Abstract
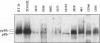
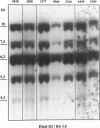
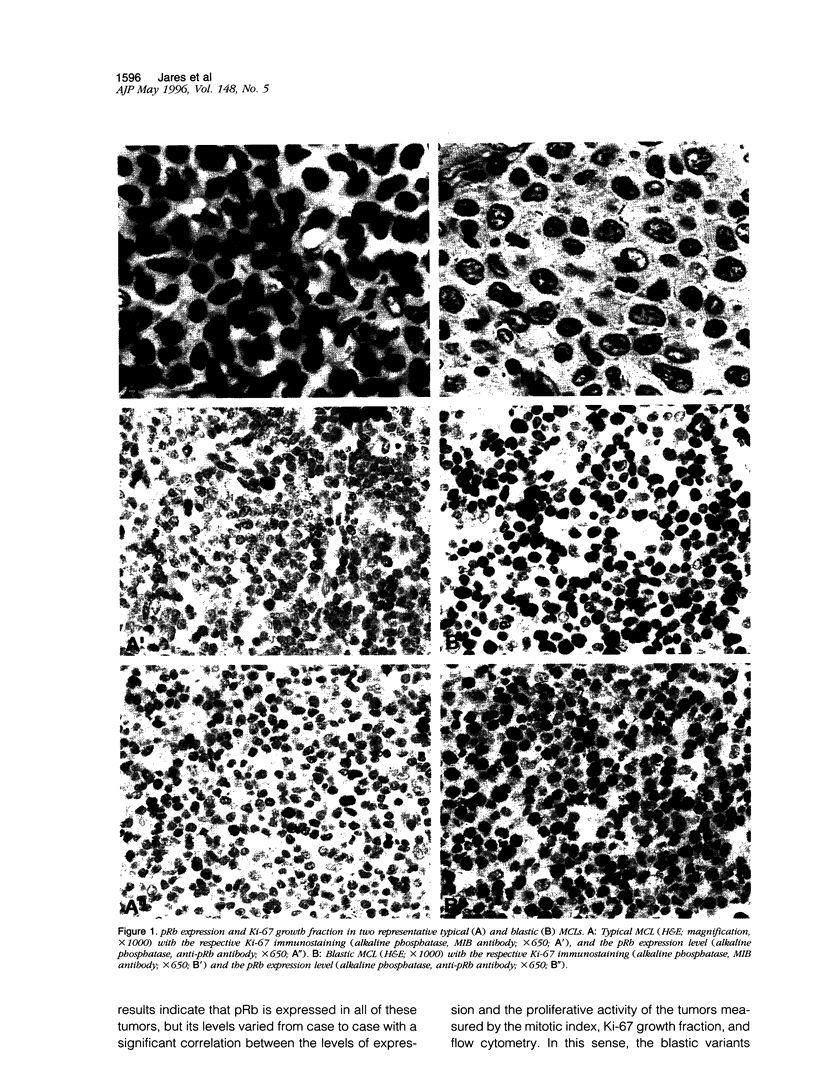
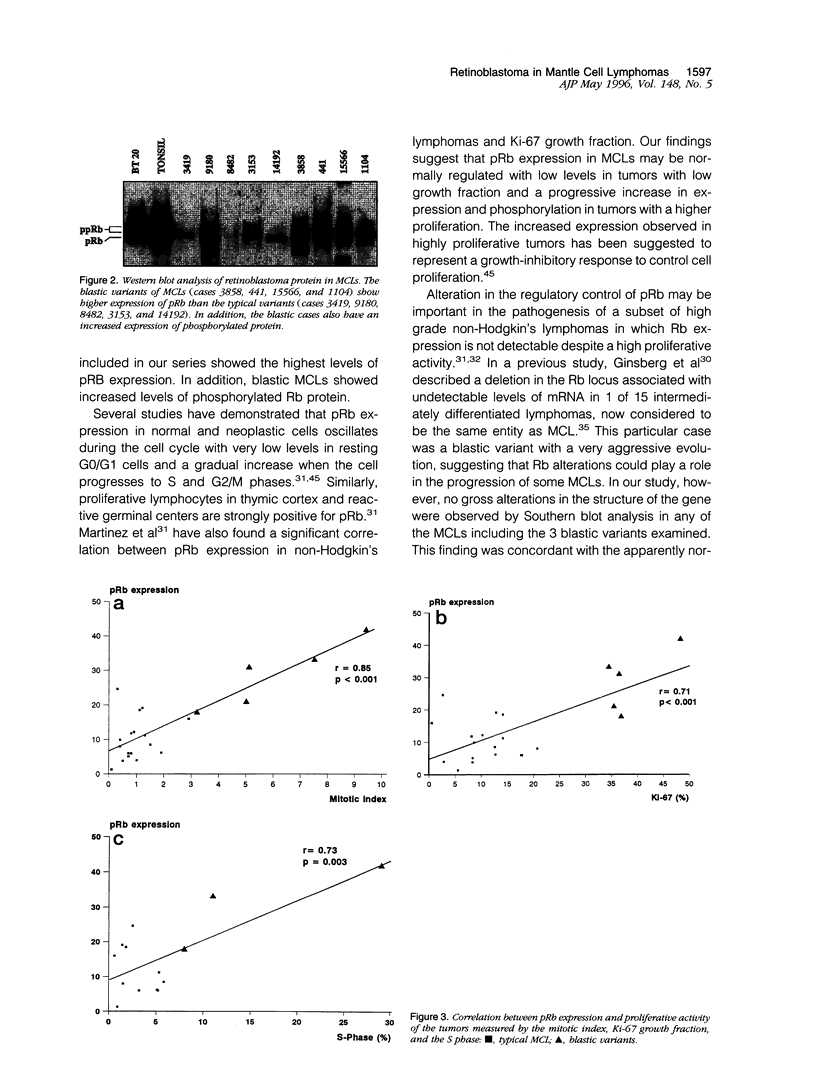
Mantle cell lymphomas (MCLs) are molecularly characterized by bcl-1 rearrangement and constant cyclin D1 (PRAD-1/CCND1) gene overexpression. Cyclin D1 is a G1 cyclin that participates in the control of the cell cycle progression by interacting with the retinoblastoma gene product (pRb). Inactivation of the Rb tumor suppressor gene has been implicated in the development of different types of human tumors including some high grade non-Hodgkin's lymphomas. To determine the role of the retinoblastoma gene in the pathogenesis of MCLs and its possible interaction with cyclin D1, pRb expression was examined in 23 MCLs including 17 typical and 6 blastic variants by immunohistochemistry and Western blot. Rb gene structure was studied in 13 cases by Southern blot. Cytogenetic analysis was performed in 5 cases. The results were compared with the cyclin D1 mRNA levels examined by Northern analysis, and the proliferative activity of the tumors was measured by Ki-67 growth fraction and flow cytometry. pRb was expressed in all MCLs. The expression varied from case to case (mean, 14.1% of positive cells; range, 1.3 to 42%) with a significant correlation with the proliferative activity of the tumors (mitotic index r = 0.85; Ki-67 r = 0.7; S phase = 0.73). Blastic variants showed higher numbers of pRb-positive cells (mean, 29%) than the typical cases (10%; P < 0.005) by immunohistochemistry and, concordantly, higher levels of expression by Western blot. In addition, the blastic cases also had an increased expression of the phosphorylated protein. No alterations in Rb gene structure were observed by Southern blot analysis. Cyclin D1 mRNA levels were independent of pRb expression and the proliferative activity of the tumors. These findings suggest that pRb in MCLs is normally regulated in relation to the proliferative activity of the tumors. Cyclin D1 overexpression may play a role in the maintenance of cell proliferation by overcoming the suppressive growth control of pRb.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Banks P. M., Chan J., Cleary M. L., Delsol G., De Wolf-Peeters C., Gatter K., Grogan T. M., Harris N. L., Isaacson P. G., Jaffe E. S. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992 Jul;16(7):637–640. doi: 10.1097/00000478-199207000-00001. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Lukas J., Müller H., Lützhøft D., Strauss M., Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994 May 1;57(3):353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- Bianchi A. B., Fischer S. M., Robles A. I., Rinchik E. M., Conti C. J. Overexpression of cyclin D1 in mouse skin carcinogenesis. Oncogene. 1993 May;8(5):1127–1133. [PubMed] [Google Scholar]
- Bosch F., Jares P., Campo E., Lopez-Guillermo A., Piris M. A., Villamor N., Tassies D., Jaffe E. S., Montserrat E., Rozman C. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood. 1994 Oct 15;84(8):2726–2732. [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg A. M., Raffeld M., Cossman J. Inactivation of the retinoblastoma gene in human lymphoid neoplasms. Blood. 1991 Feb 15;77(4):833–840. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Dowdy S. F., Eaton E. N., Arnold A., Weinberg R. A. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Jares P., Fernández P. L., Campo E., Nadal A., Bosch F., Aiza G., Nayach I., Traserra J., Cardesa A. PRAD-1/cyclin D1 gene amplification correlates with messenger RNA overexpression and tumor progression in human laryngeal carcinomas. Cancer Res. 1994 Sep 1;54(17):4813–4817. [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Zhou P., Zhang Y. J., Cacace A. M., Infante A. S., Doi S., Santella R. M., Weinstein I. B. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993 Dec;8(12):3447–3457. [PubMed] [Google Scholar]
- Jiang W., Zhang Y. J., Kahn S. M., Hollstein M. C., Santella R. M., Lu S. H., Harris C. C., Montesano R., Weinstein I. B. Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9026–9030. doi: 10.1073/pnas.90.19.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardelli P., Bookman M. A., Sundeen J., Longo D. L., Jaffe E. S. Lymphocytic lymphoma of intermediate differentiation. Morphologic and immunophenotypic spectrum and clinical correlations. Am J Surg Pathol. 1990 Aug;14(8):752–763. doi: 10.1097/00000478-199008000-00007. [DOI] [PubMed] [Google Scholar]
- Lovec H., Grzeschiczek A., Kowalski M. B., Möröy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994 Aug 1;13(15):3487–3495. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovec H., Sewing A., Lucibello F. C., Müller R., Möröy T. Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene. 1994 Jan;9(1):323–326. [PubMed] [Google Scholar]
- Lukas J., Jadayel D., Bartkova J., Nacheva E., Dyer M. J., Strauss M., Bartek J. BCL-1/cyclin D1 oncoprotein oscillates and subverts the G1 phase control in B-cell neoplasms carrying the t(11;14) translocation. Oncogene. 1994 Aug;9(8):2159–2167. [PubMed] [Google Scholar]
- Lukas J., Müller H., Bartkova J., Spitkovsky D., Kjerulff A. A., Jansen-Dürr P., Strauss M., Bartek J. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell's requirement for cyclin D1 function in G1. J Cell Biol. 1994 May;125(3):625–638. doi: 10.1083/jcb.125.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez J. C., Piris M. A., Sánchez-Beato M., Villuendas R., Orradre J. L., Algara P., Sánchez-Verde L., Martínez P. Retinoblastoma (Rb) gene product expression in lymphomas. Correlation with Ki67 growth fraction. J Pathol. 1993 Apr;169(4):405–412. doi: 10.1002/path.1711690404. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros L. J., Van Krieken J. H., Jaffe E. S., Raffeld M. Association of bcl-1 rearrangements with lymphocytic lymphoma of intermediate differentiation. Blood. 1990 Nov 15;76(10):2086–2090. [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Müller H., Lukas J., Schneider A., Warthoe P., Bartek J., Eilers M., Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka K., Ohno T., Kita K., Yamaguchi M., Takakura N., Nishii K., Miwa H., Shirakawa S. PRAD1 gene over-expression in mantle-cell lymphoma but not in other low-grade B-cell lymphomas, including extranodal lymphoma. Br J Haematol. 1994 Apr;86(4):786–791. doi: 10.1111/j.1365-2141.1994.tb04830.x. [DOI] [PubMed] [Google Scholar]
- Quelle D. E., Ashmun R. A., Shurtleff S. A., Kato J. Y., Bar-Sagi D., Roussel M. F., Sherr C. J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993 Aug;7(8):1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Raffeld M., Jaffe E. S. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991 Jul 15;78(2):259–263. [PubMed] [Google Scholar]
- Rimokh R., Berger F., Bastard C., Klein B., French M., Archimbaud E., Rouault J. P., Santa Lucia B., Duret L., Vuillaume M. Rearrangement of CCND1 (BCL1/PRAD1) 3' untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood. 1994 Jun 15;83(12):3689–3696. [PubMed] [Google Scholar]
- Rimokh R., Berger F., Delsol G., Charrin C., Berthéas M. F., Ffrench M., Garoscio M., Felman P., Coiffier B., Bryon P. A. Rearrangement and overexpression of the BCL-1/PRAD-1 gene in intermediate lymphocytic lymphomas and in t(11q13)-bearing leukemias. Blood. 1993 Jun 1;81(11):3063–3067. [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer I. E., Siriwardana S., Langan T. A., Sclafani R. A. Cyclin D1 overexpression vs. retinoblastoma inactivation: implications for growth control evasion in non-small cell and small cell lung cancer. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7827–7831. doi: 10.1073/pnas.91.16.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Yamamoto K., Iida S., Akao Y., Utsumi K. R., Kubonishi I., Miyoshi I., Ohtsuki T., Yawata Y., Namba M. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene. 1992 Jul;7(7):1401–1406. [PubMed] [Google Scholar]
- Shankey T. V., Rabinovitch P. S., Bagwell B., Bauer K. D., Duque R. E., Hedley D. W., Mayall B. H., Wheeless L., Cox C. Guidelines for implementation of clinical DNA cytometry. International Society for Analytical Cytology. Cytometry. 1993;14(5):472–477. doi: 10.1002/cyto.990140503. [DOI] [PubMed] [Google Scholar]
- Tam S. W., Theodoras A. M., Shay J. W., Draetta G. F., Pagano M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene. 1994 Sep;9(9):2663–2674. [PubMed] [Google Scholar]
- Vandenberghe E., De Wolf-Peeters C., van den Oord J., Wlodarska I., Delabie J., Stul M., Thomas J., Michaux J. L., Mecucci C., Cassiman J. J. Translocation (11;14): a cytogenetic anomaly associated with B-cell lymphomas of non-follicle centre cell lineage. J Pathol. 1991 Jan;163(1):13–18. doi: 10.1002/path.1711630104. [DOI] [PubMed] [Google Scholar]
- Weide R., Tiemann M., Pflüger K. H., Köppler H., Parvizl B., Wacker H. H., Kreipe H. H., Havemann K., Parwaresch M. R. Altered expression of the retinoblastoma gene product in human high grade non-Hodgkin's lymphomas. Leukemia. 1994 Jan;8(1):97–101. [PubMed] [Google Scholar]
- Weisenburger D. D., Sanger W. G., Armitage J. O., Purtilo D. T. Intermediate lymphocytic lymphoma: immunophenotypic and cytogenetic findings. Blood. 1987 Jun;69(6):1617–1621. [PubMed] [Google Scholar]
- Williams M. E., Westermann C. D., Swerdlow S. H. Genotypic characterization of centrocytic lymphoma: frequent rearrangement of the chromosome 11 bcl-1 locus. Blood. 1990 Oct 1;76(7):1387–1391. [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Benedict W. F. Lack of nuclear RB protein staining in G0/middle G1 cells: correlation to changes in total RB protein level. Oncogene. 1991 Jul;6(7):1139–1146. [PubMed] [Google Scholar]
- Yang W. I., Zukerberg L. R., Motokura T., Arnold A., Harris N. L. Cyclin D1 (Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol. 1994 Jul;145(1):86–96. [PMC free article] [PubMed] [Google Scholar]
- Zucca E., Stein H., Coiffier B. European Lymphoma Task Force (ELTF): Report of the workshop on Mantle Cell Lymphoma (MCL) Ann Oncol. 1994 Jul;5(6):507–511. doi: 10.1093/oxfordjournals.annonc.a058904. [DOI] [PubMed] [Google Scholar]