Abstract
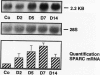
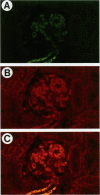
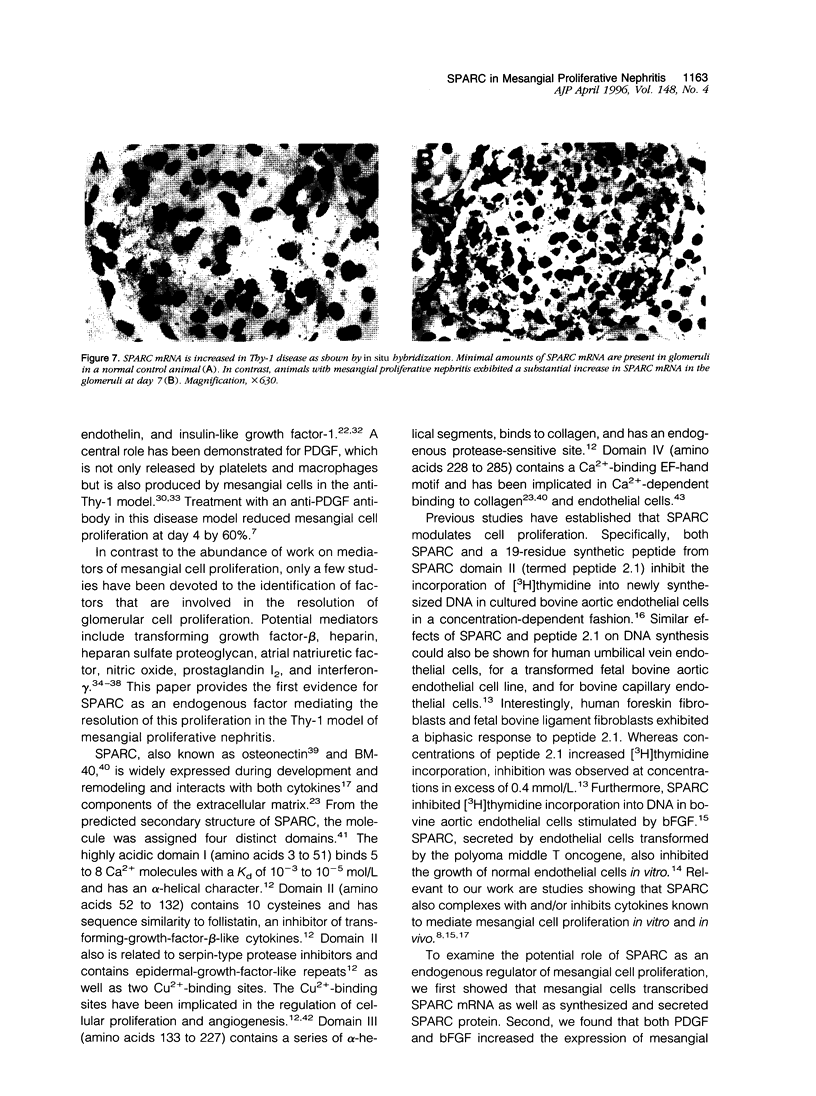
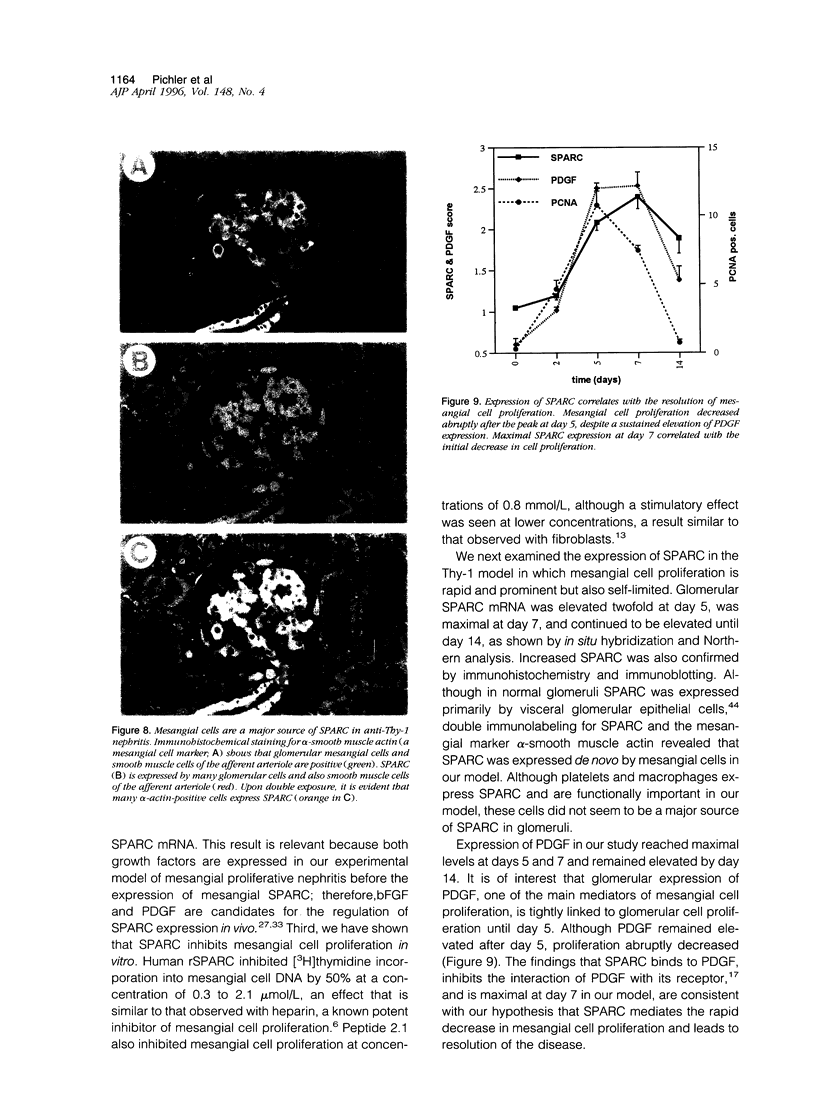
Mesangial cell proliferation is a characteristic feature of many glomerular diseases and often precedes extracellular matrix expansion and glomerulosclerosis. This study provides the first evidence that SPARC (secreted protein acidic and rich in cysteine) could be an endogenous factor mediating resolution of experimental mesangial proliferative nephritis in the rat. SPARC is a platelet-derived-growth-factor-binding glycoprotein that inhibits proliferation of endothelial cells and fibroblasts. We now show that SPARC is synthesized by mesangial cells in culture and that SPARC mRNA levels are increased by platelet-derived growth factor and basic fibroblast growth factor. Recombinant SPARC or the synthetic SPARC peptide 2.1 inhibited platelet-derived-growth-factor-induced mesangial cell DNA synthesis in vitro. In a model of experimental mesangioproliferative glomerulonephritis, SPARC mRNA was increased 5-fold by day 7 and was identified in the mesangium by in situ hybridization. Similarly, SPARC was increased in glomerular mesangial cells and visceral epithelial cells by day 5 and reached maximal expression levels by day 7. Mesangial cell proliferation increased by 36-fold on day 5 and decreased abruptly on day 7. Maximal expression of SPARC was correlated with the resolution of mesangial cell proliferation. We propose that SPARC functions in part as an endogenous inhibitor of platelet-derived-growth-factor-mediated mesangial cell proliferation in glomerulonephritis and that it could account for the resolution of cellular proliferation in this disease.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Baker P. J., Johnson R. J., Ochi R. F., Pritzl P., Couser W. G. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J Clin Invest. 1986 Mar;77(3):762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Gown A. M., Johnson R. J. Enhanced expression of "muscle-specific" actin in glomerulonephritis. Kidney Int. 1992 May;41(5):1134–1142. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- Baker A. J., Mooney A., Hughes J., Lombardi D., Johnson R. J., Savill J. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest. 1994 Nov;94(5):2105–2116. doi: 10.1172/JCI117565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk J. A., Baneyx F., Vernon R. B., Funk S. E., Sage E. H. Expression of biologically active human SPARC in Escherichia coli. Arch Biochem Biophys. 1996 Jan 1;325(1):8–19. doi: 10.1006/abbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Nakamura T., Languino L. R., Ruoslahti E. Role of TGF-beta 1 in experimental glomerulonephritis. Ciba Found Symp. 1991;157:178–193. [PubMed] [Google Scholar]
- Engel J., Taylor W., Paulsson M., Sage H., Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987 Nov 3;26(22):6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- Floege J., Alpers C. E., Burns M. W., Pritzl P., Gordon K., Couser W. G., Johnson R. J. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model. Lab Invest. 1992 Apr;66(4):485–497. [PubMed] [Google Scholar]
- Floege J., Alpers C. E., Sage E. H., Pritzl P., Gordon K., Johnson R. J., Couser W. G. Markers of complement-dependent and complement-independent glomerular visceral epithelial cell injury in vivo. Expression of antiadhesive proteins and cytoskeletal changes. Lab Invest. 1992 Oct;67(4):486–497. [PubMed] [Google Scholar]
- Floege J., Burns M. W., Alpers C. E., Yoshimura A., Pritzl P., Gordon K., Seifert R. A., Bowen-Pope D. F., Couser W. G., Johnson R. J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992 Feb;41(2):297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Lindner V., Alpers C. E., Young B. A., Reidy M. A., Johnson R. J. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest. 1992 Dec;90(6):2362–2369. doi: 10.1172/JCI116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Alpers C. E., Barrett T. B., Bowen-Pope D. F., Johnson R. J. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993 Dec;92(6):2952–2962. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Couser W. G., Johnson R. J. Heparin suppresses mesangial cell proliferation and matrix expansion in experimental mesangioproliferative glomerulonephritis. Kidney Int. 1993 Feb;43(2):369–380. doi: 10.1038/ki.1993.55. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Couser W. G., Johnson R. J. Heparin suppresses mesangial cell proliferation and matrix expansion in experimental mesangioproliferative glomerulonephritis. Kidney Int. 1993 Feb;43(2):369–380. doi: 10.1038/ki.1993.55. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Johnson R. J. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl. 1993 Jan;39:S47–S54. [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Alpers C. E., Fatemi-Nainie S., Richardson C. A., Gordon K., Couser W. G. Visceral glomerular epithelial cells can proliferate in vivo and synthesize platelet-derived growth factor B-chain. Am J Pathol. 1993 Feb;142(2):637–650. [PMC free article] [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Iida H., Pritzl P., Yoshimura A., Campbell C., Alpers C. E., Couser W. G. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991 Sep;40(3):477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Funk S. E., Sage E. H. Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol. 1993 Jan;154(1):53–63. doi: 10.1002/jcp.1041540108. [DOI] [PubMed] [Google Scholar]
- Funk S. E., Sage E. H. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum S. E., Ding X., Funk S. E., Sage E. H. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3448–3452. doi: 10.1073/pnas.91.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselaar P., Loskutoff D. J., Sawdey M., Sage E. H. SPARC induces the expression of type 1 plasminogen activator inhibitor in cultured bovine aortic endothelial cells. J Biol Chem. 1991 Jul 15;266(20):13178–13184. [PubMed] [Google Scholar]
- Hasselaar P., Sage E. H. SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J Cell Biochem. 1992 Jul;49(3):272–283. doi: 10.1002/jcb.240490310. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Seifert R., Alpers C. E., Gronwald R. G., Phillips P. E., Pritzl P., Gordon K., Gown A. M., Ross R., Bowen-Pope D. F. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Diglio C. A., Sage E. H. Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler Thromb. 1991 Jul-Aug;11(4):805–815. doi: 10.1161/01.atv.11.4.805. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Floege J., Yoshimura A., Iida H., Couser W. G., Alpers C. E. The activated mesangial cell: a glomerular "myofibroblast"? J Am Soc Nephrol. 1992 Apr;2(10 Suppl):S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Lombardi D., Eng E., Gordon K., Alpers C. E., Pritzl P., Floege J., Young B., Pippin J., Couser W. G. Modulation of experimental mesangial proliferative nephritis by interferon-gamma. Kidney Int. 1995 Jan;47(1):62–69. doi: 10.1038/ki.1995.7. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane T. F., Iruela-Arispe M. L., Johnson R. S., Sage E. H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol. 1994 May;125(4):929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. F., Iruela-Arispe M. L., Sage E. H. Regulation of gene expression by SPARC during angiogenesis in vitro. Changes in fibronectin, thrombospondin-1, and plasminogen activator inhibitor-1. J Biol Chem. 1992 Aug 15;267(23):16736–16745. [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990 Dec;111(6 Pt 2):3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994 Feb;8(2):163–173. [PubMed] [Google Scholar]
- MacKay K., Striker L. J., Stauffer J. W., Doi T., Agodoa L. Y., Striker G. E. Transforming growth factor-beta. Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest. 1989 Apr;83(4):1160–1167. doi: 10.1172/JCI113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Aumailley M., Mann K., Timpl R., Engel J. Calcium-dependent binding of basement membrane protein BM-40 (osteonectin, SPARC) to basement membrane collagen type IV. Eur J Biochem. 1991 May 23;198(1):141–150. doi: 10.1111/j.1432-1033.1991.tb15996.x. [DOI] [PubMed] [Google Scholar]
- Menè P., Abboud H. E., Dunn M. J. Regulation of human mesangial cell growth in culture by thromboxane A2 and prostacyclin. Kidney Int. 1990 Aug;38(2):232–239. doi: 10.1038/ki.1990.191. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lightner V. A., Aukhil I., Yan Y. Z., Erickson H. P., Hök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991 Nov;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce C. M., Striker L. J., Peten E., Elliot S. J., Striker G. E. Glomerulosclerosis at both early and late stages is associated with increased cell turnover in mice transgenic for growth hormone. Lab Invest. 1991 Nov;65(5):601–605. [PubMed] [Google Scholar]
- Raines E. W., Lane T. F., Iruela-Arispe M. L., Ross R., Sage E. H. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. J., Puolakkainen P., Lane T. F., Dickerson D., Bornstein P., Sage E. H. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- Sage E. H. Secretion of SPARC by endothelial cells transformed by polyoma middle T oncogene inhibits the growth of normal endothelial cells in vitro. Biochem Cell Biol. 1992 Jul;70(7):579–592. doi: 10.1139/o92-089. [DOI] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Gordon K., Alpers C. E., Floege J., Pritzl P., Ross R., Couser W. G., Bowen-Pope D. F., Johnson R. J. Demonstration of PDGF B-chain mRNA in glomeruli in mesangial proliferative nephritis by in situ hybridization. Kidney Int. 1991 Sep;40(3):470–476. doi: 10.1038/ki.1991.234. [DOI] [PubMed] [Google Scholar]
- Yost J. C., Sage E. H. Specific interaction of SPARC with endothelial cells is mediated through a carboxyl-terminal sequence containing a calcium-binding EF hand. J Biol Chem. 1993 Dec 5;268(34):25790–25796. [PubMed] [Google Scholar]