Abstract
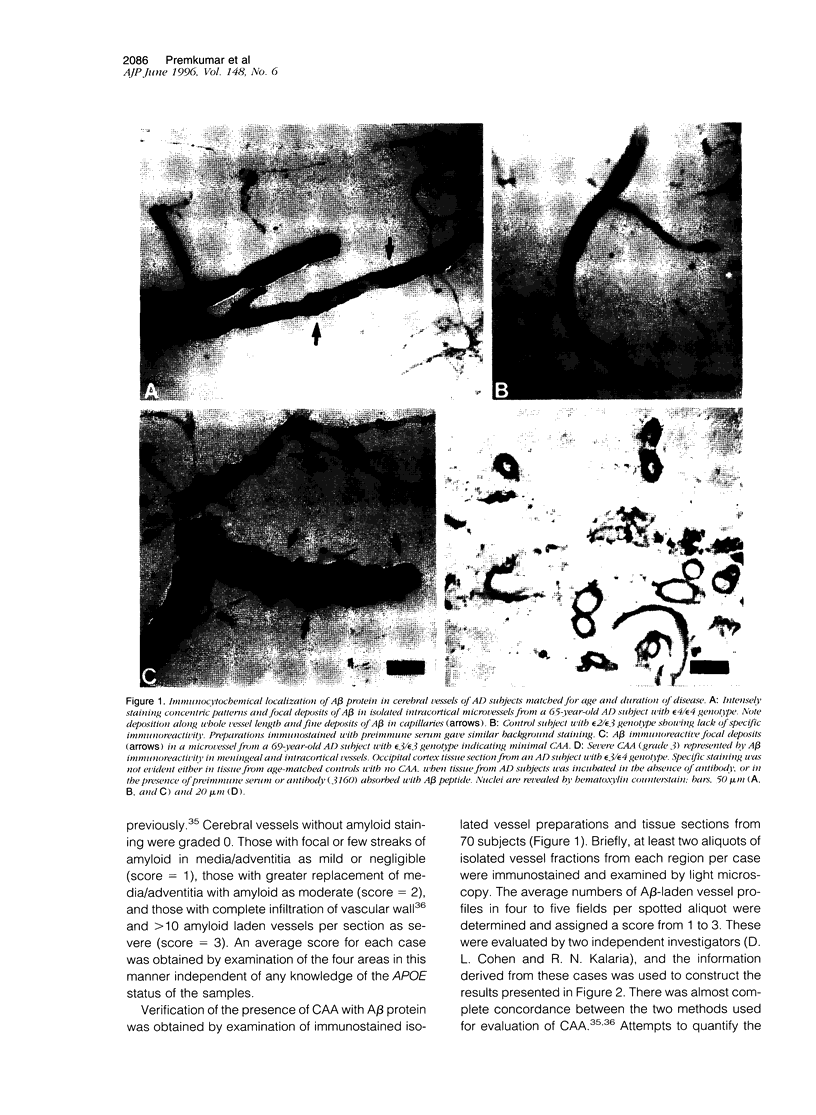
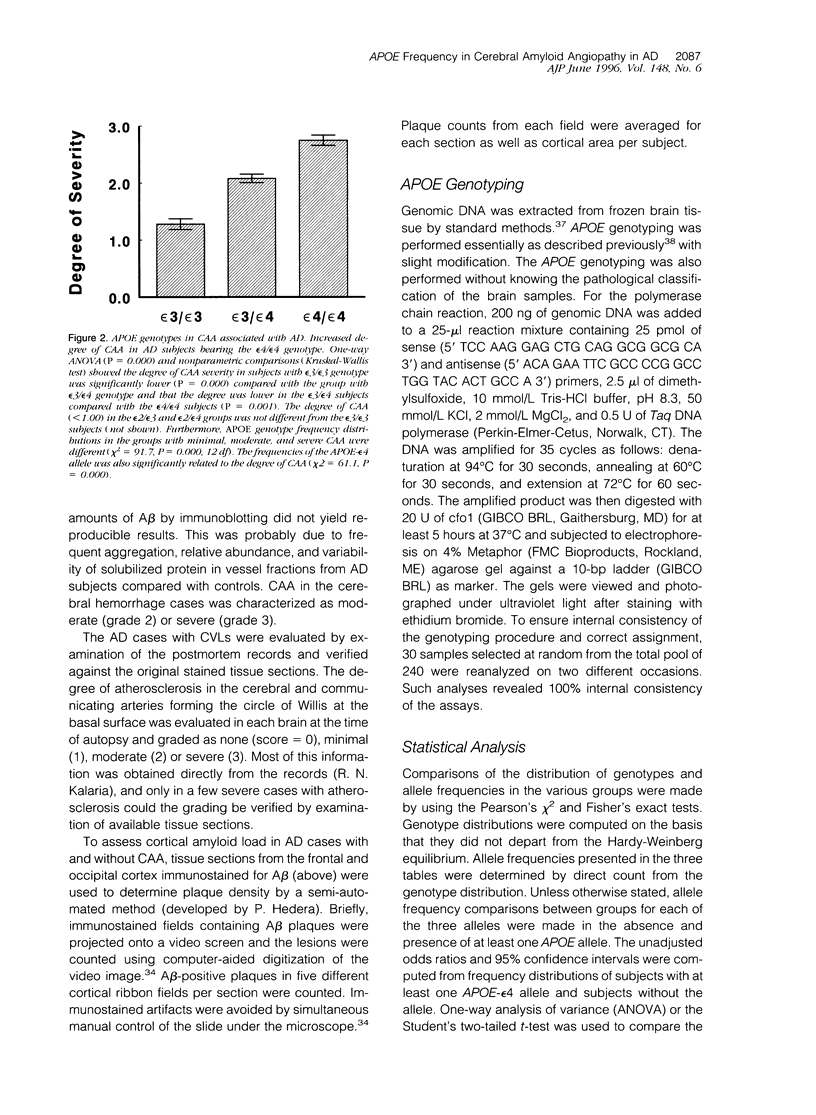
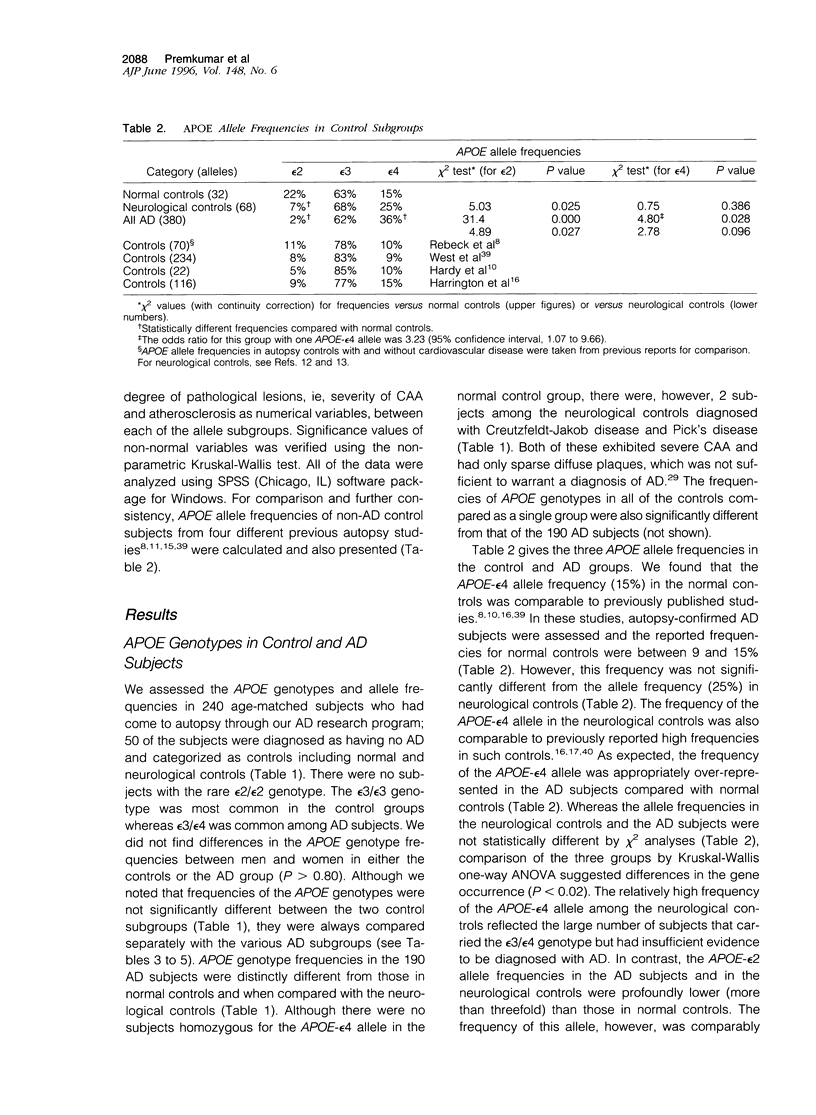
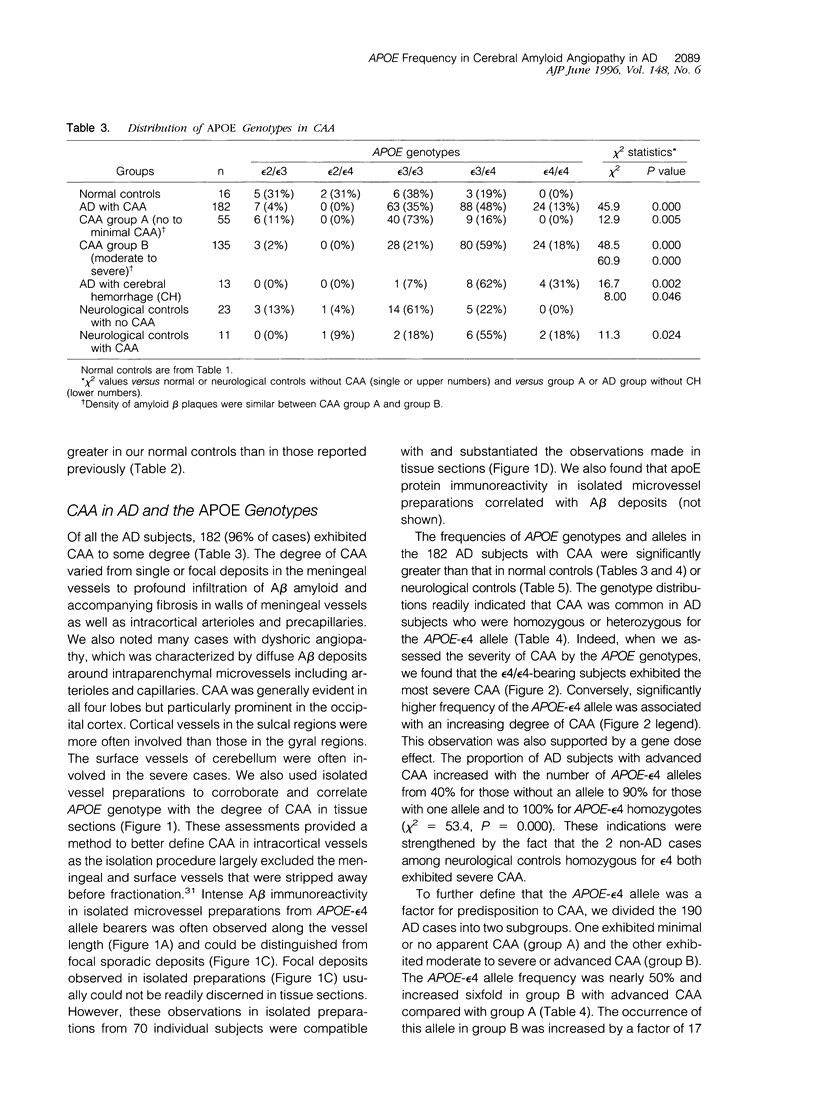
The presence of apolipoprotein E-epsilon4 (APOE-epsilon4) allele has been implicated as a risk factor for Alzheimer's disease (AD). We examined the frequencies of APOE-epsilon4 alleles in age-matched controls and subgroups of 190 AD subjects exhibited cerebral amyloid angiopathy (CAA) and other frequently associated lesions. CAA was evident in 96% of the AD subjects, which were divided into two groups, one bearing mild or no apparent CAA and the other with moderate to severe CAA. APOE-epsilon4 allele frequency (48%) in the latter advanced CAA group was six times higher than in those who exhibited mild CAA. In the advanced CAA subjects, the occurrence of an epsilon4 allele was increased by a factor of 17 (95% confidence interval, 7.56 to 38.9). This was despite the fact that neocortical amyloid-beta plaque densities in the two groups were similar and that all of the AD subjects had met the accepted neuropathological criteria. We also observed that the degree of CAA severity was greatest in the group of subjects with the epsilon4/epsilon4 genotype. The association between CAA and APOE-epsilon4 was further implicated in two non-AD subjects among neurological controls with severe CAA. These two subjects, both homozygous for the APOE-epsilon4 allele, were primarily diagnosed as having Creutzfeldt-Jakob disease and Pick's disease in the absence of significant neocortical amyloid deposition. Allele frequency comparisons between neurological control subjects with CAA and those without likewise accorded a strong relationship between the APOE-epsilon4 allele and the presence of CAA. More remarkably, the epsilon4 allele frequency was highly associated with AD subjects exhibiting lobar or intracerebral hemorrhage, all of whom had advanced CAA. We observed that 36% of the AD subjects had concomitant cerebrovascular pathology resulting from single infarcts, multiple microinfarcts, ischemic white matter lesions, or petechial hemorrhages. Although the difference in APOE genotype distribution between subjects with and without cerebrovascular lesions did not reach statistical significance, we did note that the frequency of the epsilon4 allele was significantly higher in subjects with such pathology as compared with those without. However, we found no evidence to suggest that the acquisition of an APOE-epsilon4 allele or one of the alleles, epsilon2 or epsilon3, was a factor in the occurrence of atherosclerosis localized in the basal surface arteries. Analyses of our sample also confirm that there was a lower frequency of the APOE-epsilon2 allele in AD subjects and that the frequency of the epsilon4 allele in AD subjects with concomitant diffuse Lewy body disease was intermediate between controls and AD subjects. Our results suggest that the APOE-epsilon4 allele is a significant factor in the development of CAA in AD and reveal the possibility that APOE is an independent factor in CAA and other vascular abnormalities associated with AD.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amouyel P., Vidal O., Launay J. M., Laplanche J. L. The apolipoprotein E alleles as major susceptibility factors for Creutzfeldt-Jakob disease. The French Research Group on Epidemiology of Human Spongiform Encephalopathies. Lancet. 1994 Nov 12;344(8933):1315–1318. doi: 10.1016/s0140-6736(94)90691-2. [DOI] [PubMed] [Google Scholar]
- Arai H., Higuchi S., Muramatsu T., Iwatsubo T., Sasaki H., Trojanowski J. Q. Apolipoprotein E gene in diffuse Lewy body disease with or without co-existing Alzheimer's disease. Lancet. 1994 Nov 5;344(8932):1307–1307. doi: 10.1016/s0140-6736(94)90799-4. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Risch N. J., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Jr, Rimmler J. B., Locke P. A., Conneally P. M., Schmader K. E. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994 Jun;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988 Jan-Feb;8(1):1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- Englund E., Brun A. White matter changes in dementia of Alzheimer's type: the difference in vulnerability between cell compartments. Histopathology. 1990 May;16(5):433–439. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Frisoni G. B., Calabresi L., Geroldi C., Bianchetti A., D'Acquarica A. L., Govoni S., Sirtori C. R., Trabucchi M., Franceschini G. Apolipoprotein E epsilon 4 allele in Alzheimer's disease and vascular dementia. Dementia. 1994 Sep-Oct;5(5):240–242. doi: 10.1159/000106730. [DOI] [PubMed] [Google Scholar]
- Haan J., Hardy J. A., Roos R. A. Hereditary cerebral hemorrhage with amyloidosis--Dutch type: its importance for Alzheimer research. Trends Neurosci. 1991 Jun;14(6):231–234. doi: 10.1016/0166-2236(91)90120-j. [DOI] [PubMed] [Google Scholar]
- Haan J., Van Broeckhoven C., van Duijn C. M., Voorhoeve E., van Harskamp F., van Swieten J. C., Maat-Schieman M. L., Roos R. A., Bakker E. The apolipoprotein E epsilon 4 allele does not influence the clinical expression of the amyloid precursor protein gene codon 693 or 692 mutations. Ann Neurol. 1994 Sep;36(3):434–437. doi: 10.1002/ana.410360315. [DOI] [PubMed] [Google Scholar]
- Hansen L. A., Galasko D., Samuel W., Xia Y., Chen X., Saitoh T. Apolipoprotein-E epsilon-4 is associated with increased neurofibrillary pathology in the Lewy body variant of Alzheimer's disease. Neurosci Lett. 1994 Nov 21;182(1):63–65. doi: 10.1016/0304-3940(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Hardy J., Crook R., Prihar G., Roberts G., Raghavan R., Perry R. Senile dementia of the Lewy body type has an apolipoprotein E epsilon 4 allele frequency intermediate between controls and Alzheimer's disease. Neurosci Lett. 1994 Nov 21;182(1):1–2. doi: 10.1016/0304-3940(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Harrington C. R., Louwagie J., Rossau R., Vanmechelen E., Perry R. H., Perry E. K., Xuereb J. H., Roth M., Wischik C. M. Influence of apolipoprotein E genotype on senile dementia of the Alzheimer and Lewy body types. Significance for etiological theories of Alzheimer's disease. Am J Pathol. 1994 Dec;145(6):1472–1484. [PMC free article] [PubMed] [Google Scholar]
- Kalaria R. N., Cohen D. L., Premkumar D. R. Apolipoprotein E alleles and brain vascular pathology in Alzheimer's disease. Ann N Y Acad Sci. 1996 Jan 17;777:266–270. doi: 10.1111/j.1749-6632.1996.tb34430.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Golde T., Kroon S. N., Perry G. Serine protease inhibitor antithrombin III and its messenger RNA in the pathogenesis of Alzheimer's disease. Am J Pathol. 1993 Sep;143(3):886–893. [PMC free article] [PubMed] [Google Scholar]
- Kalaria R. N., Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer's disease. Neuroreport. 1995 Feb 15;6(3):477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Sromek S. M., Grahovac I., Harik S. I. Transferrin receptors of rat and human brain and cerebral microvessels and their status in Alzheimer's disease. Brain Res. 1992 Jul 10;585(1-2):87–93. doi: 10.1016/0006-8993(92)91193-i. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N. The blood-brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1992 Fall;4(3):226–260. [PubMed] [Google Scholar]
- Kawai M., Kalaria R. N., Cras P., Siedlak S. L., Velasco M. E., Shelton E. R., Chan H. W., Greenberg B. D., Perry G. Degeneration of vascular muscle cells in cerebral amyloid angiopathy of Alzheimer disease. Brain Res. 1993 Sep 24;623(1):142–146. doi: 10.1016/0006-8993(93)90021-e. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosunen O., Talasniemi S., Lehtovirta M., Heinonen O., Helisalmi S., Mannermaa A., Paljärvi L., Ryynänen M., Riekkinen P. J., Sr, Soininen H. Relation of coronary atherosclerosis and apolipoprotein E genotypes in Alzheimer patients. Stroke. 1995 May;26(5):743–748. doi: 10.1161/01.str.26.5.743. [DOI] [PubMed] [Google Scholar]
- Lippa C. F., Smith T. W., Saunders A. M., Crook R., Pulaski-Salo D., Davies P., Hardy J., Roses A. D., Dickson D. Apolipoprotein E genotype and Lewy body disease. Neurology. 1995 Jan;45(1):97–103. doi: 10.1212/wnl.45.1.97. [DOI] [PubMed] [Google Scholar]
- Ma J., Yee A., Brewer H. B., Jr, Das S., Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mandybur T. I. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986 Jan;45(1):79–90. [PubMed] [Google Scholar]
- Mirra S. S., Heyman A., McKeel D., Sumi S. M., Crain B. J., Brownlee L. M., Vogel F. S., Hughes J. P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991 Feb 8;541(1):163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Nicoll J. A., Roberts G. W., Graham D. I. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995 Feb;1(2):135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Murakami K., Yamada N. Apolipoprotein E genotype and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):737–737. doi: 10.1016/0140-6736(93)91728-5. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown S. M., Mann D. M., Bourke J. P., Roberts D. A., Balderson D., Burns A., Byrne J., Owen F. Apolipoprotein E4 and Alzheimer's disease pathology in Lewy body disease and in other beta-amyloid-forming diseases. Lancet. 1994 May 7;343(8906):1155–1155. doi: 10.1016/s0140-6736(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown S. M., Siddons M., Mann D. M., Owen F., Neary D., Snowden J. S. Apolipoprotein E allelic frequencies in patients with lobar atrophy. Neurosci Lett. 1995 Mar 31;188(3):205–207. doi: 10.1016/0304-3940(95)11425-v. [DOI] [PubMed] [Google Scholar]
- Pirttila T., Lehtimaki T., Nikkari T., Frey H., Mattila K. Apolipoprotein E E4 allele and risk of dementia. JAMA. 1995 Feb 1;273(5):375–376. doi: 10.1001/jama.273.5.375. [DOI] [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Premkumar D. R., Kalaria R. N. Altered expression of amyloid beta precursor mRNAs in cerebral vessels, meninges, and choroid plexus in Alzheimer's disease. Ann N Y Acad Sci. 1996 Jan 17;777:288–292. doi: 10.1111/j.1749-6632.1996.tb34434.x. [DOI] [PubMed] [Google Scholar]
- Rebeck G. W., Reiter J. S., Strickland D. K., Hyman B. T. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993 Oct;11(4):575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Roses A. D. Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994 Sep;53(5):429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Schmader K., Breitner J. C., Benson M. D., Brown W. T., Goldfarb L., Goldgaber D., Manwaring M. G., Szymanski M. H., McCown N. Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer's disease and in other amyloid-forming diseases. Lancet. 1993 Sep 18;342(8873):710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak-Vance M. A., Goldgaber D., Roses A. D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot C., Lendon C., Craddock N., Shears S., Morris J. C., Goate A. Protection against Alzheimer's disease with apoE epsilon 2. Lancet. 1994 Jun 4;343(8910):1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- Vinters H. V. Cerebral amyloid angiopathy. A critical review. Stroke. 1987 Mar-Apr;18(2):311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Vonsattel J. P., Myers R. H., Hedley-Whyte E. T., Ropper A. H., Bird E. D., Richardson E. P., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991 Nov;30(5):637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- Wenham P. R., Price W. H., Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991 May 11;337(8750):1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- West H. L., Rebeck G. W., Hyman B. T. Frequency of the apolipoprotein E epsilon 2 allele is diminished in sporadic Alzheimer disease. Neurosci Lett. 1994 Jul 4;175(1-2):46–48. doi: 10.1016/0304-3940(94)91074-x. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wu E., Lipton R. B., Dickson D. W. Amyloid angiopathy in diffuse Lewy body disease. Neurology. 1992 Nov;42(11):2131–2135. doi: 10.1212/wnl.42.11.2131. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Ishiguro K., Sugihara S., Nakazato Y., Kawarabayashi T., Sun X., Hirai S. Presence of apolipoprotein E on extracellular neurofibrillary tangles and on meningeal blood vessels precedes the Alzheimer beta-amyloid deposition. Acta Neuropathol. 1994;88(5):413–419. doi: 10.1007/BF00389492. [DOI] [PubMed] [Google Scholar]
- Zerr I., Helmhold M., Armstrong V. W., Weber T. Apolipoprotein E in Creutzfeldt-Jakob disease. Lancet. 1995 Jan 7;345(8941):68–69. [PubMed] [Google Scholar]



