Abstract
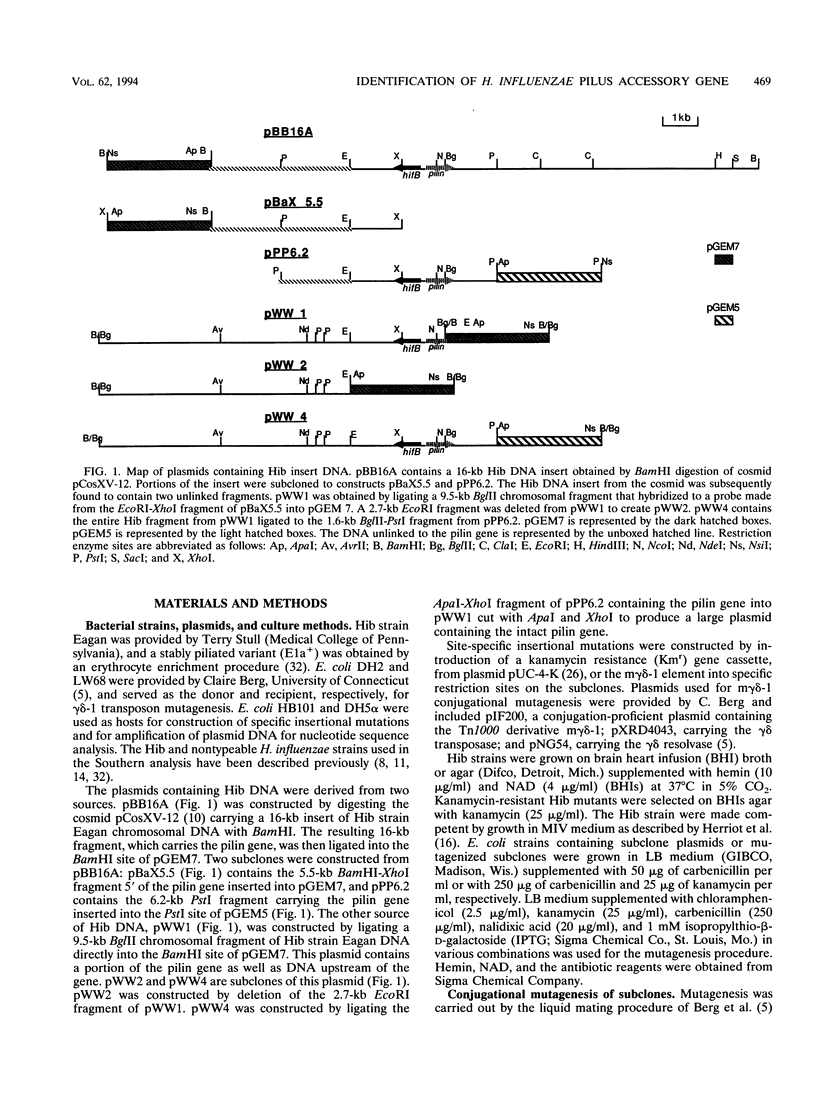
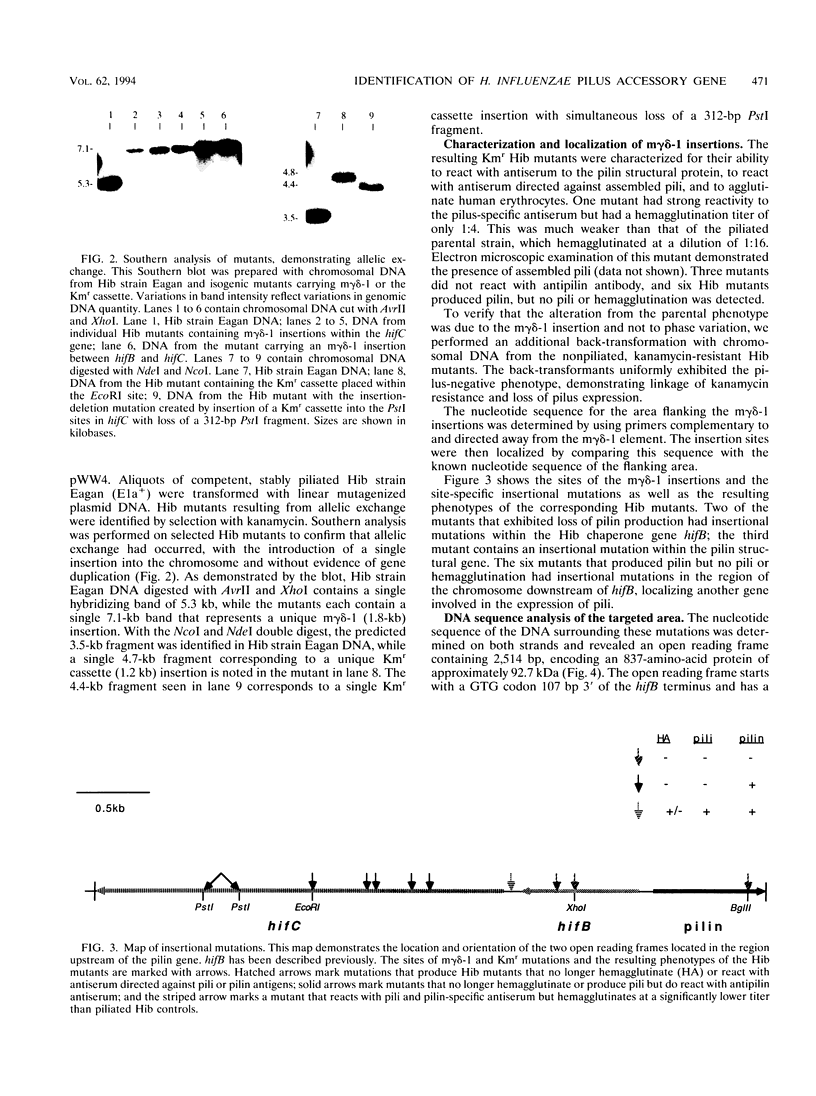
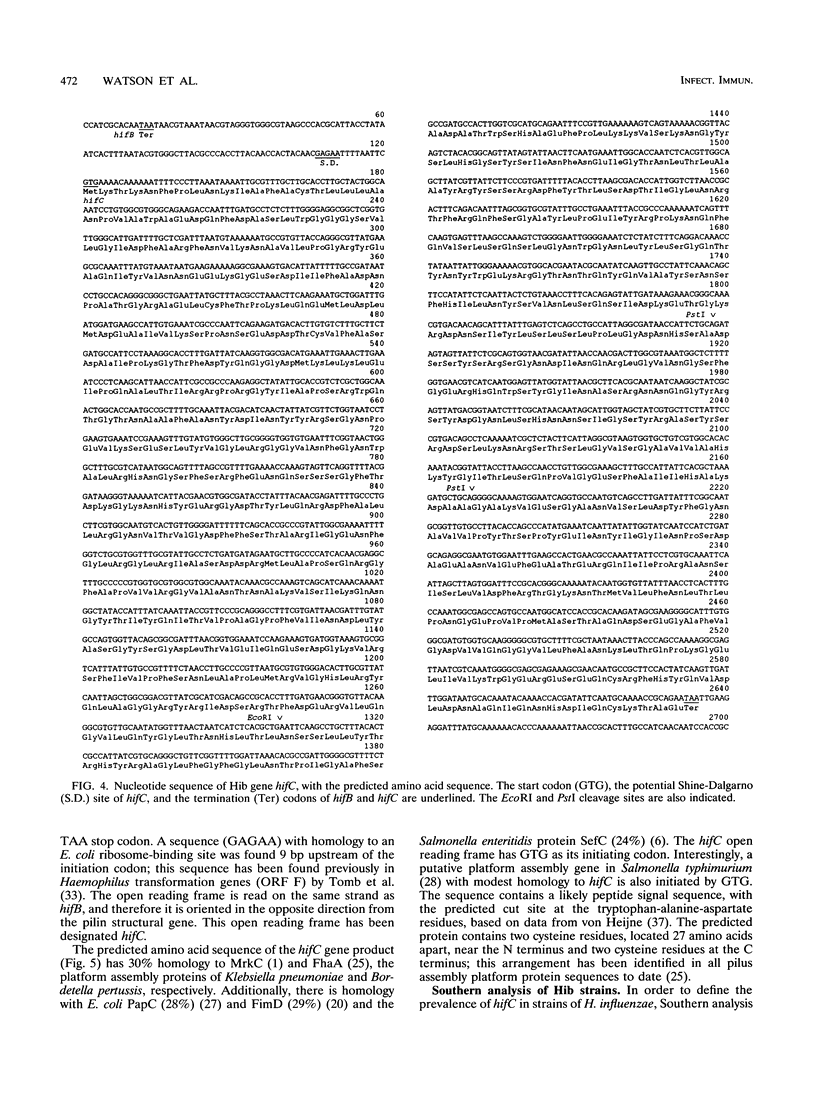
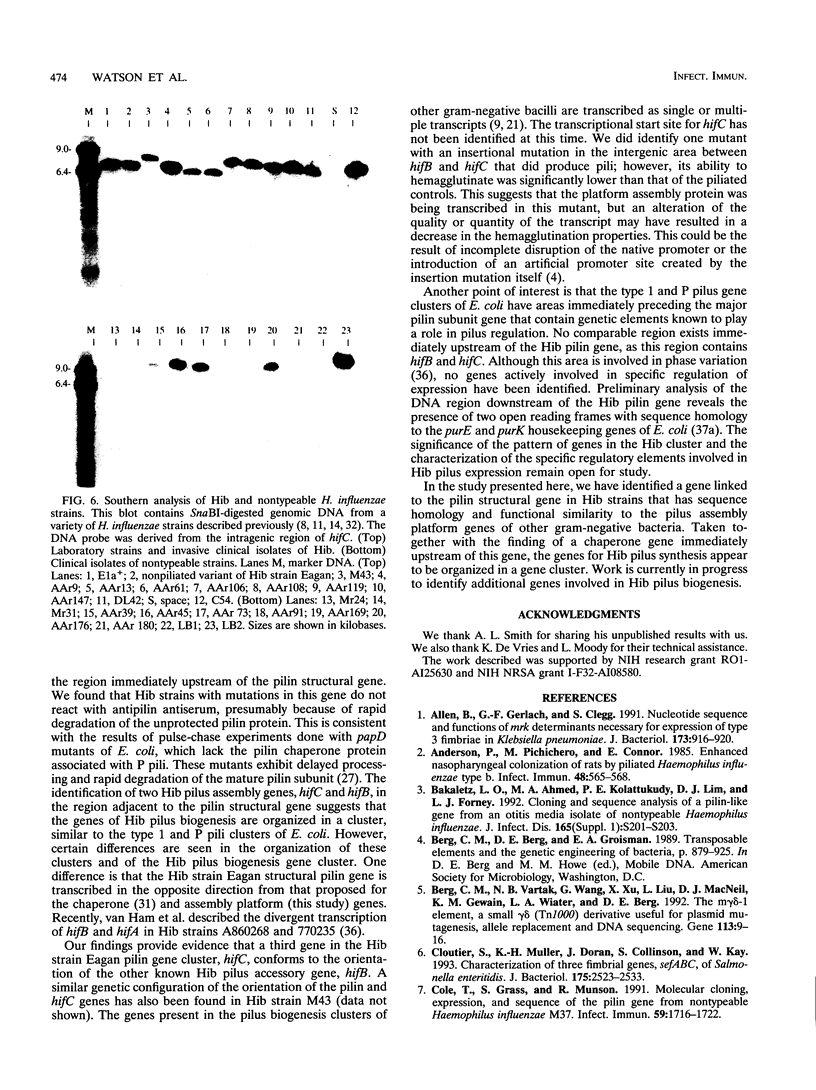
Haemophilus influenzae type b (Hib) pili are complex filamentous surface structures consisting predominantly of pilin protein subunits. The gene encoding the major pilin protein subunit of Hib adherence pili has been cloned and its nucleotide sequence has been determined. In order to identify specific accessory genes involved in pilus expression and assembly, we constructed isogenic Hib mutants containing insertional chromosomal mutations in the DNA flanking the pilin structural gene. These mutants were screened for pilin production, pilus expression, and hemagglutination. Pili and pilin production were assessed by immunoassays with polyclonal antisera specific for pilin and pili of Hib strain Eagan. Hemagglutination was semiquantitatively evaluated in a microtiter plate assay. Six Hib mutants produced proteins immunoreactive with antipilin antiserum but no longer produced structures reactive with antipilus antiserum. In addition, the mutants were unable to agglutinate human erythrocytes. Nucleotide sequence analysis localized the insertion sites in the six mutants to 2.5-kb open reading frame upstream of the pilin structural gene and immediately downstream of an Hib pilin chaperone gene. The amino acid sequence encoded by this open reading frame has significant homology to members of the pilus assembly platform protein family, including FhaA of Bordetella pertussis, MrkC of Klebsiella pneumoniae, and the Escherichia coli assembly platform proteins FimD and PapC. This open reading frame, designated hifC, appears to represent a gene essential to Hib pilus biogenesis that has genetic and functional similarity to the pilus platform assembly genes of other gram-negative rods.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. L., Gerlach G. F., Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991 Jan;173(2):916–920. doi: 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Connor E. M. Enhanced nasopharyngeal colonization of rats by piliated Haemophilus influenzae type b. Infect Immun. 1985 May;48(2):565–568. doi: 10.1128/iai.48.2.565-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaletz L. O., Ahmed M. A., Kolattukudy P. E., Lim D. J., Forney L. J. Cloning and sequence analysis of a pilin-like gene from an otitis media isolate of nontypeable Haemophilus influenzae. J Infect Dis. 1992 Jun;165 (Suppl 1):S201–S203. doi: 10.1093/infdis/165-supplement_1-s201. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Vartak N. B., Wang G., Xu X., Liu L., MacNeil D. J., Gewain K. M., Wiater L. A., Berg D. E. The m gamma delta-1 element, a small gamma delta (Tn1000) derivative useful for plasmid mutagenesis, allele replacement and DNA sequencing. Gene. 1992 Apr 1;113(1):9–16. doi: 10.1016/0378-1119(92)90664-b. [DOI] [PubMed] [Google Scholar]
- Clouthier S. C., Müller K. H., Doran J. L., Collinson S. K., Kay W. W. Characterization of three fimbrial genes, sefABC, of Salmonella enteritidis. J Bacteriol. 1993 May;175(9):2523–2533. doi: 10.1128/jb.175.9.2523-2533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T., Grass S., Munson R., Jr Molecular cloning, expression, and sequence of the pilin gene from nontypeable Haemophilus influenzae M37. Infect Immun. 1991 May;59(5):1716–1722. doi: 10.1128/iai.59.5.1716-1722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope L. D., Pelzel S. E., Latimer J. L., Hansen E. J. Characterization of a mutant of Haemophilus influenzae type b lacking the P2 major outer membrane protein. Infect Immun. 1990 Oct;58(10):3312–3318. doi: 10.1128/iai.58.10.3312-3318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Marrs C. F., Bektesh S. L., Gilsdorf J. R. Comparison and analysis of the nucleotide sequences of pilin genes from Haemophilus influenzae type b strains Eagan and M43. Infect Immun. 1991 Jun;59(6):1991–1996. doi: 10.1128/iai.59.6.1991-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., Chang H. Y., McCrea K. W., Bakaletz L. O. Comparison of hemagglutinating pili of Haemophilus influenzae type b with similar structures of nontypeable H. influenzae. Infect Immun. 1992 Feb;60(2):374–379. doi: 10.1128/iai.60.2.374-379.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., Ferrieri P. Adherence of Haemophilus influenzae to human epithelial cells. Scand J Infect Dis. 1984;16(3):271–278. doi: 10.3109/00365548409070400. [DOI] [PubMed] [Google Scholar]
- Gilsdorf J. R., Marrs C. F., McCrea K. W., Forney L. J. Cloning, expression, and sequence analysis of the Haemophilus influenzae type b strain M43p+ pilin gene. Infect Immun. 1990 Apr;58(4):1065–1072. doi: 10.1128/iai.58.4.1065-1072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., McCrea K., Forney L. Conserved and nonconserved epitopes among Haemophilus influenzae type b pili. Infect Immun. 1990 Jul;58(7):2252–2257. doi: 10.1128/iai.58.7.2252-2257.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., Tucci M., Forney L. J., Watson W., Marrs C. F., Hansen E. J. Paradoxical effect of pilus expression on binding of antibodies by Haemophilus influenzae. Infect Immun. 1993 Aug;61(8):3375–3381. doi: 10.1128/iai.61.8.3375-3381.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Kuehn M. J., Brändén C. I., Hultgren S. J. Conserved immunoglobulin-like features in a family of periplasmic pilus chaperones in bacteria. EMBO J. 1992 Apr;11(4):1617–1622. doi: 10.1002/j.1460-2075.1992.tb05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Normark S., Abraham S. N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- Jones C. H., Jacob-Dubuisson F., Dodson K., Kuehn M., Slonim L., Striker R., Hultgren S. J. Adhesin presentation in bacteria requires molecular chaperones and ushers. Infect Immun. 1992 Nov;60(11):4445–4451. doi: 10.1128/iai.60.11.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. The fimD gene required for cell surface localization of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1990 Jan;220(2):334–338. doi: 10.1007/BF00260505. [DOI] [PubMed] [Google Scholar]
- Klemm P., Krogfelt K. A., Hedegaard L., Christiansen G. The major subunit of Escherichia coli type 1 fimbriae is not required for D-mannose-specific adhesion. Mol Microbiol. 1990 Apr;4(4):553–559. doi: 10.1111/j.1365-2958.1990.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Langermann S., Wright A. Molecular analysis of the Haemophilus influenzae type b pilin gene. Mol Microbiol. 1990 Feb;4(2):221–230. doi: 10.1111/j.1365-2958.1990.tb00589.x. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Gilsdorf J. R. Structural and serological relatedness of Haemophilus influenzae type b pili. Infect Immun. 1988 May;56(5):1051–1056. doi: 10.1128/iai.56.5.1051-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Normark S. Gene products specifying adhesion of uropathogenic Escherichia coli are minor components of pili. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Geoffroy M. C., Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992 Sep;11(9):3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Keppner W., Rasched I. Versatile kanamycin-resistance cartridges for vector construction in Escherichia coli. Gene. 1986;46(1):131–133. doi: 10.1016/0378-1119(86)90176-9. [DOI] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Rioux C. R., Friedrich M. J., Kadner R. J. Genes on the 90-kilobase plasmid of Salmonella typhimurium confer low-affinity cobalamin transport: relationship to fimbria biosynthesis genes. J Bacteriol. 1990 Nov;172(11):6217–6222. doi: 10.1128/jb.172.11.6217-6222.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L., Mendelman P. M., Haas J. E., Schoenborn M. A., Mack K. D., Smith A. L. Characterization of Haemophilus influenzae type b fimbriae. Infect Immun. 1984 Dec;46(3):787–796. doi: 10.1128/iai.46.3.787-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., el-Hajj H., Smith H. O. Nucleotide sequence of a cluster of genes involved in the transformation of Haemophilus influenzae Rd. Gene. 1991 Jul 31;104(1):1–10. doi: 10.1016/0378-1119(91)90457-m. [DOI] [PubMed] [Google Scholar]
- Weber A., Harris K., Lohrke S., Forney L., Smith A. L. Inability to express fimbriae results in impaired ability of Haemophilus influenzae b to colonize the nasopharynx. Infect Immun. 1991 Dec;59(12):4724–4728. doi: 10.1128/iai.59.12.4724-4728.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R. J., van der Heide H. G., Mooi F. R. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992 Sep;6(18):2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Krenn B. E., Klaasen P. Organization and expression of genes involved in the biosynthesis of K99 fimbriae. Infect Immun. 1984 Feb;43(2):508–514. doi: 10.1128/iai.43.2.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham S. M., Mooi F. R., Sindhunata M. G., Maris W. R., van Alphen L. Cloning and expression in Escherichia coli of Haemophilus influenzae fimbrial genes establishes adherence to oropharyngeal epithelial cells. EMBO J. 1989 Nov;8(11):3535–3540. doi: 10.1002/j.1460-2075.1989.tb08519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham S. M., van Alphen L., Mooi F. R., van Putten J. P. Phase variation of H. influenzae fimbriae: transcriptional control of two divergent genes through a variable combined promoter region. Cell. 1993 Jun 18;73(6):1187–1196. doi: 10.1016/0092-8674(93)90647-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]