.
*Adjusted for age, ischaemic changes on ECG, cholesterol, systolic blood pressure, adjusted FEV1, smoking, social class, Rose myocardial infarction, history of diabetes.
Discussion
This population‐based study describes, in a more complete way than previous studies, the mortality and morbidity burden associated with angina over a long period of follow‐up. In particular, fatal and non‐fatal outcomes are described in a large cohort of women who have generally been excluded from previous epidemiological studies on angina.
Although angina is suggested to be associated with a relatively good prognosis, the present analysis clearly shows otherwise.26 Consideration of only fatal outcomes underestimates the adverse prognostic effect of angina.9,27 Whereas about 40% of men and 25% of women reporting this symptom in middle age died from a cardiovascular cause, almost half of the women and two thirds of the men went on to experience a major fatal or non‐fatal cardiovascular event over the next two decades.
Our findings in men closely support those of previous studies such as the Reykjavik Study,7 the Goteborg Primary Prevention Study8 and the British Regional Heart Study.9 We found that women had a better prognosis than men, as noted in the few prior studies to include both sexes.3,5 This was especially true for coronary events (both fatal and non‐fatal). Although men had a greater prevalence of certain adverse coronary prognostic factors (especially ECG evidence of ischaemia and infarction), women remained at lower risk after adjusting for these differences. Interestingly, however, women were less protected from non‐coronary cardiovascular events including stroke, heart failure and atrial fibrillation. We do not know of any other reports of this discrepancy. Consequently, although women had only a 1 in 5 twenty‐year risk of fatal or non‐fatal myocardial infarction compared with a 1 in 3 risk in men, the risks in women of stroke and heart failure were 1 in 8 and 1 in 12, respectively, compared with 1 in 12 and 1 in 13, respectively, in men. These differences may reflect the notably greater systolic blood pressure (a powerful risk factor for stroke) and cardiothoracic ratio (a powerful risk factor for heart failure and atrial fibrillation) in women at baseline.
Why do women have a better survival than men? One explanation is that a higher proportion of women who are “Rose positive” for angina do not have cardiac disease.18,28,29 Alternatively, women with definite angina and coronary heart disease have better left ventricular systolic function than men (although they have as much heart failure).30 Left ventricular systolic function is a very powerful predictor of death.
As described by others previously (and alluded to above), we found ischaemic changes on the ECG to be a powerful predictor of coronary death.7,25 In addition, we found that these changes were also strong predictors of non‐fatal coronary events and of other non‐fatal and fatal cardiovascular events such as heart failure and stroke. Indeed, an abnormality on the ECG was a more powerful predictor of all these adverse outcomes than the symptom of angina alone (ie, in the absence of ischaemic changes on the ECG). People with both an abnormality on the ECG and angina had the worst outcome, with a risk of 1 in 2 for myocardial infarction and 1 in 10 for stroke and heart failure in men and corresponding values of 1 in 3, 1 in 5 and 1 in 8 in women. Indeed, >75% of men and >66% of women in this category experienced a major fatal or non‐fatal cardiovascular event during follow‐up.
It should be emphasised that angina without associated changes on an ECG (similar to uncomplicated angina in prior studies2,6,7,8) was still predictive of a poor outcome, even in women. However, as in the overall cohort, the risk of death due to coronary disease in these patients was increased to a greater extent in men (71%) than in women (33%), whereas this difference was less for death from cardiovascular causes (46% in men and 31% in women). We also found much less difference between men and women when combined fatal and non‐fatal outcomes were considered, with an approximately 70–80% increase in risk of coronary events and a 40–50% increase in risk of cardiovascular events in both sexes in this category (ie, those with angina but without changes on ECG).
Our study has several limitations. Angina was documented at only one time point and spontaneous variation is well recognised.27,31 The validity of the Rose Questionnaire and which Rose categories should be included have been debated.32,33,34 Our study was conducted in an area with a high prevalence of coronary heart disease.35 We did not measure morbidity (eg, symptoms or functional limitation) not associated with hospital admission.27 Nor did we have records of coronary revascularisation either as an outcome or a treatment; similarly, we had no access to information on medical treatment. The prevalence of angina increases with increasing age36; however, by design, our study looks at outcome in a group of middle‐aged people. A lack of applicability to other populations is possible, as this is a cohort from Scotland and because the inception of the cohort occurred 30 years ago. By necessity, this is a historical cohort, demographics and risk factor profiles have changed in the time interval, and it could not benefit from several recent treatments that reduce morbidity and mortality in patients with angina.37,38,39,40 The mortality of those with angina in our study is higher than that reported in contemporary pharmaceutical trials. For example, the Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial reported 8.1% mortality at 5 years,41 and in the ACTION trial (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) the mortality was 1.53 per 100 patient‐years42 (Renfrew–Paisley angina cohort: 5‐year mortality 11.6% and 2.62 per 100 patient‐years). The mean age of patients was higher in both these studies (about 63 v 56 years in our study). Contemporary pharmacological treatments and revascularisation options may have improved outcome in patients with angina.
In summary, angina, as identified by the Rose Questionnaire, is common and is associated with substantial long‐term mortality and morbidity in both men and women. Consideration of only death (or death and myocardial infarction) greatly underestimates the public health burden related to angina. Regarding middle‐aged patients with angina, almost two thirds of the men and half of the women will die or require hospital admission for a cardiovascular reason over the subsequent 20 years. If these people have ischaemic changes on ECG, the proportions rise to more than three quarters for men and two thirds for women. Vigorous efforts to identify and treat patients with angina (many of which, especially women,43 are not known to the healthcare system4,44) could lead to substantial reductions in the direct and indirect burden of cardiovascular disease.45,46
Abbreviations
ECG - electrocardiography
ICD9 - International classification of diseases, 9th revision
Footnotes
Funding: NFM is funded by the British Heart Foundation, SS is supported by the NH&MRC and the NHF of Australia.
Competing interests: None.
References
- 1.Lampe F C, Morris R W, Walker M.et al Trends in rates of different forms of diagnosed coronary heart disease, 1978 to 2000: prospective, population based study of British men. BMJ 20053301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel W B, Feinleib M. Natural history of angina pectoris in the Framingham Study. Prognosis and survival. Am J Cardiol 197229154–163. [DOI] [PubMed] [Google Scholar]
- 3.Orencia A, Bailey K, Yawn B P.et al Effect of gender on long‐term outcome of angina pectoris and myocardial infarction/sudden unexpected death. JAMA 19932692392–2397. [PubMed] [Google Scholar]
- 4.Hemingway H, Shipley M, Britton A.et al Prognosis of angina with and without a diagnosis: 11 year follow up in the Whitehall II Prospective Cohort Study. BMJ 2003327895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murabito J M, Evans J C, Larson M G.et al Prognosis after the onset of coronary heart disease. An investigation of differences in outcome between the sexes according to initial coronary disease presentation. Circulation 1993882548–2555. [DOI] [PubMed] [Google Scholar]
- 6.Rose G, Hamilton P S, Keen H.et al Myocardial ischaemia, risk factors and death from coronary heart‐disease. Lancet 19771105–109. [DOI] [PubMed] [Google Scholar]
- 7.Sigurdsson E, Sigfusson N, Agnarsson U.et al Long‐term prognosis of different forms of coronary heart disease: the Reykjavik Study. Int J Epidemiol 19952458–68. [DOI] [PubMed] [Google Scholar]
- 8.Rosengren A, Wilhelmsen L, Hagman M.et al Natural history of myocardial infarction and angina pectoris in a general population sample of middle‐aged men: a 16‐year follow‐up of the Primary Prevention Study, Goteborg, Sweden. J Intern Med 1998244495–505. [DOI] [PubMed] [Google Scholar]
- 9.Lampe F C, Whincup P H, Wannamethee S G.et al The natural history of prevalent ischaemic heart disease in middle‐aged men. Eur Heart J 2000211052–1062. [DOI] [PubMed] [Google Scholar]
- 10.Campbell M J, Elwood P C, Abbas S.et al Chest pain in women: a study of prevalence and mortality follow up in south Wales. J Epidemiol Community Health 19843817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen‐Smith V, Hannaford P C, Elliott A M. Increased mortality among women with Rose angina who have not presented with ischaemic heart disease. Br J Gen Pract 200353784–789. [PMC free article] [PubMed] [Google Scholar]
- 12.Hawthorne V M, Watt G C, Hart C L.et al Cardiorespiratory disease in men and women in urban Scotland: baseline characteristics of the Renfrew/Paisley (midspan) study population. Scott Med J 199540102–107. [DOI] [PubMed] [Google Scholar]
- 13.Hart C L, Watt G C, Davey S G.et al Pre‐existing ischaemic heart disease and ischaemic heart disease mortality in women compared with men. Int J Epidemiol 199726508–515. [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Hart C L, Hole D J.et al A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med 2002113359–364. [DOI] [PubMed] [Google Scholar]
- 15.Murphy N F, MacIntyre K, Stewart S.et al Long‐term cardiovascular consequences of obesity: 20‐year follow‐up of more than 15 000 middle‐aged men and women (the Renfrew‐Paisley study). Eur Heart J 20052796–106. [DOI] [PubMed] [Google Scholar]
- 16.Rose G A. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 196227645–658. [PMC free article] [PubMed] [Google Scholar]
- 17.Rose G, McCartney P, Reid D D. Self‐administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med 19773142–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bass E B, Follansbee W P, Orchard T J. Comparison of a supplemented Rose Questionnaire to exercise thallium testing in men and women. J Clin Epidemiol 198942385–394. [DOI] [PubMed] [Google Scholar]
- 19.Blackwelder W C, Kagan A, Gordon T.et al Comparison of methods for diagnosing angina pectoris: the Honolulu Heart Study. Int J Epidemiol 198110211–215. [DOI] [PubMed] [Google Scholar]
- 20.Heyden S, Bartel A G, Tabesh E.et al Angina pectoris and the Rose Questionnaire. Arch Intern Med 1971128961–964. [PubMed] [Google Scholar]
- 21.Simpson R J, Jr, White A. Getting a handle on the prevalence of coronary heart disease. Br Heart J 199064291–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prineas R J, Crow R S, Blackburn H.The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Boston, MA: John Wright, 1982
- 23.World Health Organization Manual of the international statistical classification of diseases, injuries and causes of death. 9th revision. Geneva: WHO, 1977
- 24.Harley K, Jones C. Quality of Scottish Morbidity Record (SMR) data. Health Bull (Edinb) 199654410–417. [PubMed] [Google Scholar]
- 25.Daviglus M L, Liao Y, Greenland P.et al Association of nonspecific minor ST‐T abnormalities with cardiovascular mortality: the Chicago Western Electric Study. JAMA 1999281530–536. [DOI] [PubMed] [Google Scholar]
- 26.Task Force of the European Society of Cardiology Management of stable angina pectoris. Recommendations of the Task Force of the European Society of Cardiology. Eur Heart J 199718394–413. [DOI] [PubMed] [Google Scholar]
- 27.Rosengren A, Hagman M, Pennert K.et al Clinical course and symptomatology of angina pectoris in a population study. Acta Med Scand 1986220117–126. [DOI] [PubMed] [Google Scholar]
- 28.Garber C E, Carleton R A, Heller G V. Comparison of “Rose Questionnaire Angina” to exercise thallium scintigraphy: different findings in males and females. J Clin Epidemiol 199245715–720. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor D A, Adamson J, Ebrahim S. Performance of the WHO Rose Angina Questionnaire in post‐menopausal women: are all of the questions necessary? J Epidemiol Community Health 200357538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson S V, Bjorkander I, Held C.et al Age and gender differences in left ventricular function among patients with stable angina and a matched control group. A report from the Angina Prognosis Study in Stockholm. Cardiology 199687287–293. [DOI] [PubMed] [Google Scholar]
- 31.Rose G. Variability of angina. Some implications for epidemiology. Br J Prev Soc Med 19682212–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodegard J, Erikssen G, Bjornholt J V.et al Possible angina detected by the WHO Angina Questionnaire in apparently healthy men with a normal exercise ECG: coronary heart disease or not? A 26 year follow up study. Heart 200490627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook D G, Shaper A G, Macfarlane P W. Using the WHO (Rose) Angina Questionnaire in cardiovascular epidemiology. Int J Epidemiol 198918607–613. [DOI] [PubMed] [Google Scholar]
- 34.LaCroix A Z, Guralnik J M, Curb J D.et al Chest pain and coronary heart disease mortality among older men and women in three communities. Circulation 199081437–446. [DOI] [PubMed] [Google Scholar]
- 35.Smith W C, Kenicer M B, Tunstall‐Pedoe H.et al Prevalence of coronary heart disease in Scotland: Scottish Heart Health Study. Br Heart J 199064295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy N F, Simpson C R, MacIntyre K.et al Prevalence, incidence, primary care burden and medical treatment of angina in Scotland: age, sex and socioeconomic disparities—a population based study Heart2006921047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The ALLHAT Officers and Coordinators, for the ALLHAT Collaborative Research Group Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 20022882981–2997. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen T R, Kjekshus J, Berg K.et al Cholesterol lowering and the use of healthcare resources. Results of the Scandinavian Simvastatin Survival Study. Circulation 1996931796–1802. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf S, Sleight P, Pogue J.et al Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000342145–153. [DOI] [PubMed] [Google Scholar]
- 40.The IONA Study Group Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet 20023591269–1275. [DOI] [PubMed] [Google Scholar]
- 41.Braunwald E, Domanski M J, Fowler S E.et al Angiotensin‐converting‐enzyme inhibition in stable coronary artery disease. N Engl J Med 20043512058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole‐Wilson P A, Lubsen J, Kirwan B A.et al Effect of long‐acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004364849–857. [DOI] [PubMed] [Google Scholar]
- 43.Richards H, McConnachie A, Morrison C.et al Social and gender variation in the prevalence, presentation and general practitioner provisional diagnosis of chest pain. J Epidemiol Community Health 200054714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner K, Chapple A. Barriers to referral in patients with angina: qualitative study. BMJ 1999319418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capewell S, Pell J P, Morrison C.et al Increasing the impact of cardiological treatments. How best to reduce deaths. Eur Heart J 1999201386–1392. [DOI] [PubMed] [Google Scholar]
- 46.Mukherjee D, Fang J, Chetcuti S.et al Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004109745–749. [DOI] [PubMed] [Google Scholar]