Abstract
This review examines the use of cardiac resynchronisation therapy (CRT) for chronic, severe, systolic heart failure. Left ventricular (LV) remodelling is the final common pathway of systolic heart failure and portends a poor prognosis. It is characterised by progressive LV dilatation, deterioration of ventricular contractile function and distortion of LV cavity shape. The LV remodelling process is triggered by prolonged pressure or volume overload, loss of contracting myocytes from myocardial infarction, genetic abnormalities of contractile proteins or exposure to cardiotoxic agents. Current therapeutic strategies for systolic heart failure aim to slow or halt the remodelling process. “Reverse remodelling” is a relatively new concept, where progressive LV dilatation and deterioration in contractile function are not simply arrested, but partially reversed. Cardiac resynchronisation therapy is a novel and effective treatment for systolic heart failure, and is associated with reverse remodelling of the LV.
Keywords: heart failure, resynchronisation, ventricular remodelling, echocardiography
Congestive heart failure is a major health problem involving almost 22 million people world wide. Heart failure is also the most common hospital discharge diagnosis in patients over 65.1 Approximately two thirds of patients presenting with symptoms of congestive heart failure have impaired systolic function with left ventricular ejection fraction (LVEF<50%), and one third have diastolic dysfunction but preserved systolic function (LVEF>50%).2 This review is confined to patients with chronic severe systolic heart failure.
Left ventricular remodelling
Systolic heart failure is characterised by left ventricular (LV) remodelling, triggered by prolonged pressure or volume overload, loss of contracting myocytes from coronary occlusive disease and myocardial infarction, genetically determined abnormalities in the sarcomeric contractile proteins, or cardiotoxic agents. LV remodelling describes the dynamic process of progressive LV dilatation, deterioration in ventricular contractile function and distortion of both LV cavity shape and geometry of the mitral subvalve apparatus which results in mitral regurgitation. The LV remodelling process is the final common pathway for all of the above causes of heart failure and portends a poor prognosis. A number of neurohormones and local trophic factors modulate the dynamic balance between distending forces that favour dilatation and restraining forces imposed by the extracellular collagen matrix that prevent LV dilatation. As such, these represent potential targets for new therapeutic interventions to attenuate progressive LV remodelling to heart failure. “Reverse” remodelling is a relatively new concept, where progressive LV dilatation and deterioration in contractile function in patients with heart failure are not simply arrested, but partially reversed.
Contemporary treatment of heart failure
The major aims of treatment in chronic systolic heart failure are to relieve symptoms and improve exercise capacity and quality of life. Traditionally, treatment of congestive heart failure has involved three different strategies.3,4 First, reducing LV loading conditions with vasodilator therapy (nitrates/hydralazine), surgical epicardial restraint devices, or ventricular assist devices decreases LV size and therefore LV load. As contractile function varies inversely with load, the greater the reduction in loading conditions, the greater the improvement in contractile function. Second, ionotropic agents, including catecholamines and phosphodiesterase inhibitors, augment myocardial contraction. Third, neurohormonal activation can be blunted with angiotensin converting enzyme inhibitors, angiotensin receptor blockers, adrenergic receptor blockers and aldosterone receptor blockers. In spite of all these therapeutic options, the prognosis of congestive heart failure has not improved significantly over the last two decades, and the 5‐year survival for patients with New York Heart Association (NYHA) symptom class III/IV is still almost 50%.
Cardiac resynchronisation therapy in advanced heart failure
Cardiac resynchronisation therapy (CRT) has recently become an additional established treatment for a highly selected population of patients with NYHA class III/IV chronic systolic heart failure and LV dyssynchrony, as evidenced by a prolonged QRS duration beyond 130 ms.5,6,7 Prolonged QRS duration beyond 130 ms occurs in approximately one third to one half of all patients with congestive heart failure resulting from chronic systolic LV dysfunction. It is associated with a mortality that is directly proportional to the QRS duration and independent of other baseline characteristics.8 Thus, the longer the QRS duration, the greater are the morbidity and mortality. The purpose of CRT in patients with heart failure with LV dyssynchrony is to optimise atrioventricular conduction and LV filling, coordinate right and left ventricular contraction by minimising interventricular and intraventricular mechanical delay, and facilitate interventricular dependence.
Symptomatic improvement and survival benefits with resynchronisation therapy
Consistent findings both in small, uncontrolled, open‐label clinical trials and in large, randomised, double‐blind CRT trials have been the improvement in exercise capacity (6‐min hall walk distance), NYHA symptom class, and quality of life in most suitable patients with heart failure. Importantly, the incremental beneficial effects of CRT have been achieved in patients already receiving optimal traditional medical heart failure therapy with angiotensin converting enzyme inhibitors, angiotensin receptor blockers, diuretics and β blockers. The changes in structural and functional LV remodelling resulting from CRT have been documented in a number of longitudinal Doppler echocardiographic studies, and these are coincident with improvement in symptoms and exercise capacity.
Unchecked LV remodelling in advanced (NYHA symptom class III/IV) heart failure leads to progressive LV dilatation and decreasing ejection fraction, both of which correlate with ventricular arrhythmias,9 which are a common cause of sudden death in patients with heart failure. The combination of an internal cardiac defibrillator with CRT not surprisingly affords incremental survival benefit over CRT alone,10 by protecting against sudden death from ventricular dysrhythmias. In refractory heart failure of ischaemic aetiology with LV dyssynchrony and an ejection fraction <35%, a strong case can be made for placement of an internal cardiac defibrillator together with a CRT device (MADIT II trial11).
Reverse LV remodelling
Although multiple studies have documented improved survival, NYHA symptom class, exercise capacity and quality of life, few have examined the impact of CRT on LV remodelling, explored the relationship between structural LV remodelling and symptomatic improvement, or investigated the mechanism by which LV reverse remodelling is mediated.
Three recent CRT studies have used serial transthoracic Doppler echocardiography to characterise reverse LV remodelling in advanced systolic heart failure and determine whether remodelling is sustained 1 year beyond baseline. The effects of CRT on LV reverse remodelling have been compared in patients randomised to CRT under optimal medical management with those randomised to optimal medical management alone.6,12 Overall follow‐up has ranged from 1 month to 29 months,13 with the most common duration of follow‐up being 3–6 months.
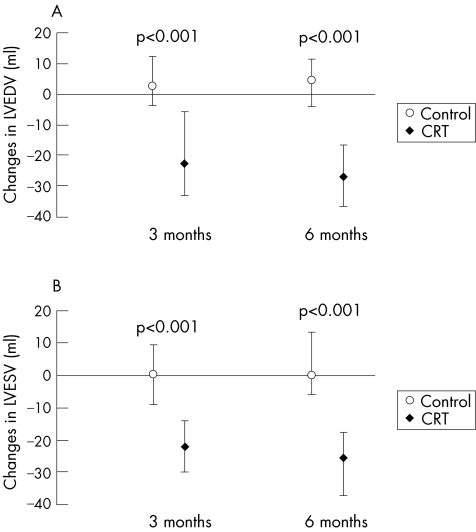
CRT results in a significant decrease in LV size, assessed as LV end‐diastolic and end‐systolic diameters or as LV volumes as early as 1 month,13,14,15 compared with control patients. There is further progressive reduction in LV diameter and LV volume at 6 months (fig 1), which are sustained at 1 year in 65–75% of patients.16,17,18 Differences in LV volumes at 18 months remained significant, and values at a mean of 29 months follow‐up13 remain to be reported from the Cardiac Resynchronisation in Heart Failure (CARE‐HF) study. The Multicenter InSync Randomised Clinical Evaluation (MIRACLE) study was a prospective, double‐blind, randomised, controlled trial designed to determine the efficacy of CRT in more than 400 patients with heart failure of NYHA III/IV symptom class.6 All patients were on an optimal medical treatment regimen and all had implantation of a biventricular pacing device. Patients were randomised to CRT programmed to active pacing mode “on” or to inactive pacing mode “off”, and were followed for a minimum of 6 months. After completion of the MIRACLE trial, clinical and echocardiographic information was available for patients in whom CRT was continued without interruption from baseline to 1 year. Before the MIRACLE trial, the few studies of the effects of CRT on echocardiographic variables were small, open‐label and uncontrolled. The MIRACLE programme demonstrated that the benefits from CRT on 6‐min hall walk distance, NYHA symptom class and quality of life occurred predominantly, but not exclusively, in those patients with objective changes in LV geometry and function.19
Figure 1 Median change (with 95% confidence intervals) in left ventricular end‐diastolic volume (LVEDV) (A) and left ventricular end‐systolic volume (LVESV) (B) at 3 and 6 months after randomisation in the control group and the cardiac resynchronisation group (CRT). (St John Sutton M, et al. Circulation 2003;107:2577–2582; reproduced with permission.)
The MIRACLE and MIRACLE ICD (internal cardiac defibrillator) studies,6,20 similarly to most smaller CRT studies, showed significant reductions in LV end‐diastolic and end‐systolic volumes at 3 and 6 months in the CRT group compared with the control group, in which no changes from baseline were observed at either 3 or 6 months of follow‐up. The reduction in LV linear diameters and LV cavity volumes mediated by CRT resulted in a decrease in LV loading conditions (wall stress), which was a stimulus for regression of LV mass and improved contractile function. LV mass decreased significantly from baseline to 6 months in the CRT group but not in the control group. The regression of LV mass occurred more slowly than the reduction in LV volume.
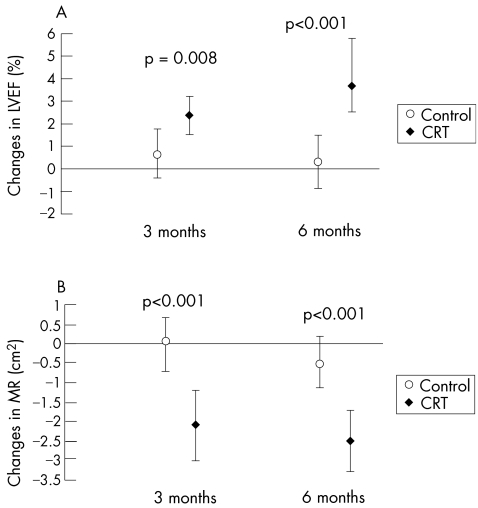
The time‐dependent progressive reduction in LV volume and regression of LV mass with CRT are associated with mechanically advantageous alterations in LV architecture, especially in restoration of mitral valve annular diameter and mitral subvalve geometry towards normal. The changes in LV cavity shape and geometry of the mitral valve apparatus are associated with reduction in the severity of mitral regurgitation. In the MIRACLE study, the severity of mitral regurgitation had decreased significantly with CRT at 3 months, and this improvement was maintained at 6 and 12 months (fig 2).21 In contrast, no change in the severity of mitral regurgitation was observed in the control group. There is some evidence that the decrease in the severity of mitral regurgitation precedes the reduction in LV volume and the associated changes in LV and mitral valve annulus and subvalve architecture.22 An alternative mechanism that may explain the early reduction in severity of mitral regurgitation by CRT is the restoration of the temporal coordination of mechanical activation of the papillary muscle insertions, allowing an increased area of mitral leaflet coaptation.23 Concomitant with the reduction in LV volume and load, regression of LV mass, favourable changes in LV architecture, and decrease in severity of mitral regurgitation, there was a significant progressive increase in LVEF at 3 and 6 months from baseline in the CRT‐ON treatment arm, whereas there was no change in the control (CRT‐OFF) treatment arm (fig 2). LVEF had increased further at 1 year with active CRT, a finding concordant with other trials.13,22
Figure 2 Median change (with 95% confidence intervals) in left ventricular ejection fraction (LVEF) (A) and mitral regurgitation (MR) (B) at 3 and 6 months after randomisation in the control group and the cardiac resynchronisation group (CRT). (St John Sutton M, et al. Circulation 2003;107:2577–2582; reproduced with permission.)
An important observation in CRT was that a progressive reduction in LV size and volumes continues and is sustained at least short term, provided that CRT is maintained. Until the results of the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) and CARE‐HF studies were published,10,13 little was known about how long the structural and functional LV remodelling with CRT is sustained. The longest follow‐up study of remodelling with CRT was the MUltisite STimulation In Cardiomyopathies (MUSTIC) trial, which showed sustained reduction in LV size (linear dimensions) measured echocardiographically at 1 year in a small patient cohort, which was associated with continued clinical benefit.14 It is now evident that reversed LV remodelling and associated symptomatic improvement continues for at least 18 months after initiation of CRT.19
The need for continuous rather than intermittent CRT to induce reverse LV remodelling was demonstrated clearly when CRT was discontinued after 3 months in one small open‐label study.22 After 3 months of CRT‐induced reverse LV remodelling, cessation of CRT resulted in rapid abolition of the LV volume reduction achieved over the preceding 3 months with concomitant recurrent LV dilatation and progressive deterioration in LVEF towards baseline values.22 Furthermore, mitral regurgitation returned with cessation of CRT. Over the course of 1 week, the severity of mitral regurgitation increased quickly to pre‐CRT (baseline) levels.
Diastolic ventricular function with resynchronisation therapy
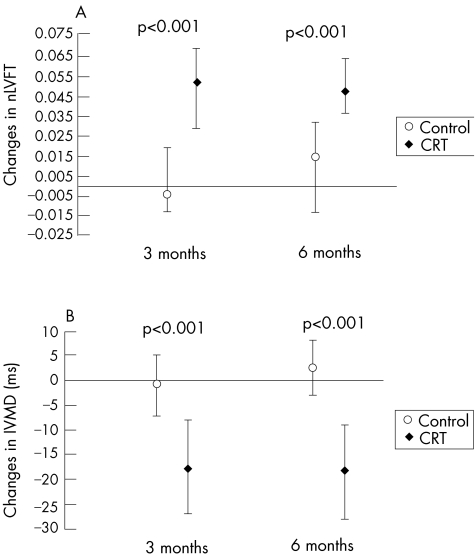
Although CRT studies have been conducted exclusively in patients with advanced systolic heart failure, improvements in diastolic function have been unequivocally demonstrated in these patients.12 Optimisation of atrio‐ventricular (AV) delay and synchronous biventricular pacing in the MIRACLE study resulted in prolongation of the duration of LV filling, separation of the rapid filling phase from atrial systolic contraction, concomitant shortening of interventricular mechanical delay, and simultaneous ventricular depolarisation which coordinates contraction and relaxation (fig 3). In spite of prolongation of LV diastolic filling time, the transmitral flow velocity during atrial contraction (peak A‐wave velocity), the flow velocity ratio of rapid LV filling and atrial contraction (E/A wave velocity ratio), and isovolumic relaxation time had not changed significantly after 6 months of continuous CRT. However, deceleration slope and deceleration time of the E‐wave (during rapid filling), which are more robust measures of myocardial diastolic properties, were significantly increased, while there was no change in the control group. Myocardial performance (Tei) index, which represents a combination of LV diastolic and systolic function, had improved significantly at 3 months, and this improvement continued between 6 and 12 months in the CRT group, whereas there were no changes in the control group.12,21 However, patients with systolic heart failure and restrictive LV filling characterised by a short deceleration time and peak E‐wave velocity >1.0 m/s show little or no remodelling response to CRT.
Figure 3 Median change (with 95% confidence intervals) in normalised left ventricular filling time (nLVFT) (A) and interventricular mechanical delay (IVMD) (B) at 3 and 6 months after randomisation to the control or the cardiac resynchronisation group (CRT). (St John Sutton M, et al. Circulation 2003;107:2577–2582; reproduced with permission.).
Historically, exercise capacity, assessed as either peak VO2 or treadmill time or distance, in patients with advanced heart failure have not correlated with changes in LV volume or LVEF. Analysis of the interactions between 6‐min hall walk, quality of life and NYHA symptom class with echocardiographic changes in LV geometry and function in the MIRACLE study revealed that symptomatic benefits from CRT occurred predominantly in those patients with the greatest structural and functional LV remodelling.12
Responders and non‐responders to resynchronisation therapy
The proportion of NYHA symptom class III/IV patients with heart failure who responded favourably to CRT by exhibiting LV reverse remodelling was similar in patients with ischaemic and non‐ischaemic aetiology of heart failure. However, the extent of LV remodelling varied according to the aetiology of heart failure. Reduction in LV volume, decrease in severity of mitral regurgitation, and increase in LVEF were consistently 2–3‐fold greater in patients with non‐ischaemic than ischaemic heart failure in spite of the significantly larger baseline volumes and lower ejection fractions in the non‐ischaemic patients.12,21 However, in spite of the 2–3‐fold difference in LV volume reduction in the non‐ischaemic patients at 6 months, both ischaemic and non‐ischaemic heart failure showed similar improvement in NYHA symptom class, quality of life and 6‐min hall walk distance.
Similarly to a number of previous small studies, the MIRACLE study showed that CRT has a differential effect on both the magnitude and temporal schedule of LV remodelling in patients with ischaemic versus non‐ischaemic/idiopathic aetiology of heart failure. After adjustment for differences in baseline characteristics (including age, sex, ejection fraction, LV volumes), the beneficial reduction in LV volumes seen at 6 months had partially regressed to baseline by 12 months in the non‐ischaemic patients with heart failure. At between 6 and 12 months of continuous CRT, patients with heart failure due to ischaemic cardiomyopathy underwent recurrent LV dilatation to the point that LV volumes had returned to baseline values.21 The less extensive reverse LV remodelling in patients with ischaemic heart failure may be due to the progressive regional loss of myocardium caused by recurring episodes of ischaemia, which characterise the natural history of ischaemic cardiomyopathy, rather than to primary loss of efficacy of CRT.
Predictors of optimal response to resynchronisation therapy
Although the structural changes in LV architecture and function achieved by reverse remodelling with CRT and the accompanying symptomatic improvement occur in patients refractory to optimal medical treatment, LV reverse remodelling with CRT does not occur in all patients. Between two thirds and three quarters of all patients studied exhibited reverse LV remodelling, but the reasons for this are not entirely clear. There is no current consensus on baseline clinical data that distinguish responders from non‐responders to CRT or predict an optimal response to CRT before device implantation. A number of possible mechanisms have been proposed. Firstly, suboptimal placement of the right ventricular or LV pacing electrodes has been suggested, especially in ischaemic patients with heart failure with scar formation subjacent to the pacing electrode that is constrained by the anatomical distribution of the coronary sinus and its branches. Secondly, LV dyssynchrony may be absent, in spite of prolonged QRS duration. Thirdly, CRT may be delayed until LV dysfunction has become terminal and irreversible. The most likely causes are suboptimal lead placement or lead dislocation and absence of LV dyssynchrony.
New techniques for detection of LV dyssynchrony
In the efforts to identify patients who respond to CRT, new non‐invasive imaging technology has recently become available with which LV dyssynchrony can, not only be detected, but also quantified.24,25 Baseline clinical data, QRS duration, echocardiographic measures of LV size and end‐systolic volume, which are normally powerful predictors of clinical outcome, do not reliably predict response to CRT. What appears to be fundamental for a good response to CRT is the presence of regional LV dyssynchrony, which was initially assessed as QRS prolongation. The time interval or delay between maximal posterior excursion of the septum and peak anterior excursion of the posterior by M‐mode echocardiography of the LV wall has been used to identify dyssynchrony between the free wall and septum. This method is restricted to two opposing walls of the left ventricle. In contrast, Doppler tissue imaging, regional endocardial displacement, strain and strain rate imaging allow assessment of the timing of peak myocardial systolic velocity, peak displacement and/or peak strain, respectively, throughout the entire left ventricle. Dyssynchrony can be detected throughout the left ventricle as regional variation in the relative timing of contraction greater than the normal ranges using cut off points calculated from the mean and standard deviation in normal subjects. These parameters are more robust estimates of LV dyssynchrony than measurement of the QRS duration.
Real‐time three‐dimensional echocardiography using the conventional 16‐segment model of the left ventricle can demonstrate LV dyssynchrony by the regional variation in the timing of acquisition of minimal systolic and maximal diastolic volumes for each of the 16 segments before device implantation. In addition, after resynchronisation therapy, restoration of coordinated contraction occurs with minimal differences in the timing of onset of contraction and peak shortening among the 16‐segment regions.
Conclusions
In summary, CRT has emerged as an effective treatment that improves symptoms, exercise capacity and quality of life in most patients with advanced congestive heart failure who are refractory to optimal medical treatment. The beneficial clinical outcomes associated with CRT are sustained for at least to 18 months. When combined with an internal cardiac defibrillator, the incidence of sudden cardiac death from life‐threatening ventricular arrhythmias, which are a common mode of death in advanced heart failure, are reduced. What remains to be elucidated is the recognition of patients who will have an optimal response to CRT before device implantation, which will be achieved with the new developments in non‐invasive imaging technologies.
Abbreviations
CARE‐HF - Cardiac Resynchronisation in Heart Failure
CRT - cardiac resynchronisation therapy
LV - left ventricular
LVEF - left ventricular ejection fraction
MIRACLE - Multicenter InSync Randomised Clinical Evaluation
NYHA - New York Heart Association
Footnotes
Competing interests: None.
References
- 1.American Heart Association 2001 heart and stroke statistical update. Dallas, TX: American Heart Association, 2000
- 2.Vasan R S, Benjamin E J, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 1995261565–1574. [DOI] [PubMed] [Google Scholar]
- 3.Nohria A, Lewis E, Stevenson L W. Medical management of advanced heart failure. JAMA 2002287628–640. [DOI] [PubMed] [Google Scholar]
- 4.Hunt S A, Baker D W, Chin M H.et al ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure). Circulation 20011042996–3007. [DOI] [PubMed] [Google Scholar]
- 5.Stellbrink C, Breithardt O, Franke A.et al Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol 2001381957–1965. [DOI] [PubMed] [Google Scholar]
- 6.Abraham W T, Fisher W G, Smith A L.et al Cardiac resynchronization in chronic heart failure. N Engl J Med 20023461845–1853. [DOI] [PubMed] [Google Scholar]
- 7.Cazeau S, Leclercq C, Lavergne T.et al Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001344873–880. [DOI] [PubMed] [Google Scholar]
- 8.Shamin W, Francis D P, Yousufuddin M.et al Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol 199931171–178. [DOI] [PubMed] [Google Scholar]
- 9.St John Sutton M, Lee D, Rouleau JL, et al. Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation 20031072577–2582. [DOI] [PubMed] [Google Scholar]
- 10.Bristow M R, Saxon L A, Boehmer J.et al Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 20043502140–2150. [DOI] [PubMed] [Google Scholar]
- 11.Moss A J, Zareba W, Hall W J, for the Multicenter Automatic Defibrillator Implantation Trial II Investigators et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002346877–883. [DOI] [PubMed] [Google Scholar]
- 12.Messenger J, Kruger K, Hilpisch K E.et al Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 20031071985–1990. [DOI] [PubMed] [Google Scholar]
- 13.Cleland J G F, Daubert J C, Erdmann E.et al The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 20053521539–1549. [DOI] [PubMed] [Google Scholar]
- 14.Linde C, Leclercq C, Rex S.et al Long‐term benefits of biventricular pacing in congestive heart failure: results from the Multisite Stimulation in Cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 200240111–118. [DOI] [PubMed] [Google Scholar]
- 15.Molhoek S G, Bax J J, van Erven L.et al Comparison of benefits from cardiac resynchronization therapy in patients with ischemic cardiomyopathy versus idiopathic dilated cardiomyopathy. Am J Cardiol 200493860–863. [DOI] [PubMed] [Google Scholar]
- 16.Chuang M L, Hibberd M G, Salton C J.et al Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two‐ and three‐dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardio 200035477–484. [DOI] [PubMed] [Google Scholar]
- 17.Burns R J, Gibbons R J, Yi Q.et al The relationship of left ventricular ejection fraction, end systolic volume index and infarct size to six‐month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 20023930–36. [DOI] [PubMed] [Google Scholar]
- 18.St John Sutton M, Pfeffer M A, Plappert T.et al Quantitative two‐dimensional echocardiographic measurments are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 19948968–75. [DOI] [PubMed] [Google Scholar]
- 19.Abraham W T, Leon A R, Young J B. Benefits of cardiac resynchronization therapy sustained for 18 months: results from the MIRACLE program [abstract]. Circulation 2003108(Suppl IV)IV629 [Google Scholar]
- 20.Young J B, Abraham W T, Smith A L.et al Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) trial investigators. Combined cardiac resynchronization and implantable cardioverter defibrillation in advanced chronic heart failure. The MIRACLE ICD trial. JAMA 20032892865–3694. [DOI] [PubMed] [Google Scholar]
- 21.St John Sutton M, Plappert T, Hilpisch K E.et al Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology. Circulation 2006113266–272. [DOI] [PubMed] [Google Scholar]
- 22.Yu C M, Chau E, Sanderson J E.et al Tissue Doppler echoardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002105438–445. [DOI] [PubMed] [Google Scholar]
- 23.Kanzaki H, Bazaz R, Schwartzman D.et al A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol 2004441619–1625. [DOI] [PubMed] [Google Scholar]
- 24.Bax J J, Abraham T, Barold S S.et al Cardiac resynchronization therapy. Part 1. Issues before device implantation. J Am Coll Cardiol 2005452153–2167. [DOI] [PubMed] [Google Scholar]
- 25.Bax J J, Abraham T, Barold S S.et al Cardiac resynchronization therapy. Part 2. Issues during and after device implantation and unresolved questions. J Am Coll Cardiol 2005462168–2182. [DOI] [PubMed] [Google Scholar]