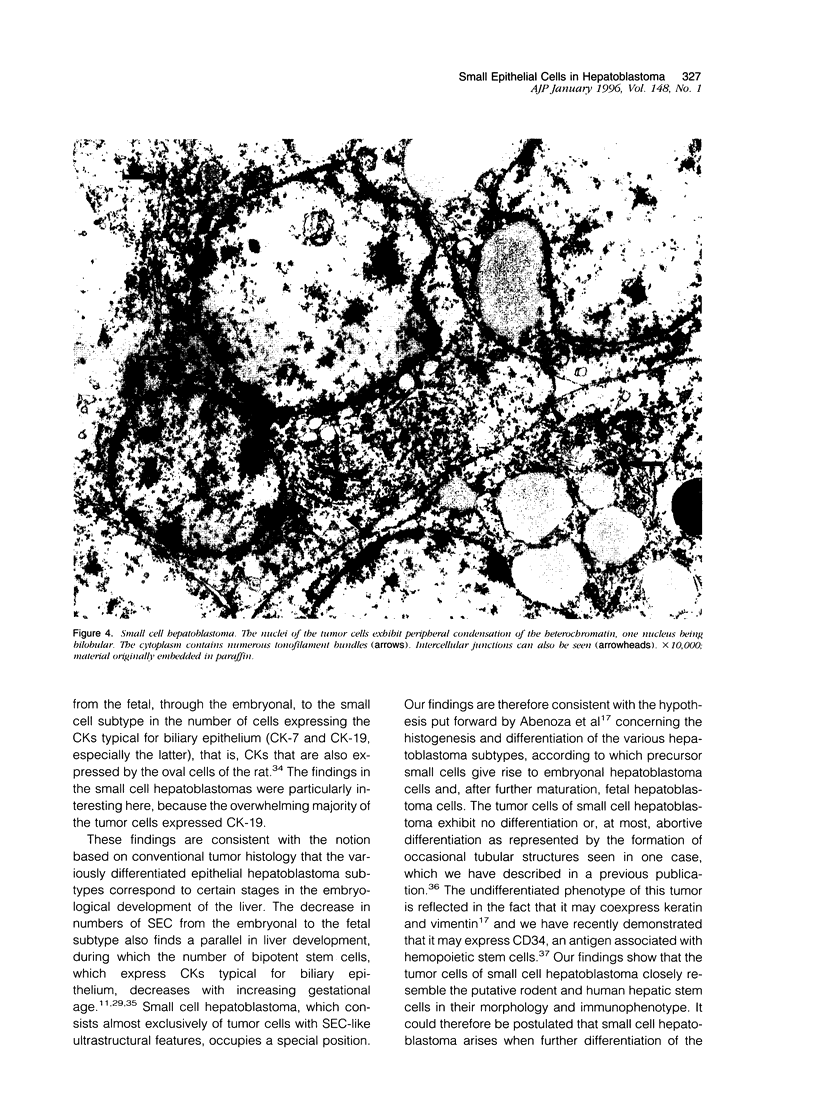
Abstract
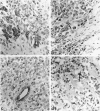
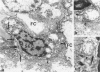
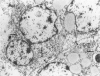
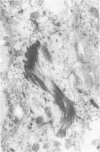
The wide range of epithelial and mesenchymal lines of differentiation seen in hepatoblastoma suggests that this tumor derives from a pluripotent stem cell. To test this hypothesis, seven hepatoblastomas of various subtypes were investigated for the presence of cells with the features of the oval cells found during hepatocarcinogenesis in rodents that are thought to be closely related to hepatic stem cells. Because similar cells, referred to as "small cells," have been described in human liver disease with chronic ductular reaction, five liver biopsies from infants with biliary atresia were also investigated. The specimens were investigated by electron microscopy, immunoelectron microscopy, and immunostaining for cytokeratins 7, 8, 18, and 19. Small epithelial cells (SEC) corresponding to the oval cells of the rat and the "small cells" in humans were found in both biliary atresia and hepatoblastoma. These cells were oval and exhibited intercellular junctions, tonofilament bundles, and a biliary epithelium-type cytokeratin profile. SEC were found in small numbers in fetal hepatoblastoma and in moderate numbers in embryonal hepatoblastoma. In small cell hepatoblastoma, nearly all the tumor cells exhibited SEC-like ultrastructural features and a corresponding cytokeratin profile. Thus, cells exhibiting morphological and immunophenotypic features of hepatic stem cells are detectable in hepatoblastoma. Their numbers vary according to the subtype, reflecting the differing degrees of differentiation of the various subtypes, consistent with the theory propounded in the literature that embryonal and, with further differentiation, fetal tumor cells derive from precursor small cells. The findings support the hypothesis that hepatoblastoma derives from a pluripotent, probably entodermal or even less committed, stem cell.
Full text
PDF
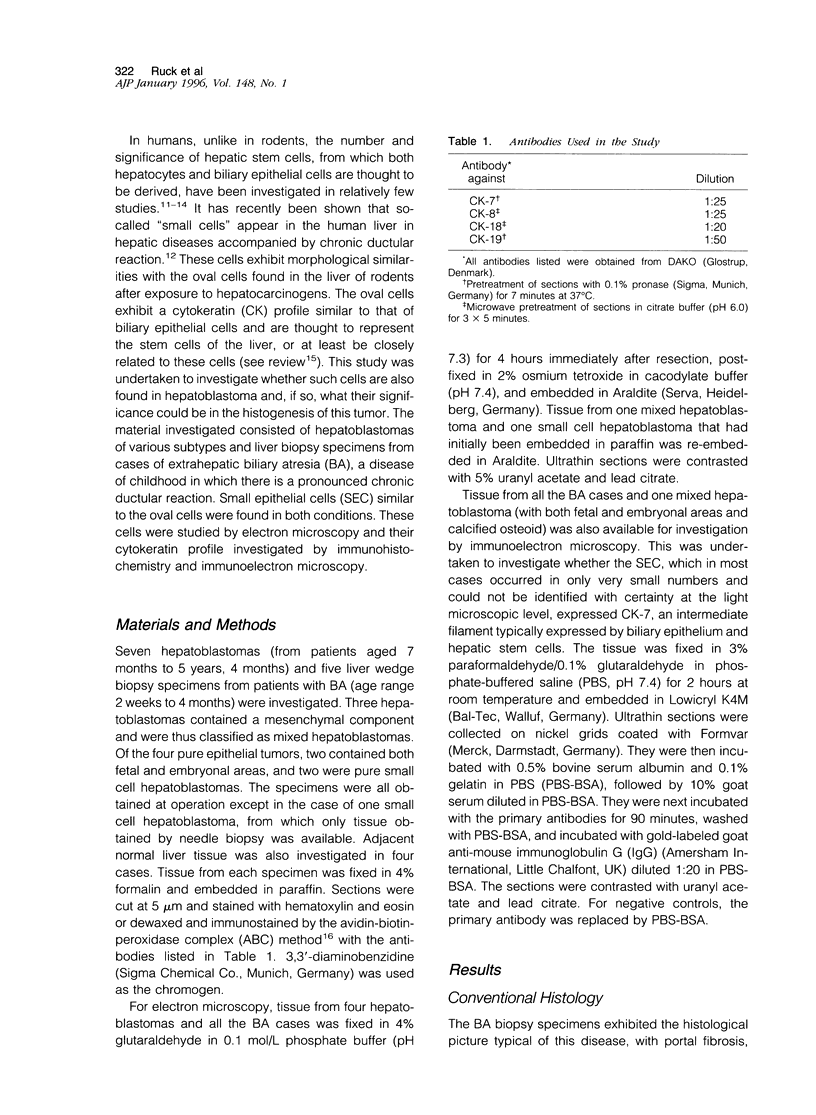

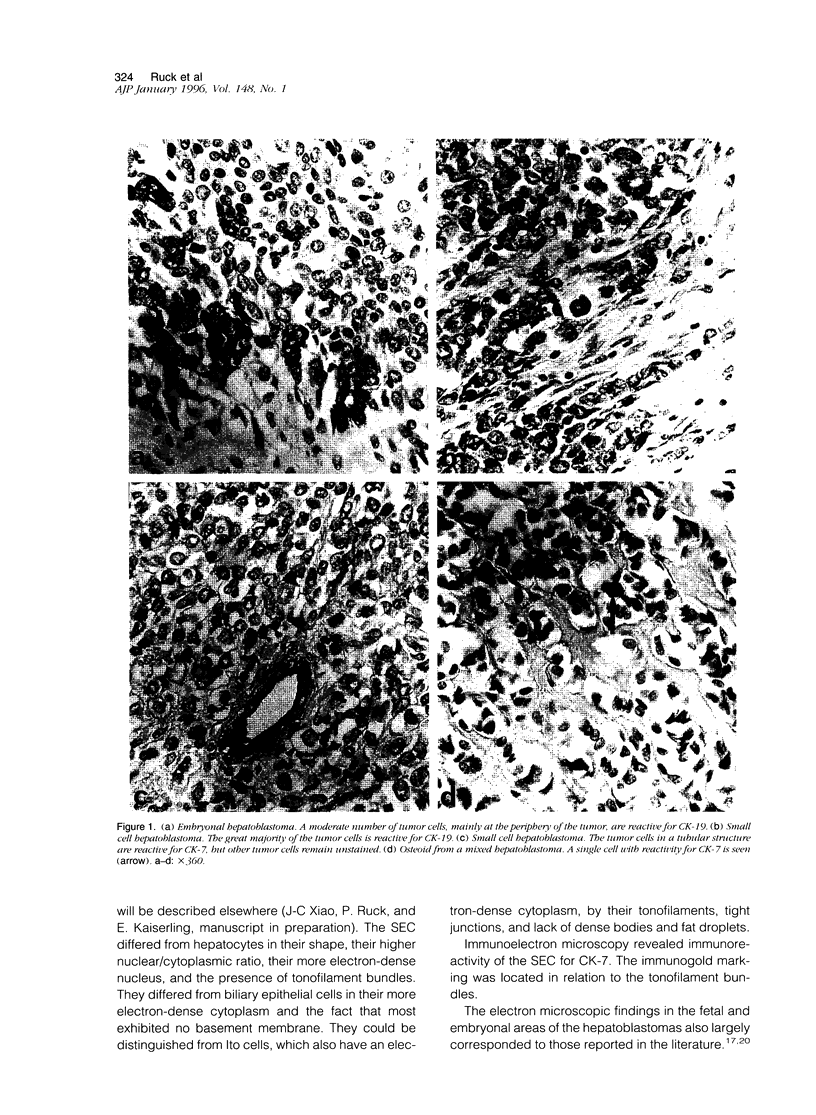
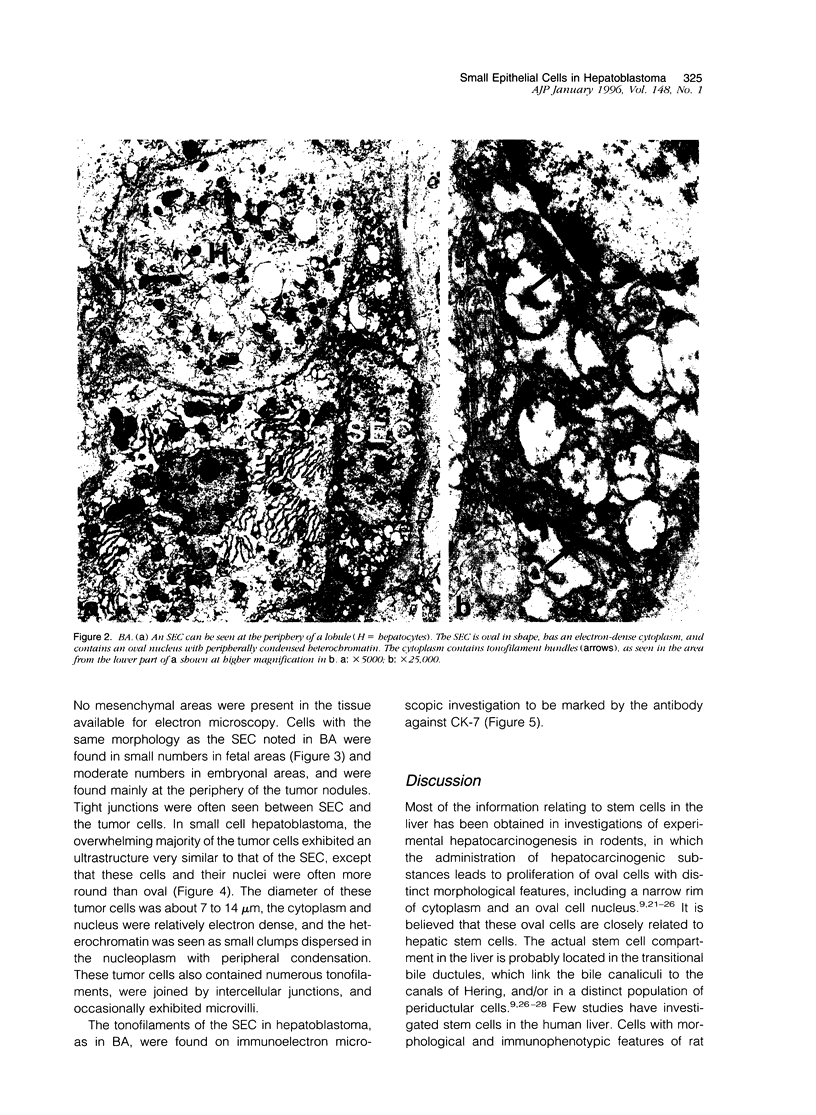


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abenoza P., Manivel J. C., Wick M. R., Hagen K., Dehner L. P. Hepatoblastoma: an immunohistochemical and ultrastructural study. Hum Pathol. 1987 Oct;18(10):1025–1035. doi: 10.1016/s0046-8177(87)80219-8. [DOI] [PubMed] [Google Scholar]
- Charlotte F., L'Herminé A., Martin N., Geleyn Y., Nollet M., Gaulard P., Zafrani E. S. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994 Mar;144(3):460–465. [PMC free article] [PubMed] [Google Scholar]
- Conrad R. J., Gribbin D., Walker N. I., Ong T. H. Combined cystic teratoma and hepatoblastoma of the liver. Probable divergent differentiation of an uncommitted hepatic precursor cell. Cancer. 1993 Nov 15;72(10):2910–2913. doi: 10.1002/1097-0142(19931115)72:10<2910::aid-cncr2820721009>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Conran R. M., Hitchcock C. L., Waclawiw M. A., Stocker J. T., Ishak K. G. Hepatoblastoma: the prognostic significance of histologic type. Pediatr Pathol. 1992 Mar-Apr;12(2):167–183. doi: 10.3109/15513819209023293. [DOI] [PubMed] [Google Scholar]
- De Vos R., Desmet V. Ultrastructural characteristics of novel epithelial cell types identified in human pathologic liver specimens with chronic ductular reaction. Am J Pathol. 1992 Jun;140(6):1441–1450. [PMC free article] [PubMed] [Google Scholar]
- Desmet V. J., Van Eyken P., Sciot R. Cytokeratins for probing cell lineage relationships in developing liver. Hepatology. 1990 Nov;12(5):1249–1251. doi: 10.1002/hep.1840120530. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Factor V. M., Radaeva S. A., Thorgeirsson S. S. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994 Aug;145(2):409–422. [PMC free article] [PubMed] [Google Scholar]
- Farber E. Clonal adaptation during carcinogenesis. Biochem Pharmacol. 1990 Jun 15;39(12):1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- Fausto N. Oval cells and liver carcinogenesis: an analysis of cell lineages in hepatic tumors using oncogene transfection techniques. Prog Clin Biol Res. 1990;331:325–334. [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N. Liver stem cells and development. Lab Invest. 1993 Mar;68(3):253–254. [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N., Shen S., Stromeyer F. W., Ishak K. G. Phenotypic characterization of hepatic proliferation. Antigenic expression by proliferating epithelial cells in fetal liver, massive hepatic necrosis, and nodular transformation of the liver. Am J Pathol. 1983 Jan;110(1):70–74. [PMC free article] [PubMed] [Google Scholar]
- Germain L., Blouin M. J., Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988 Sep 1;48(17):4909–4918. [PubMed] [Google Scholar]
- Hollander M., Schaffner F. Electron microscopic studies in biliary atresia. I. Bile ductular proliferation. Am J Dis Child. 1968 Jul;116(1):49–56. doi: 10.1001/archpedi.1968.02100020051007. [DOI] [PubMed] [Google Scholar]
- Hollander M., Schaffner F. Electron microscopic studies in biliary atresia. II. Hepatocellular alterations. Am J Dis Child. 1968 Jul;116(1):57–65. doi: 10.1001/archpedi.1968.02100020059008. [DOI] [PubMed] [Google Scholar]
- Horie A., Kotoo Y., Hayashi I. Ultrastructural comparison of hepatoblastoma and hepatocellular carcinoma. Cancer. 1979 Dec;44(6):2184–2193. doi: 10.1002/1097-0142(197912)44:6<2184::aid-cncr2820440631>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hsia C. C., Evarts R. P., Nakatsukasa H., Marsden E. R., Thorgeirsson S. S. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992 Dec;16(6):1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ishak K. G., Glunz P. R. Hepatoblastoma and hepatocarcinoma in infancy and childhood. Report of 47 cases. Cancer. 1967 Mar;20(3):396–422. doi: 10.1002/1097-0142(1967)20:3<396::aid-cncr2820200308>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kasai M., Watanabe I. Histologic classification of liver-cell carcinoma in infancy and childhood and its clinical evaluation. A study of 70 cases collected in Japan. Cancer. 1970 Mar;25(3):551–563. doi: 10.1002/1097-0142(197003)25:3<551::aid-cncr2820250309>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lai Y. S., Thung S. N., Gerber M. A., Chen M. L., Schaffner F. Expression of cytokeratins in normal and diseased livers and in primary liver carcinomas. Arch Pathol Lab Med. 1989 Feb;113(2):134–138. [PubMed] [Google Scholar]
- Lenzi R., Liu M. H., Tarsetti F., Slott P. A., Alpini G., Zhai W. R., Paronetto F., Lenzen R., Tavoloni N. Histogenesis of bile duct-like cells proliferating during ethionine hepatocarcinogenesis. Evidence for a biliary epithelial nature of oval cells. Lab Invest. 1992 Mar;66(3):390–402. [PubMed] [Google Scholar]
- Manivel C., Wick M. R., Abenoza P., Dehner L. P. Teratoid hepatoblastoma. The nosologic dilemma of solid embryonic neoplasms of childhood. Cancer. 1986 Jun 1;57(11):2168–2174. doi: 10.1002/1097-0142(19860601)57:11<2168::aid-cncr2820571115>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Ruck P., Harms D., Kaiserling E. Neuroendocrine differentiation in hepatoblastoma. An immunohistochemical investigation. Am J Surg Pathol. 1990 Sep;14(9):847–855. doi: 10.1097/00000478-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Ruck P., Xiao J. C., Kaiserling E. Immunoreactivity of sinusoids in hepatoblastoma: an immunohistochemical study using lectin UEA-1 and antibodies against endothelium-associated antigens, including CD34. Histopathology. 1995 May;26(5):451–455. doi: 10.1111/j.1365-2559.1995.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Shah K. D., Gerber M. A. Development of intrahepatic bile ducts in humans. Immunohistochemical study using monoclonal cytokeratin antibodies. Arch Pathol Lab Med. 1989 Oct;113(10):1135–1138. [PubMed] [Google Scholar]
- Sigal S. H., Brill S., Fiorino A. S., Reid L. M. The liver as a stem cell and lineage system. Am J Physiol. 1992 Aug;263(2 Pt 1):G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson S. S. Hepatic stem cells. Am J Pathol. 1993 May;142(5):1331–1333. [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W. Hepatocarcinomas, cholangiocarcinomas, and hepatoblastomas produced by chemically transformed cultured rat liver epithelial cells. A light- and electron-microscopic analysis. Am J Pathol. 1987 Apr;127(1):168–181. [PMC free article] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Callea F., Ramaekers F., Schaart G., Desmet V. J. A cytokeratin-immunohistochemical study of hepatoblastoma. Hum Pathol. 1990 Mar;21(3):302–308. doi: 10.1016/0046-8177(90)90231-s. [DOI] [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- van Eyken P., Sciot R., van Damme B., de Wolf-Peeters C., Desmet V. J. Keratin immunohistochemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):63–72. doi: 10.1007/BF00750732. [DOI] [PubMed] [Google Scholar]