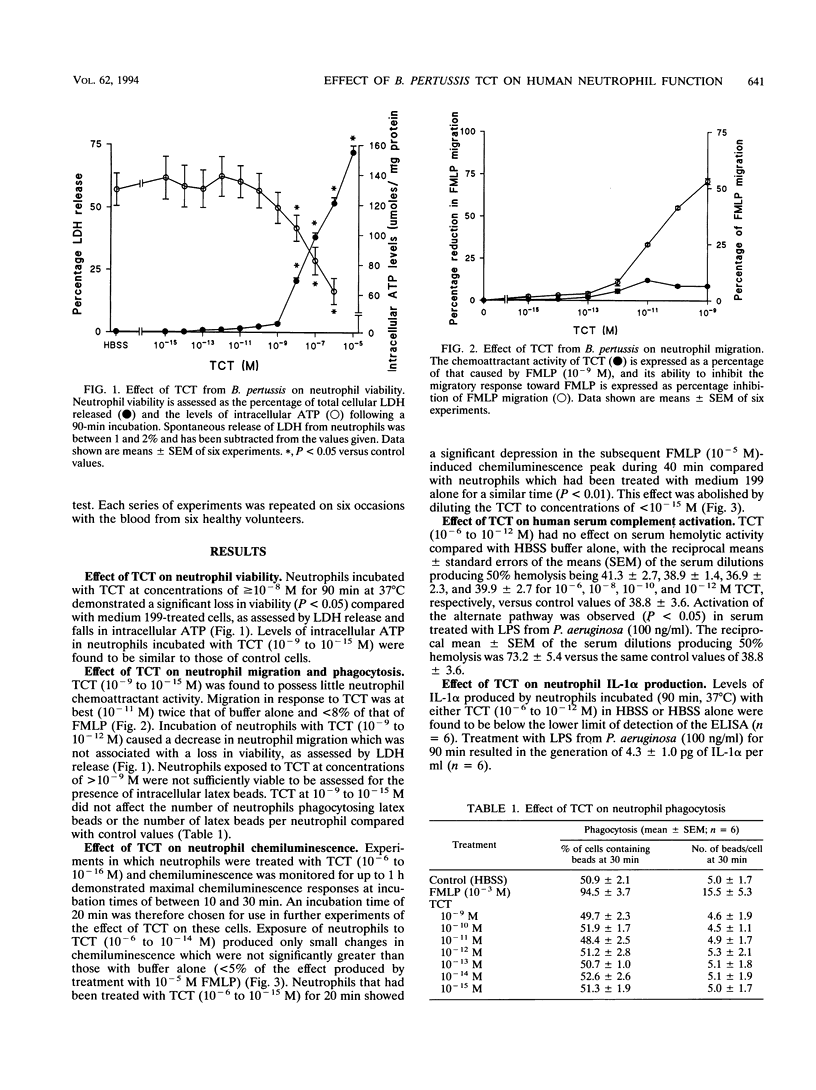
Abstract
The infiltration of neutrophils which phagocytose and kill microorganisms is an important defense mechanism against infections of the airways. Bordetella pertussis is a human respiratory pathogen which colonizes ciliated epithelium, causing whooping cough. We have investigated the effects of the peptidoglycan fragment tracheal cytotoxin (TCT) of B. pertussis on human neutrophil function in vitro. TCT (10(-6) to 10(-8) M) was toxic for human neutrophils, as measured by lactate dehydrogenase release and levels of intracellular ATP. TCT (10(-9) to 10(-15) M) did not stimulate neutrophil migration or chemiluminescence and did not affect neutrophil phagocytosis. Incubation of neutrophils for 20 min with TCT (10(-9) to 10(-11) M) significantly inhibited (P < 0.05) their subsequent migration toward the chemotactic factor N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP; 10(-9) M). Incubation of neutrophils for 20 min with TCT (10(-9) to 10(-15) M) significantly inhibited (P < 0.05) chemiluminescence stimulated by FMLP (10(-5) M). TCT (10(-6) to 10(-12) M) did not stimulate interleukin-1 alpha production by neutrophils or serum complement activation by the alternate pathway. We conclude that TCT at concentrations of < 10(-8) M affects important neutrophil functions and at higher concentrations is toxic. TCT may therefore contribute to the survival of B. pertussis within the airways in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Yoder M., Bonsib S., Schmidt M., Ho P., Jersild R., Baehner R. L. Effects of a chemotactic factor, N-formylmethionyl peptide, on adherence, superoxide anion generation, phagocytosis, and microtubule assembly of human polymorphonuclear leukocytes. J Lab Clin Med. 1979 Mar;93(3):506–514. [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cookson B. T., Cho H. L., Herwaldt L. A., Goldman W. E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989 Jul;57(7):2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundell D. R., Taylor G. W., Kanthakumar K., Wilks M., Tabaqchali S., Dorey E., Devalia J. L., Roberts D. E., Davies R. J., Wilson R. Inhibition of human neutrophil migration in vitro by low-molecular-mass products of nontypeable Haemophilus influenzae. Infect Immun. 1993 Jun;61(6):2419–2424. doi: 10.1128/iai.61.6.2419-2424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie D. C., Peters A. M., Garbett N. D., George P., Strickland B., Lavender J. P., Cole P. J. Indium-111 labelled granulocyte scanning to detect inflammation in the lungs of patients with chronic sputum expectoration. Thorax. 1990 Jul;45(7):541–544. doi: 10.1136/thx.45.7.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian H. Human mononuclear cells exposed to staphylococci rapidly produce an inhibitor of neutrophil chemotaxis. J Infect Dis. 1985 Jul;152(1):24–32. doi: 10.1093/infdis/152.1.24. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontán P. A., Amura C. R., García V. E., Cerquetti M. C., Sordelli D. O. Preliminary characterization of Pseudomonas aeruginosa peptide chemotactins for polymorphonuclear leukocytes. Infect Immun. 1992 Jun;60(6):2465–2469. doi: 10.1128/iai.60.6.2465-2469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett N. D., Matharu G. S., Cole P. J. Defective opsonization of Haemophilus influenzae by sera of elderly patients. Clin Exp Immunol. 1989 Apr;76(1):73–75. [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss L. N., Moser S. A., Unanue E. R., Goldman W. E. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect Immun. 1993 Aug;61(8):3123–3128. doi: 10.1128/iai.61.8.3123-3128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger K. E., Kharazmi A., Høiby N. Extracellular lipase of Pseudomonas aeruginosa: biochemical characterization and effect on human neutrophil and monocyte function in vitro. Microb Pathog. 1991 Mar;10(3):173–182. doi: 10.1016/0882-4010(91)90052-c. [DOI] [PubMed] [Google Scholar]
- Koivuranta-Vaara P., Banda D., Goldstein I. M. Bacterial-lipopolysaccharide-induced release of lactoferrin from human polymorphonuclear leukocytes: role of monocyte-derived tumor necrosis factor alpha. Infect Immun. 1987 Dec;55(12):2956–2961. doi: 10.1128/iai.55.12.2956-2961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee T. H., Nagy L., Nagakura T., Walport M. J., Kay A. B. Identification and partial characterization of an exercise-induced neutrophil chemotactic factor in bronchial asthma. J Clin Invest. 1982 Apr;69(4):889–899. doi: 10.1172/JCI110528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Ras G., Wilson R., Todd H., Taylor G., Cole P. Effect of bacterial products on neutrophil migration in vitro. Thorax. 1990 Apr;45(4):276–280. doi: 10.1136/thx.45.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C., Hansen E. J. Role of C5 and recruited neutrophils in early clearance of nontypable Haemophilus influenzae from murine lungs. Infect Immun. 1985 Oct;50(1):207–212. doi: 10.1128/iai.50.1.207-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Rich R., Zak O. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis. 1987 Apr;135(4):869–874. doi: 10.1164/arrd.1987.135.4.869. [DOI] [PubMed] [Google Scholar]
- Wilson R., Read R., Thomas M., Rutman A., Harrison K., Lund V., Cookson B., Goldman W., Lambert H., Cole P. Effects of Bordetella pertussis infection on human respiratory epithelium in vivo and in vitro. Infect Immun. 1991 Jan;59(1):337–345. doi: 10.1128/iai.59.1.337-345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen G. S., Haslett C., Rees A. J., Gumbay R. S., Henson J. E., Henson P. M. Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis. 1987 Jul;136(1):19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- Young S. K., Worthen G. S., Haslett C., Tonnesen M. G., Henson P. M. Interaction between chemoattractants and bacterial lipopolysaccharide in the induction and enhancement of neutrophil adhesion. Am J Respir Cell Mol Biol. 1990 Jun;2(6):523–532. doi: 10.1165/ajrcmb/2.6.523. [DOI] [PubMed] [Google Scholar]


