Abstract
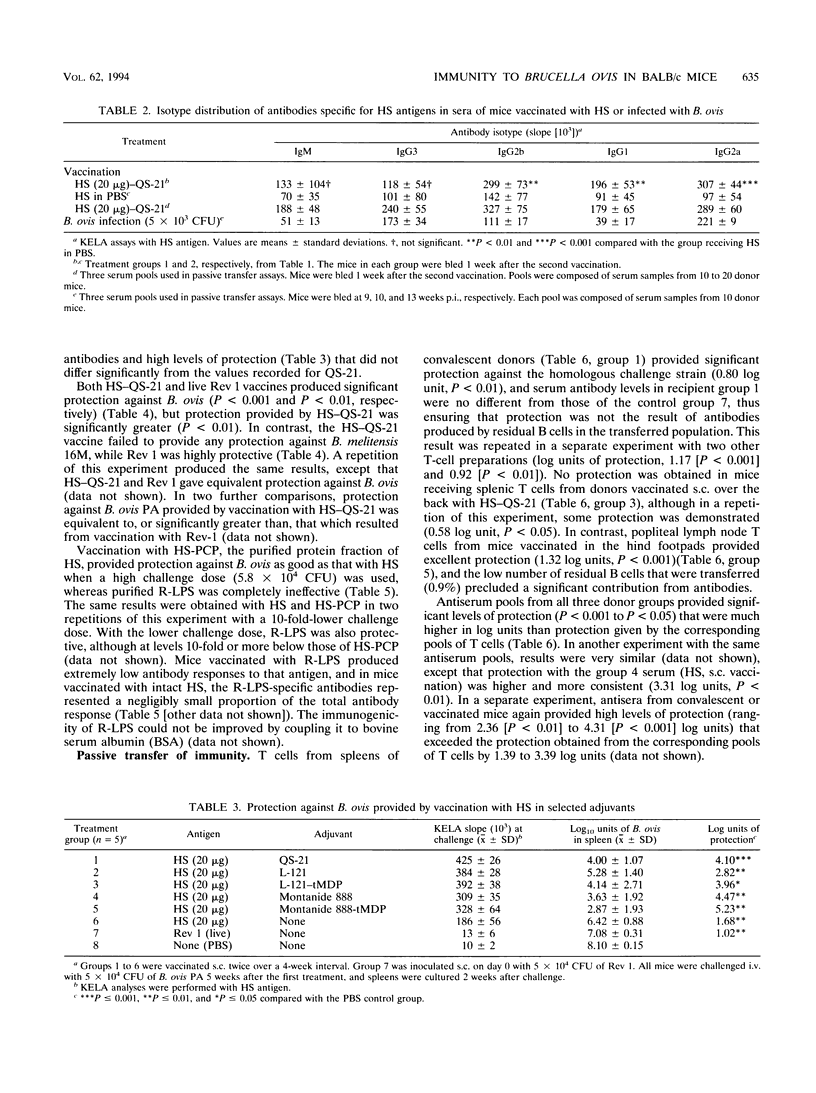
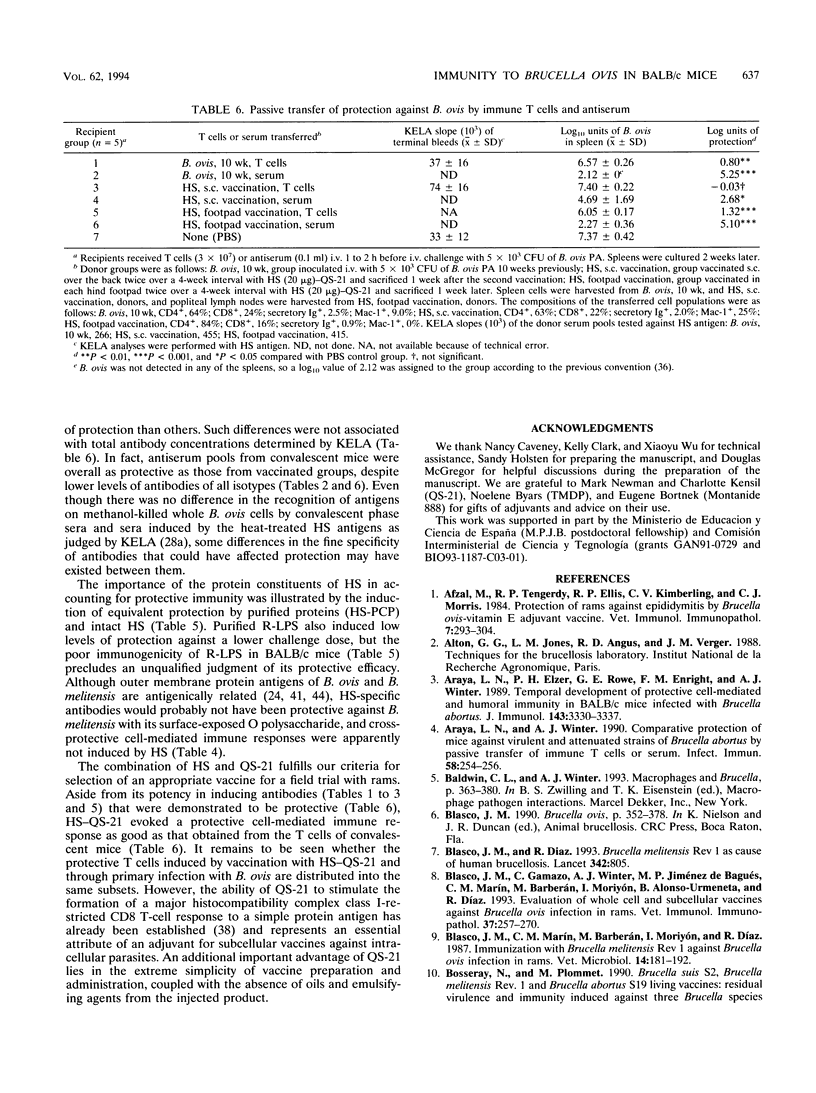
Experiments were performed with BALB/c mice to elucidate the roles of humoral and cell-mediated immune responses in the acquisition of protective immunity to Brucella ovis and to compare infection immunity with immunity developed through vaccination with a hot saline extract (HS) of B. ovis. Mice convalescing from a primary infection with B. ovis displayed a high level of resistance to reinfection, as evidenced by splenic bacterial counts decreased over 10,000-fold from control groups at 2 weeks after challenge. Passive transfer assays revealed that protection was mediated by both T lymphocytes and antibodies but that antibodies had a substantially greater role on the basis of log units of protection that were transferred. Antibodies specific for HS proteins in sera from convalescent mice were predominantly of the immunoglobulin G 2a and 3 isotypes. Vaccination with HS conferred good protection against B. ovis, but protection was greatly enhanced by the incorporation of QS-21 or other adjuvants. Protection provided by the HS vaccine resulted largely from immune responses to its protein moieties. A critical evaluation of the protective efficacy of the rough lipopolysaccharide component of HS was precluded by its poor immunogenicity in BALB/c mice. HS-QS-21 afforded protection against challenge infection with B. ovis as good as that which developed after a primary infection and as good as or better than that provided by attenuated Brucella melitensis vaccine strain Rev 1. Passive transfer experiments confirmed that the magnitudes of both humoral and cell-mediated forms of protective immunity were equivalent in mice vaccinated with HS-QS-21 and those recovering from a primary infection. Protective immunity to B. ovis in mice therefore resembled that to Brucella abortus, except that the relative roles of humoral and cell-mediated immunity, rather than being equivalent, were shifted toward a greater role for antibodies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzal M., Tengerdy R. P., Ellis R. P., Kimberling C. V., Morris C. J. Protection of rams against epididymitis by a Brucella ovis-vitamin E adjuvant vaccine. Vet Immunol Immunopathol. 1984 Oct;7(3-4):293–304. doi: 10.1016/0165-2427(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Araya L. N., Elzer P. H., Rowe G. E., Enright F. M., Winter A. J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989 Nov 15;143(10):3330–3337. [PubMed] [Google Scholar]
- Araya L. N., Winter A. J. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect Immun. 1990 Jan;58(1):254–256. doi: 10.1128/iai.58.1.254-256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco J. M., Díaz R. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. Lancet. 1993 Sep 25;342(8874):805–805. doi: 10.1016/0140-6736(93)91571-3. [DOI] [PubMed] [Google Scholar]
- Blasco J. M., Gamazo C., Winter A. J., Jiménez de Bagüs M. P., Marín C., Barberán M., Moriyón I., Alonso-Urmeneta B., Díaz R. Evaluation of whole cell and subcellular vaccines against Brucella ovis in rams. Vet Immunol Immunopathol. 1993 Aug;37(3-4):257–270. doi: 10.1016/0165-2427(93)90198-d. [DOI] [PubMed] [Google Scholar]
- Bulgin M. S. Epididymitis in rams and lambs. Vet Clin North Am Food Anim Pract. 1990 Nov;6(3):683–690. doi: 10.1016/s0749-0720(15)30840-9. [DOI] [PubMed] [Google Scholar]
- Burgess G. W. Ovine contagious epididymitis: a review. Vet Microbiol. 1982 Dec;7(6):551–575. doi: 10.1016/0378-1135(82)90049-9. [DOI] [PubMed] [Google Scholar]
- Carpenter T. E., Berry S. L., Glenn J. S. Economics of Brucella ovis control in sheep: computerized decision-tree analysis. J Am Vet Med Assoc. 1987 Apr 15;190(8):983–987. [PubMed] [Google Scholar]
- Claxton P. D. Brucella ovis vaccination of rams. A comparison of two commercial vaccines and two methods of vaccination. Aust Vet J. 1968 Feb;44(2):48–54. doi: 10.1111/j.1751-0813.1968.tb04953.x. [DOI] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of Brucella ovis and Brucella melitensis. J Bacteriol. 1967 Apr;93(4):1262–1268. doi: 10.1128/jb.93.4.1262-1268.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterbank R., Pardon P., Marly J. Efficacy of Brucella melitensis Rev. 1 vaccine against Brucella ovis infection in rams. Ann Rech Vet. 1982;13(2):185–190. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gamazo C., Winter A. J., Moriyón I., Riezu-Boj J. I., Blasco J. M., Díaz R. Comparative analyses of proteins extracted by hot saline or released spontaneously into outer membrane blebs from field strains of Brucella ovis and Brucella melitensis. Infect Immun. 1989 May;57(5):1419–1426. doi: 10.1128/iai.57.5.1419-1426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carrillo C. Protection of rams against Brucella ovis infection by Brucella melitensis Rev. 1 vaccine. Zentralbl Veterinarmed B. 1981;28(6):425–431. doi: 10.1111/j.1439-0450.1981.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Gradwell D. V., Van Zyl F. E. Effectivity of Rev 1 vaccine in rams against brucella ovis infection. J S Afr Vet Assoc. 1975 Dec;46(4):349–351. [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. L. Experimental Brucella ovis infection in ewes. 1. Breeding performance of infected ewes. Aust Vet J. 1972 Jan;48(1):12–17. doi: 10.1111/j.1751-0813.1972.tb02200.x. [DOI] [PubMed] [Google Scholar]
- Jiménez de Bagüs M. P., Marín C. M., Barberán M., Blasco J. M. Evaluation of vaccines and of antigen therapy in a mouse model for Brucella ovis. Vaccine. 1993;11(1):61–66. doi: 10.1016/0264-410x(93)90340-4. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Winter A. J. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect Immun. 1992 Jul;60(7):3011–3014. doi: 10.1128/iai.60.7.3011-3014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensil C. R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991 Jan 15;146(2):431–437. [PubMed] [Google Scholar]
- Libal M. C., Kirkbride C. A. Brucella ovis-induced abortion in ewes. J Am Vet Med Assoc. 1983 Sep 1;183(5):553–554. [PubMed] [Google Scholar]
- Marín C. M., Barberán M., Jiménez de Bagués M. P., Blasco J. M. Comparison of subcutaneous and conjunctival routes of Rev 1 vaccination for the prophylaxis of Brucella ovis infection in rams. Res Vet Sci. 1990 Mar;48(2):209–215. [PubMed] [Google Scholar]
- McGowan B., Harrold D. R. Epididymitis in rams: studies on vaccine efficacy. Cornell Vet. 1979 Jan;69(1):73–76. [PubMed] [Google Scholar]
- Meinershagen W. A., Frank F. W., Waldhalm D. G. Brucella ovis as a cause of abortion in ewes. Am J Vet Res. 1974 May;35(5):723–724. [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Jones L. M., Berman D. T. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. 1984 Mar;43(3):779–782. doi: 10.1128/iai.43.3.779-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Wu J. Y., Gardner B. H., Munroe K. J., Leombruno D., Recchia J., Kensil C. R., Coughlin R. T. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T lymphocyte responses. J Immunol. 1992 Apr 15;148(8):2357–2362. [PubMed] [Google Scholar]
- Plant J. W., Eamens G. J., Seaman J. T. Serological, bacteriological and pathological changes in rams following different routes of exposure to Brucella ovis. Aust Vet J. 1986 Dec;63(12):409–412. doi: 10.1111/j.1751-0813.1986.tb15919.x. [DOI] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Gamazo C., Díaz R. Antibody response to Brucella ovis outer membrane proteins in ovine brucellosis. Infect Immun. 1990 Feb;58(2):489–494. doi: 10.1128/iai.58.2.489-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Marín C. M., Diaz R. Comparison of lipopolysaccharide and outer membrane protein-lipopolysaccharide extracts in an enzyme-linked immunosorbent assay for the diagnosis of Brucella ovis infection. J Clin Microbiol. 1986 May;23(5):938–942. doi: 10.1128/jcm.23.5.938-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris D. R. The persistence of antibodies against Brucella ovis and Brucella abortus in rams following vaccination: a field study. N Z Vet J. 1967 Jun;15(6):94–98. doi: 10.1080/00480169.1967.33702. [DOI] [PubMed] [Google Scholar]
- Roberts A. D., Ordway D. J., Orme I. M. Listeria monocytogenes infection in beta 2 microglobulin-deficient mice. Infect Immun. 1993 Mar;61(3):1113–1116. doi: 10.1128/iai.61.3.1113-1116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Slack J., Der-Balian G. P., Nahm M., Davie J. M. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980 Apr 1;151(4):853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., McIntyre T. M., Mandler R., Pecanha L. M., Finkelman F. D., Lees A., Mond J. J. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992 May 1;175(5):1367–1371. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Swift B. L., Maki L. R. Immunologic studies on three ram epididymitis bacterins. Cornell Vet. 1968 Oct;48(4):659–665. [PubMed] [Google Scholar]
- Tsang V. C., Wilson B. C., Maddison S. E. Kinetic studies of a quantitative single-tube enzyme-linked immunosorbent assay. Clin Chem. 1980 Aug;26(9):1255–1260. [PubMed] [Google Scholar]
- Winter A. J., Duncan J. R., Santisteban C. G., Douglas J. T., Adams L. G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989 Nov;57(11):3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Rowe G. E., Duncan J. R., Eis M. J., Widom J., Ganem B., Morein B. Effectiveness of natural and synthetic complexes of porin and O polysaccharide as vaccines against Brucella abortus in mice. Infect Immun. 1988 Nov;56(11):2808–2817. doi: 10.1128/iai.56.11.2808-2817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


