Abstract
Objective
To analyse the long‐term course of QRS duration after pulmonary valve replacement in patients with a previous correction for tetralogy of Fallot.
Setting
Tertiary referral centres.
Methods
In a retrospective study, 99 adult patients with tetralogy of Fallot, who had undergone a first pulmonary valve replacement late after initial total correction, were identified from the CONCOR (CONgenital CORvitia) registry. Computer‐generated QRS durations were obtained from 12‐lead electrocardiogram ECG reports in the medical records. A mixed linear regression model was used to analyse the course of QRS duration over time and to identify risk factors for increase in QRS duration over time. Composite end point was created from sudden cardiac death, ventricular tachycardia or implantable cardioverter–defibrillator discharge.
Results
In total, 99 patients (57% men, mean (SD) age at pulmonary valve replacement 29 (11) years) with a median follow‐up of 4.9 (0.1–16) years were analysed. In patients with preoperative QRS <120 ms, surgery caused no significant change in QRS duration (increase 1.3 (7.9) ms; p = 0.65), and after surgery, QRS duration remained stable over time (increase 0.0064 (0.059) ms/year; p = 0.98). By contrast, in patients with a preoperative QRS of 150–180 ms or QRS ⩾180 ms, surgery resulted in QRS shortening (mean decrease 9.9 (SE 4.3) ms, p = 0.021, and 12.2 (SE 2) ms; p<0.001, respectively). During follow‐up, a QRS widening 1.1(1.3) ms/year (p<0.001) in both groups was observed. In patients with a preoperative QRS ⩾180 ms, no significant difference was observed in the number of patients reaching the composite end point compared with patients with a preoperative QRS of 150–180 ms (25% vs 7%; p = 0.08). However, the former more often reached QRS ⩾180 ms again after surgery compared with the latter (53% vs13%; p = 0.02, respectively). None of the patients with a preoperative QRS ⩾180 ms died during follow‐up.
Conclusion
In our study, we observed a decrease in QRS duration directly after surgery, followed by a steady increase, in patients with a preoperative QRS >150 ms. The beneficial effect of pulmonary valve replacement on QRS duration was transient. The risk of developing ventricular arrhythmias after surgery was substantial when preoperative QRS was⩾180 ms, but mortality remained low.
In timing pulmonary valve replacement late after initial correction for tetralogy of Fallot, the benefits of pulmonary valve replacement have to be weighed against the need for future pulmonary valve replacements for homograft failure. Besides improvement in right ventricle dimensions,1 pulmonary valve replacement seems to decrease the incidence of pre‐existing ventricular tachyarrhythmias (in conjunction with intraoperative cryoablation) and to stabilise QRS duration.2 However, in a recent study by van Huysduynen et al,3 a reduction in QRS duration was observed in conjunction with the decrease in end‐diastolic volume. It remains uncertain whether pulmonary valve replacement leads to long‐term reduction or stabilisation of the QRS complex, as the previous studies only analysed ECGs at one time interval after surgery. Therefore, we aimed to study the effect of pulmonary valve replacement on QRS duration over time in adult patients with corrected tetralogy of Fallot.
Methods
In a retrospective study, 158 adult patients with a diagnosis of tetralogy of Fallot, who received a pulmonary valve replacement late after initial total correction, were identified through the CONCOR (CONgenital CORvitia) registry4 in The Netherlands.5 Patients with a homograft used for initial correction with multiple pulmonary valve replacements and patients without electronically stored ECGs were excluded from the analysis, leaving 99 patients for study.
Data on patient and surgical characteristics, echocardiography, magnetic resonance imaging, and ECG before pulmonary valve replacement (n = 83) and all available ECGs (n = 497) after pulmonary valve replacements were acquired between 1 week and 15 years after surgery. Computer‐generated QRS durations were acquired from 12‐lead ECG reports, present in the medical records. A composite end point for arrhythmic events included sudden cardiac death, ventricular tachycardia or appropriate implantable cardioverter–defibrillator (ICD) discharge. Appropriate shock was defined as an ICD shock, delivered in response to ventricular arrhythmia.
Data are described as numbers with frequency, median with range and mean with standard deviation (SD). Multivariable linear regression analysis was used to analyse predictors for pre‐operative QRS duration. To analyse the effect of preoperative QRS duration on QRS increase after surgery, the preoperative QRS durations were divided into four groups (<120, 120–150, 150–180 and ⩾180 ms).6,7 Repeated measurements of QRS durations were modelled with linear mixed‐effects regression models. The effect of surgery on QRS duration was analysed in a model with time after first ECG and a dichotomous variable, indicating whether the ECG was performed before or after surgery, as covariates. The effect of surgery was presented as mean with standard error (SE). In a separate model, the increase in QRS duration was analysed with time after pulmonary valve replacement of QRS measurement as a covariate. Differences between the pre‐operative groups 150–180 and ⩾180 ms were sought with Fisher's exact test and analysis of variance with post hoc Bonferroni correction.
Results
Pulmonary valve replacement
Table 1 lists the patient and surgical characteristics of pulmonary valve replacement.
Table 1 Patient characteristics.
| All patients (n = 99) | |
|---|---|
| Patient characteristics | |
| Male/female | 56/43 |
| Previous shunt procedure, n (%) | 48 (49) |
| Mean SD age at previous shunt procedure (years) | 2.3 (2.4) |
| Median range age at initial correction (years) | 4.9 (0.3–30) |
| Type of RVOT reconstruction at initial correction (n = 93) | |
| Myectomy/valvulotomy, n (%) | 9/93 (10) |
| RVOT patch, n (%) | 13/93 (14) |
| Transannular patch, n (%) | 65/93 (70) |
| Valved conduit*, n (%) | 6/93 (7) |
| Preoperative CMR (n = 49) and echocardiographic data (n = 82) | |
| Mean (SD) pulmonary regurgitation (grades 1–4) | 3.2(0.92) |
| Mean (SD) pulmonary stenosis (mm Hg) | 22 (24) |
| Mean (SD) RV end‐diastolic volume (ml/m2) | 168 (37) |
| Mean (SD) RV end‐systolic volume (ml/m2) | 101 (32) |
| Mean (SD) RV ejection fraction (%) | 41 (9) |
| Surgical parameters | |
| Concomitant RV aneurysm/patch resection, n (%) | 46 (47) |
| Concomitant branch pulmonary artery angioplasty, n (%) | 22 (22) |
| Proximal extension of the homograft with patch, n (%) | 12 (12) |
| Concomitant cryoablation, n (%) | 1 (1) |
CMR, cardiac magnetic resonance; RV, right ventricle; RVOT, right ventricular outflow tract; VSD, ventricular septal defect.
*No homograft was used during initial correction.
Two patients died late after pulmonary valve replacement, resulting in a death rate of 0.0039 deaths/year. One patient died 2.4 years after surgery from excessive ventricular arrhythmias during hospitalisation for terminal heart failure with a QRS duration of 170 ms. The other patient died suddenly 17 months after surgery, without symptoms of right ventricular failure, and a QRS duration of 156 ms at the latest follow‐up.
Preoperative predictors for QRS duration
The mean (SD) QRS duration before surgery was 150 (30) ms. No difference in QRS duration was observed in patients with or without transannular patching at initial correction (150 (31) vs 154 (29) ms). However, in multivariable analysis, the only significant predictors for a greater preoperative QRS duration included higher right ventricular end‐diastolic volume (β = 0.52; p<0.001) and male sex (β = 0.33; p = 0.01).
Effect of pulmonary valve replacement on QRS duration
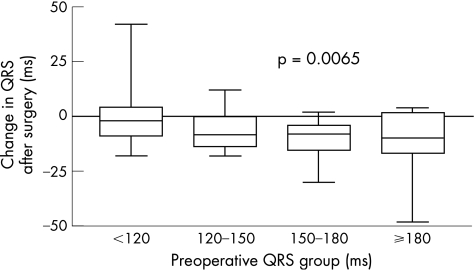
The median time (range) after surgery until the first QRS measurement was 1 month (1 week to 8.8 years). The mean (SE) decrease in QRS duration directly after surgery was 7.4 (0.9) ms (p<0.001). Patients with a longer preoperative QRS duration showed a greater decrease in QRS duration after surgery (fig 1). We observed a significant QRS decrease in patients with a preoperative QRS of 150–180 ms (decrease 9.9 (SE 4.3) ms; p = 0.021), and a preoperative QRS ⩾180 ms (decrease 12.3 (SE 2) ms; p<0.001) after surgery.
Figure 1 Change in QRS duration after surgery is plotted against preoperative QRS duration. Patients with longer QRS duration preoperatively showed a larger decrease in QRS duration (p = 0.0065).
QRS duration during follow‐up after pulmonary valve surgery
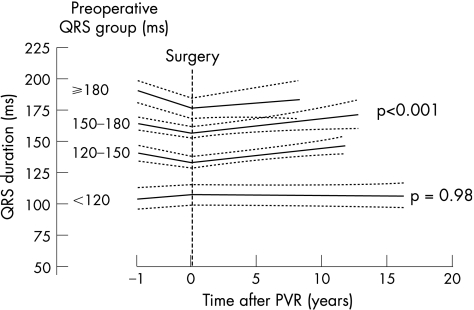
During a median (range) follow‐up period of 4.9 (0.1–16) years, the average (SD) QRS increase was 1.0 (1.3) ms/year after surgery. When QRS was ⩾120 ms before surgery, the mean increase in QRS was 1.1 (1.3) ms/year (p<0.001; fig 2). No significant difference in QRS increase was observed between patients with preoperative QRS ⩾180, 150–180 or 120–150 ms. No significant increase was observed in patients with a preoperative QRS <120 ms 0.0064 (0.059) ms/year, p = 0.98).
Figure 2 Change in QRS duration after pulmonary valve replacement is plotted with the 95% CIs, as analysed by mixed linear regression modelling. Patients were divided into four groups according to their preoperative QRS groups. When preoperative QRS was >120 ms, the average (SD) increase for all groups was 1.1 (1.3) ms/year (p<0.001) after surgery, whereas no increase was observed in patients with a preoperative QRS <120 ms (p = 0.98). A significant decrease was observed in patients with a preoperative QRS of 150–180 ms (mean decrease 9.9 (SE 4.3) ms; p = 0.021), and a preoperative QRS ⩾180 ms (mean decrease 12.2 (SE 2) ms; p<0.001) after surgery.
Arrhythmias after surgery
Before surgery, 3/99 (3%) patients were treated with an ICD. After surgery, in 4/99 (4%) patients an ICD was implanted at a median range of 1.6 years (1 week to 5.5 years). Table 2 lists the clinical events after surgery according to the preoperative QRS value.
Table 2 Clinical events after surgery.
| Preoperative QRS (ms) | |||||
|---|---|---|---|---|---|
| Missing preoperative QRS (n = 16) | <120 (n = 18) | 120–150 (n = 19) | 150–180 (n = 30) | ⩾180 (n = 16) | |
| Composite end point ventricular arrhythmia (%) | 0 | 6 | 5 | 7 | 25 |
| Sudden cardiac death (%) | 0 | 0 | 0 | 3 | 0 |
| (Non) sustained VT/appropriate ICD discharge (%) | 0 | 6 | 5 | 3 | 25† |
| Mean (SD) duration to QRS⩾180 ms (years) | NA | 68 (4.5) | 42 (4.4) | 23 (4.3)* | 2 (2)*† |
| Patients reaching >180 ms after surgery (%) | 6 | 0 | 0 | 13 | 56† |
| ICD implantation after surgery (%) | 0 | 0 | 0 | 3 | 19 |
| Homograft explantation after surgery (%) | 19 | 11 | 5 | 7 | 6 |
ICD, implantable cardioverter‐defibrillator; VT, ventricular tachycardia.
*Calculated from mixed linear regression analysis (fig 2).
†Significant difference between patients with pre‐operative QRS 150–180 (p<0.05).
Discussion
In this study on the long‐term course of QRS duration after pulmonary valve replacement, we confirmed that QRS duration mainly relates to right ventricular volume. QRS duration decreases initially after surgery in patients with a preoperative QRS >150 ms. In contrast to previous findings, QRS duration in these patients did not remain stable over the following years, but slowly increased by 1.09 ms/year. The risk of developing QRS ⩾180 ms, and ventricular arrhythmias was substantial if preoperative QRS was ⩾180 ms. However, the mortality remained low in these patients.
Sudden cardiac death
Gatzoulis et al. showed6 that patients with a QRS ⩾180 ms have a 42‐fold increased risk of developing sustained ventricular tachycardia and a 2.2‐fold increased risk of sudden cardiac death during a 10‐year follow‐up. However, this study and others have shown that QRS duration may be a proxy for right ventricle volume load.3 Furthermore, a multicentre retrospective study showed that although QRS duration is a univariate predictor of mortality, other variables dominate using multivariate Cox analysis.8 Although QRS duration is an easy and useful predictor of outcome, it is part of a complicated picture and may only be a proxy.
The death rate reported in our study (0.0039 deaths/patient‐year) was slightly higher than other larger follow‐up studies on patients with corrected tetralogy of Fallot (0.0021–0.0034 deaths/patient‐year). However, the population of patients with corrected tetralogy of Fallot in our report was a selection of patients presenting with severe pulmonary regurgitation and right ventricle dilatation, which required pulmonary valve replacement.
QRS duration after pulmonary valve replacement
The study of van Huysduynen et al3 was the first to report a reduction in QRS duration, 14.3 months after pulmonary valve replacement, in conjunction with improvement in right ventricle end‐diastolic volume. In another study by Doughan et al7 on 21 patients undergoing pulmonary valve replacement, these findings were confirmed, mainly in patients with a preoperative QRS >155 ms. In our study, the decrease after surgery was significant in patients with a preoperative QRS >150 ms. In our study, this change was already apparent in the first month after pulmonary valve replacement, followed by a linear increase during the following years. However, van Straten et al9 have shown that postoperative right ventricular end‐diastolic volumes were larger in the presence of direct postoperative pulmonary regurgitation. Therefore, a decrease in QRS duration after surgery will become less likely if a patient develops recurrent postoperative pulmonary regurgitation.
Therrien et al2 showed no significant decrease in QRS duration after surgery at a mean follow‐up of 4 years after surgery. Our study shows that the decrease in QRS duration is transient and eventually QRS duration returns to its preoperative value. This gradual increase in QRS duration is probably accompanied by an increase in right ventricle volumes with increasing homograft dysfunction.
In previous studies, it has been shown that QRS duration increased by a mean of 2 ms/year in patients without pulmonary valve replacement.2,6,10 Therefore, the growth observed in our operated patient population (after pulmonary valve replacement) was still lower than these studies. The lower increase of QRS duration in our study population is probably the result of the lower degree of pulmonary regurgitation after surgery. However, recurrent pulmonary regurgitation after pulmonary valve replacement due to homograft failure is not uncommon,11 with a subsequent effect on right ventricle dilatation and QRS duration.
Optimal timing of pulmonary valve replacement
After pulmonary valve replacement, the right ventricle probably becomes less vulnerable to arrhythmias, owing to the decrease in QRS duration. In timing pulmonary valve replacement, it may be helpful if a preoperative threshold value can be observed, above which right ventricular mechanical and electrical reverse remodelling does not take place any longer and, there is no reduction in the risk of malignant arrhythmias. In our study, we observed that there was no real cut‐off value and that the longer the preoperative QRS duration, the greater the reduction after surgery. Although performing valve surgery before QRS duration reaches 180 ms did not result in a marked lower combined end point for sudden cardiac death and arrhythmia in our study, it did keep the QRS duration under 180 ms in most patients (87%) and the change of ventricular tachycardia or ICD discharge low (7%). In our opinion, pulmonary valve replacement before QRS reaches 180 ms should be recommended. An additional argument for performing pulmonary valve replacement before QRS reaches 180 ms is that in the absence of severe pulmonary regurgitation after surgery, ventricular tachycardias may be better tolerated haemodynamically.
Limitation
The results of this study are limited by the retrospective nature of the data collection and reliance on medical records over a relatively long period of time. QRS duration was not manually measured, but was obtained from computer‐generated reports in the medical records.
Conclusion
The beneficial effect of pulmonary valve replacement on QRS duration was transient after pulmonary valve replacement. Although the mortality was low, the risk of developing ventricular arrhythmias after surgery was substantial when preoperative QRS was ⩾180 ms.
Abbreviations
ICD - implantable cardioverter‐defibrillator
Footnotes
Conflict of interests: None.
References
- 1.Vliegen H W, van Straten A, de Roos A.et al Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation 20021061703–1707. [DOI] [PubMed] [Google Scholar]
- 2.Therrien J, Siu S C, Harris L.et al Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation 20011032489–2494. [DOI] [PubMed] [Google Scholar]
- 3.van Huysduynen B H, van Straten A, Swenne C A.et al Reduction of QRS duration after pulmonary valve replacement in adult Fallot patients is related to reduction of right ventricular volume. Eur Heart J 200526928–932. [DOI] [PubMed] [Google Scholar]
- 4.Van der Velde E T, Vriend J W J, Mannens M M A M.et al CONCOR, an initiative towards a national registry and DNA‐bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol 200520549–557. [DOI] [PubMed] [Google Scholar]
- 5.Oosterhof T, Meijboom F J, Vliegen H W.et al Long‐term follow up of homograft function after pulmonary valve replacement in patients with tetralogy of Fallot. Eur Heart J 2006271478–1484. [DOI] [PubMed] [Google Scholar]
- 6.Gatzoulis M A, Balaji S, Webber S A.et al Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000356975–981. [DOI] [PubMed] [Google Scholar]
- 7.Doughan A R, McConnell M E, Lyle T A.et al Effects of pulmonary valve replacement on QRS duration and right ventricular cavity size late after repair of right ventricular outflow tract obstruction. Am J Cardiol 2005951511–1514. [DOI] [PubMed] [Google Scholar]
- 8.Khairy P, Landzberg M J, Gatzoulis M A.et al Value of programmed ventricular stimulation after tetralogy of Fallot repair: a multicenter study. Circulation 20041091994–2000. [DOI] [PubMed] [Google Scholar]
- 9.van Straten A, Vliegen H W, Hazekamp M G.et al Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology 2004233824–829. [DOI] [PubMed] [Google Scholar]
- 10.Neffke J G, Tulevski I I, van der Wall E E.et al ECG determinants in adult patients with chronic right ventricular pressure overload caused by congenital heart disease: relation with plasma neurohormones and MRI parameters. Heart 200288266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J W, Ruzmetov M, Rodefeld M D.et al Right ventricular outflow tract reconstruction with an allograft conduit in non‐Ross patients: risk factors for allograft dysfunction and failure. Ann Thorac Surg 200580655–664. [DOI] [PubMed] [Google Scholar]