Abstract
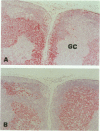
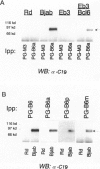
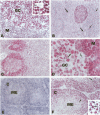
The human BCL-6 gene, which is rearranged in approximately 30% of diffuse large B cell lymphomas, encodes a 706-amino-acid nuclear protein of the Kruppel-type zinc finger transcription factors mainly expressed in normal germinal center B cells and related lymphomas. Four monoclonal antibodies (PG-B6, PG-B6a, PG-B6p, and PG-B6m), specifically directed against the human BCL-6 protein, were generated by immunizing BALB/c mice with a recombinant protein corresponding to the BCL-6 amino-terminal region (amino acids 3 to 484). The PG-B6 monoclonal antibody reacted with a BCL-6 epitope sensitive to fixatives and preserved in all mammalian species. PG-B6a (a is for avian) recognized the most evolutionarily conserved BCL-6 epitope (expressed in all animal species including avian). PG-B6p (p is for paraffin) recognized a fixative-resistant epitope of BCL-6 that was detectable on paraffin sections after microwave heating in 1 mmol/L EDTA buffer. PG-B6m (m is for mantle) was the least specific monoclonal antibody as, in addition to BCL-6, it reacted with a yet undefined antigen selectively located in the cytoplasm of mantle and marginal zone B cells. All monoclonal antibodies detected strong nuclear expression of BCL-6 in follicular lymphomas, diffuse large B cell lymphomas, Burkitt's lymphomas, and nodular, lymphocyte-predominance Hodgkin's disease. In diffuse large B cell lymphomas, BCL-6 expression was independent of BCL-6 gene rearrangements and did not correlate with expression of other markers or the proliferation index. BCL-6 was not expressed in B-CLL, hairy cell leukemia, mantle-cell- and marginal-zone-derived lymphomas. Labeling of paraffin sections with PG-B6p proved useful for differentiating proliferation centers in B-CLL (BCl-2+/BCL-6-) from trapped germinal centers in mantle cell lymphomas (BCL-2-/BCL-6+) and for identifying neoplastic cells in cases of nodular, lymphocyte-predominance Hodgkin's disease. Because of their high specificity, wide reactivity in humans and animal species including avians (PG-B6a), and suitability for labeling routine paraffin sections (PG-B6p), the reagents described in this paper should prove valuable in both research and diagnostics.
Full text
PDF



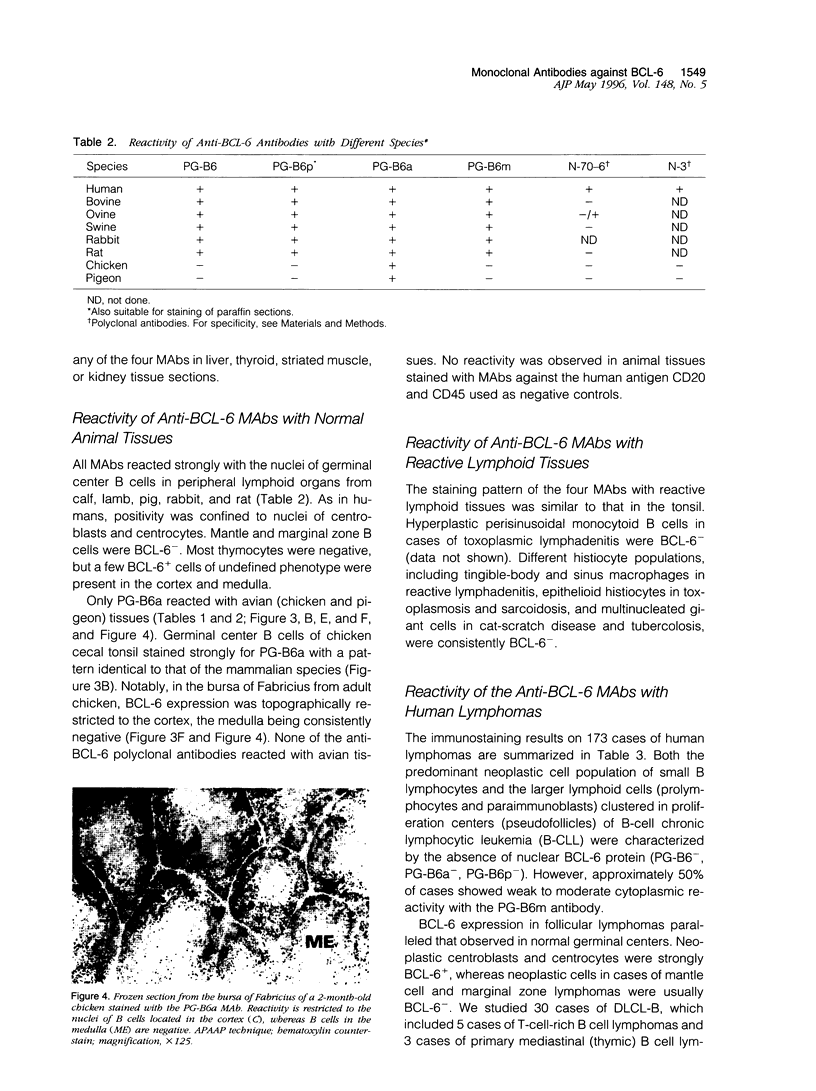
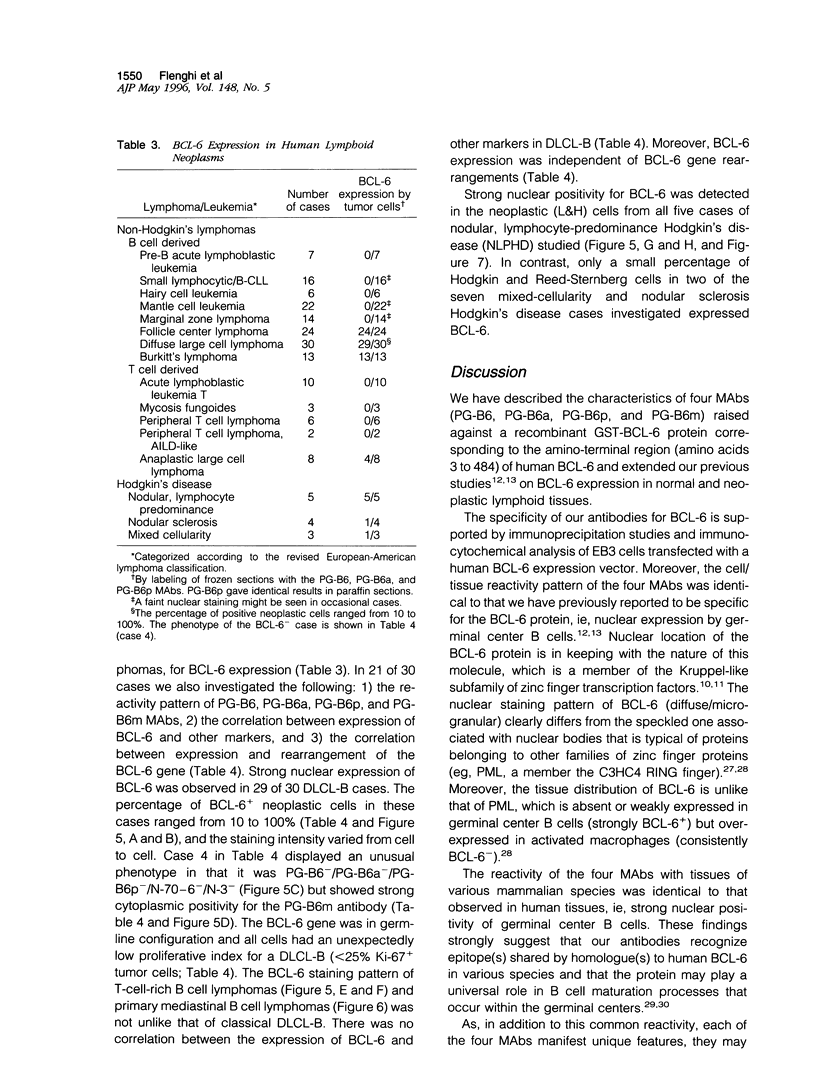
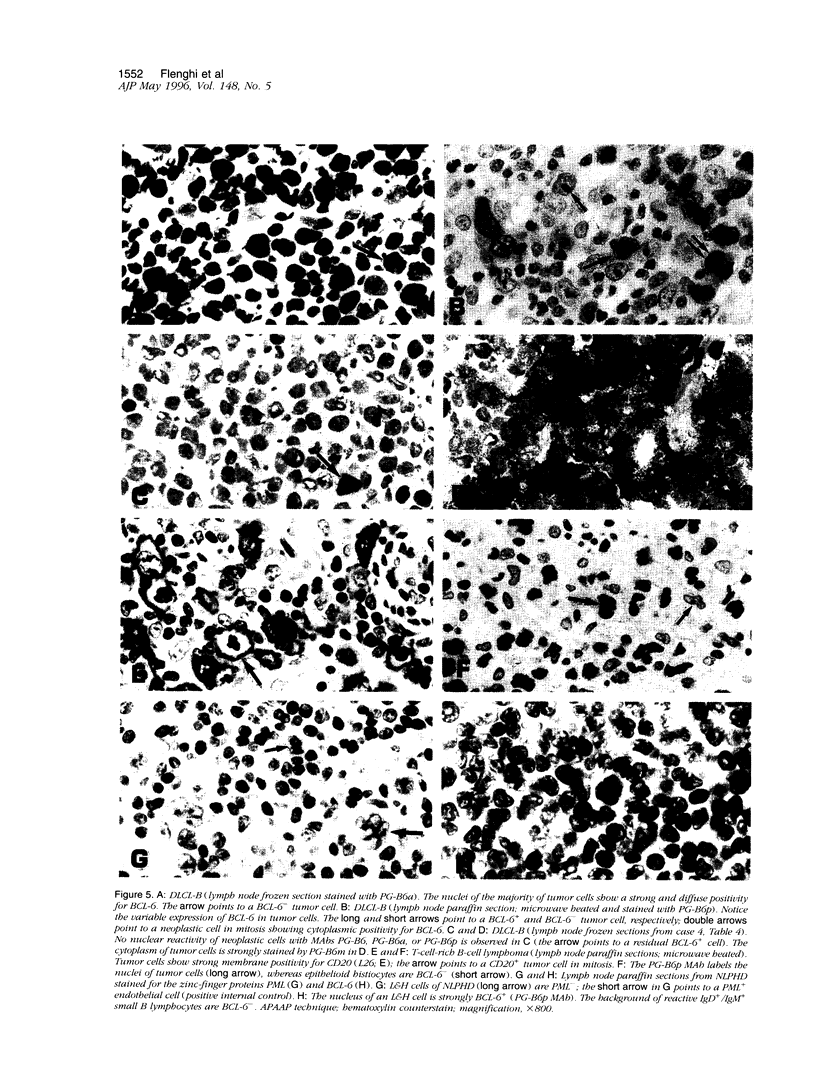



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron B. W., Nucifora G., McCabe N., Espinosa R., 3rd, Le Beau M. M., McKeithan T. W. Identification of the gene associated with the recurring chromosomal translocations t(3;14)(q27;q32) and t(3;22)(q27;q11) in B-cell lymphomas. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5262–5266. doi: 10.1073/pnas.90.11.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard C., Deweindt C., Kerckaert J. P., Lenormand B., Rossi A., Pezzella F., Fruchart C., Duval C., Monconduit M., Tilly H. LAZ3 rearrangements in non-Hodgkin's lymphoma: correlation with histology, immunophenotype, karyotype, and clinical outcome in 217 patients. Blood. 1994 May 1;83(9):2423–2427. [PubMed] [Google Scholar]
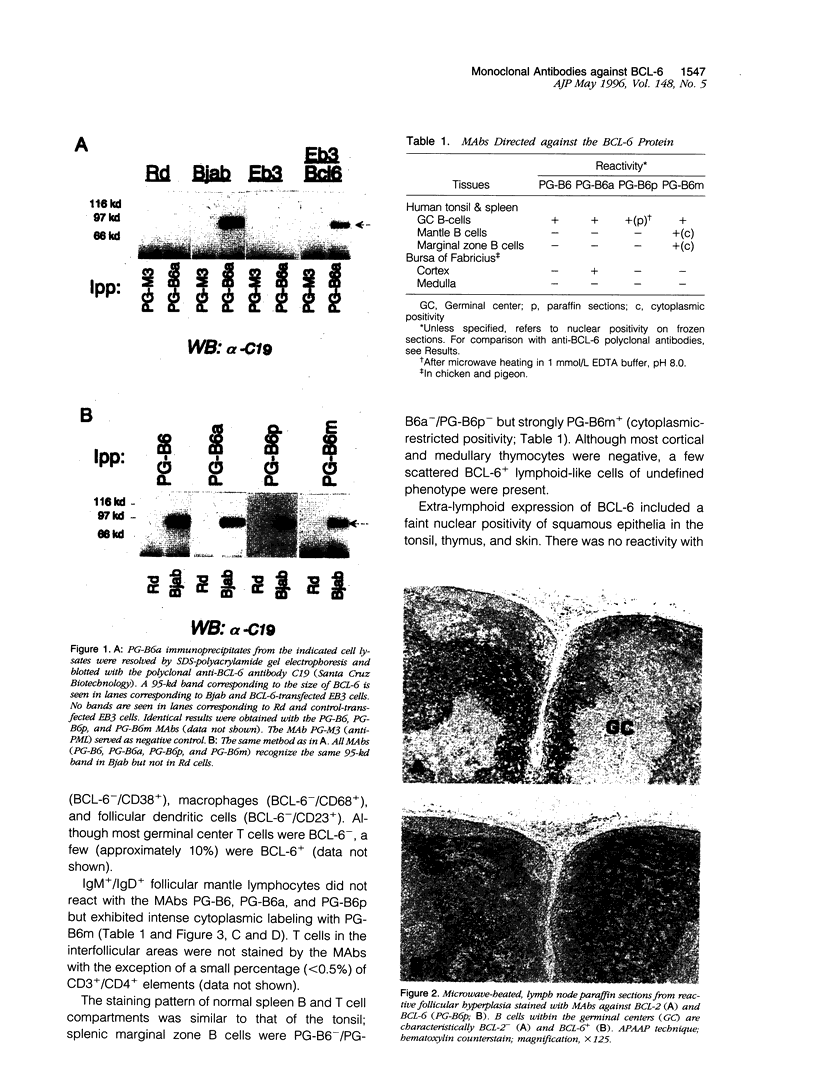
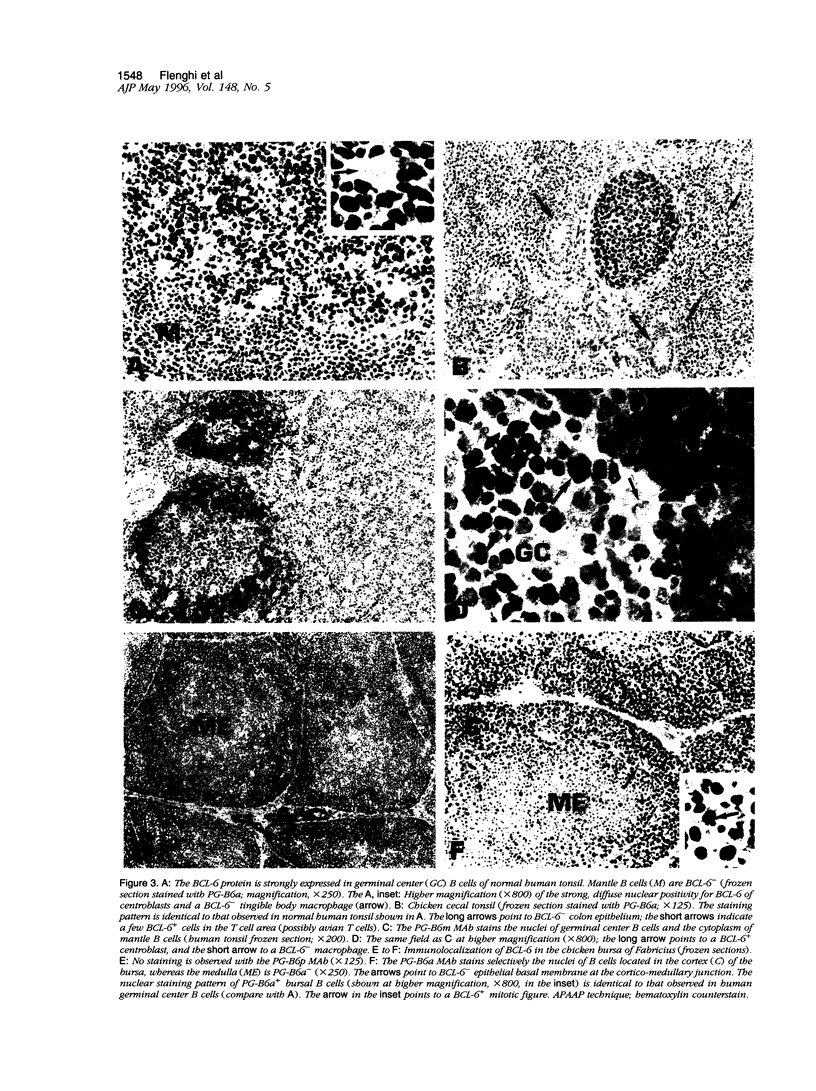
- Cattoretti G., Chang C. C., Cechova K., Zhang J., Ye B. H., Falini B., Louie D. C., Offit K., Chaganti R. S., Dalla-Favera R. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995 Jul 1;86(1):45–53. [PubMed] [Google Scholar]
- Cattoretti G., Pileri S., Parravicini C., Becker M. H., Poggi S., Bifulco C., Key G., D'Amato L., Sabattini E., Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993 Oct;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Pizzolo G., Caligaris-Cappio F., Ambrosetti A., Vinante F., Morittu L., Bonetti F., Fiore-Donati L., Janossy G. Immunohistochemical demonstration of follicular dendritic cells in bone marrow involvement of B-cell chronic lymphocytic leukemia. Cancer. 1985 Jul 15;56(2):328–332. doi: 10.1002/1097-0142(19850715)56:2<328::aid-cncr2820560221>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Delabie J., Vandenberghe E., Kennes C., Verhoef G., Foschini M. P., Stul M., Cassiman J. J., De Wolf-Peeters C. Histiocyte-rich B-cell lymphoma. A distinct clinicopathologic entity possibly related to lymphocyte predominant Hodgkin's disease, paragranuloma subtype. Am J Surg Pathol. 1992 Jan;16(1):37–48. [PubMed] [Google Scholar]
- Deweindt C., Kerckaert J. P., Tilly H., Quief S., Nguyen V. C., Bastard C. Cloning of a breakpoint cluster region at band 3q27 involved in human non-Hodgkin's lymphoma. Genes Chromosomes Cancer. 1993 Nov;8(3):149–154. doi: 10.1002/gcc.2870080303. [DOI] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Fagioli M., Stein H., Schwarting R., Riccardi C., Manocchio I., Pileri S., Pelicci P. G., Lanfrancone L. Evolutionary conservation in various mammalian species of the human proliferation-associated epitope recognized by the Ki-67 monoclonal antibody. J Histochem Cytochem. 1989 Oct;37(10):1471–1478. doi: 10.1177/37.10.2476477. [DOI] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Pileri S., Gambacorta M., Bigerna B., Durkop H., Eitelbach F., Thiele J., Pacini R., Cavaliere A. PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol. 1993 May;142(5):1359–1372. [PMC free article] [PubMed] [Google Scholar]
- Falini B., Pileri S., Martelli M. F. Histological and immunohistological analysis of human lymphomas. Crit Rev Oncol Hematol. 1989;9(4):351–419. doi: 10.1016/s1040-8428(89)80018-6. [DOI] [PubMed] [Google Scholar]
- Falini B., Venturi S., Martélli M., Santucci A., Pileri S., Pescarmona E., Giovannini M., Mazza P., Martelli M. F., Pasqualucci L. Mediastinal large B-cell lymphoma: clinical and immunohistological findings in 18 patients treated with different third-generation regimens. Br J Haematol. 1995 Apr;89(4):780–789. doi: 10.1111/j.1365-2141.1995.tb08415.x. [DOI] [PubMed] [Google Scholar]
- Flenghi L., Fagioli M., Tomassoni L., Pileri S., Gambacorta M., Pacini R., Grignani F., Casini T., Ferrucci P. F., Martelli M. F. Characterization of a new monoclonal antibody (PG-M3) directed against the aminoterminal portion of the PML gene product: immunocytochemical evidence for high expression of PML proteins on activated macrophages, endothelial cells, and epithelia. Blood. 1995 Apr 1;85(7):1871–1880. [PubMed] [Google Scholar]
- Flenghi L., Ye B. H., Fizzotti M., Bigerna B., Cattoretti G., Venturi S., Pacini R., Pileri S., Lo Coco F., Pescarmona E. A specific monoclonal antibody (PG-B6) detects expression of the BCL-6 protein in germinal center B cells. Am J Pathol. 1995 Aug;147(2):405–411. [PMC free article] [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Inghirami G., Foitl D. R., Sabichi A., Zhu B. Y., Knowles D. M. Autoantibody-associated cross-reactive idiotype-bearing human B lymphocytes: distribution and characterization, including Ig VH gene and CD5 antigen expression. Blood. 1991 Sep 15;78(6):1503–1515. [PubMed] [Google Scholar]
- Kerckaert J. P., Deweindt C., Tilly H., Quief S., Lecocq G., Bastard C. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nat Genet. 1993 Sep;5(1):66–70. doi: 10.1038/ng0993-66. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Lo Coco F., Ye B. H., Lista F., Corradini P., Offit K., Knowles D. M., Chaganti R. S., Dalla-Favera R. Rearrangements of the BCL6 gene in diffuse large cell non-Hodgkin's lymphoma. Blood. 1994 Apr 1;83(7):1757–1759. [PubMed] [Google Scholar]
- Mason D. Y., Comans-Bitter W. M., Cordell J. L., Verhoeven M. A., van Dongen J. J. Antibody L26 recognizes an intracellular epitope on the B-cell-associated CD20 antigen. Am J Pathol. 1990 Jun;136(6):1215–1222. [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Cordell J. L., Abdulaziz Z., Naiem M., Bordenave G. Preparation of peroxidase: antiperoxidase (PAP) complexes for immunohistological labeling of monoclonal antibodies. J Histochem Cytochem. 1982 Nov;30(11):1114–1122. doi: 10.1177/30.11.6183312. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Thompson C. B. Somatic diversification of the chicken immunoglobulin light-chain gene. Adv Immunol. 1990;48:41–67. doi: 10.1016/s0065-2776(08)60751-8. [DOI] [PubMed] [Google Scholar]
- Miki T., Kawamata N., Hirosawa S., Aoki N. Gene involved in the 3q27 translocation associated with B-cell lymphoma, BCL5, encodes a Krüppel-like zinc-finger protein. Blood. 1994 Jan 1;83(1):26–32. [PubMed] [Google Scholar]
- Morgan J. M., Navabi H., Schmid K. W., Jasani B. Possible role of tissue-bound calcium ions in citrate-mediated high-temperature antigen retrieval. J Pathol. 1994 Dec;174(4):301–307. doi: 10.1002/path.1711740410. [DOI] [PubMed] [Google Scholar]
- Offit K., Jhanwar S., Ebrahim S. A., Filippa D., Clarkson B. D., Chaganti R. S. t(3;22)(q27;q11): a novel translocation associated with diffuse non-Hodgkin's lymphoma. Blood. 1989 Nov 1;74(6):1876–1879. [PubMed] [Google Scholar]
- Offit K., Lo Coco F., Louie D. C., Parsa N. Z., Leung D., Portlock C., Ye B. H., Lista F., Filippa D. A., Rosenbaum A. Rearrangement of the bcl-6 gene as a prognostic marker in diffuse large-cell lymphoma. N Engl J Med. 1994 Jul 14;331(2):74–80. doi: 10.1056/NEJM199407143310202. [DOI] [PubMed] [Google Scholar]
- Onizuka T., Moriyama M., Yamochi T., Kuroda T., Kazama A., Kanazawa N., Sato K., Kato T., Ota H., Mori S. BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood. 1995 Jul 1;86(1):28–37. [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M. Inhibition of endogenous tissue alkaline phosphatase with the use of alkaline phosphatase conjugates in immunohistochemistry. J Histochem Cytochem. 1981 Aug;29(8):981–984. doi: 10.1177/29.8.7024402. [DOI] [PubMed] [Google Scholar]
- Ramsay A. D., Smith W. J., Isaacson P. G. T-cell-rich B-cell lymphoma. Am J Surg Pathol. 1988 Jun;12(6):433–443. doi: 10.1097/00000478-198806000-00003. [DOI] [PubMed] [Google Scholar]
- Ratech H., Sheibani K., Nathwani B. N., Rappaport H. Immunoarchitecture of the "pseudofollicles" of well-differentiated (small) lymphocytic lymphoma: a comparison with true follicles. Hum Pathol. 1988 Jan;19(1):89–94. doi: 10.1016/s0046-8177(88)80322-8. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Bertocci B., Dahan A., Weill J. C. Formation of the chicken B-cell repertoire: ontogenesis, regulation of Ig gene rearrangement, and diversification by gene conversion. Adv Immunol. 1994;57:353–378. doi: 10.1016/s0065-2776(08)60676-8. [DOI] [PubMed] [Google Scholar]
- Ruppert J. M., Kinzler K. W., Wong A. J., Bigner S. H., Kao F. T., Law M. L., Seuanez H. N., O'Brien S. J., Vogelstein B. The GLI-Kruppel family of human genes. Mol Cell Biol. 1988 Aug;8(8):3104–3113. doi: 10.1128/mcb.8.8.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Isaacson P. G. Proliferation centres in B-cell malignant lymphoma, lymphocytic (B-CLL): an immunophenotypic study. Histopathology. 1994 May;24(5):445–451. doi: 10.1111/j.1365-2559.1994.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Stansfeld A. G., Diebold J., Noel H., Kapanci Y., Rilke F., Kelényi G., Sundstrom C., Lennert K., van Unnik J. A., Mioduszewska O. Updated Kiel classification for lymphomas. Lancet. 1988 Feb 6;1(8580):292–293. doi: 10.1016/s0140-6736(88)90367-4. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Thorbecke G. J., Amin A. R., Tsiagbe V. K. Biology of germinal centers in lymphoid tissue. FASEB J. 1994 Aug;8(11):832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- Timens W., Visser L., Poppema S. Nodular lymphocyte predominance type of Hodgkin's disease is a germinal center lymphoma. Lab Invest. 1986 Apr;54(4):457–461. [PubMed] [Google Scholar]
- Weis K., Rambaud S., Lavau C., Jansen J., Carvalho T., Carmo-Fonseca M., Lamond A., Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994 Jan 28;76(2):345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Ye B. H., Lista F., Lo Coco F., Knowles D. M., Offit K., Chaganti R. S., Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993 Oct 29;262(5134):747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., de Wolf-Peeters C., Desmet V. J. Marginal zone lymphocytes in the lymph node. Hum Pathol. 1989 Dec;20(12):1225–1227. doi: 10.1016/s0046-8177(89)80023-1. [DOI] [PubMed] [Google Scholar]