Abstract
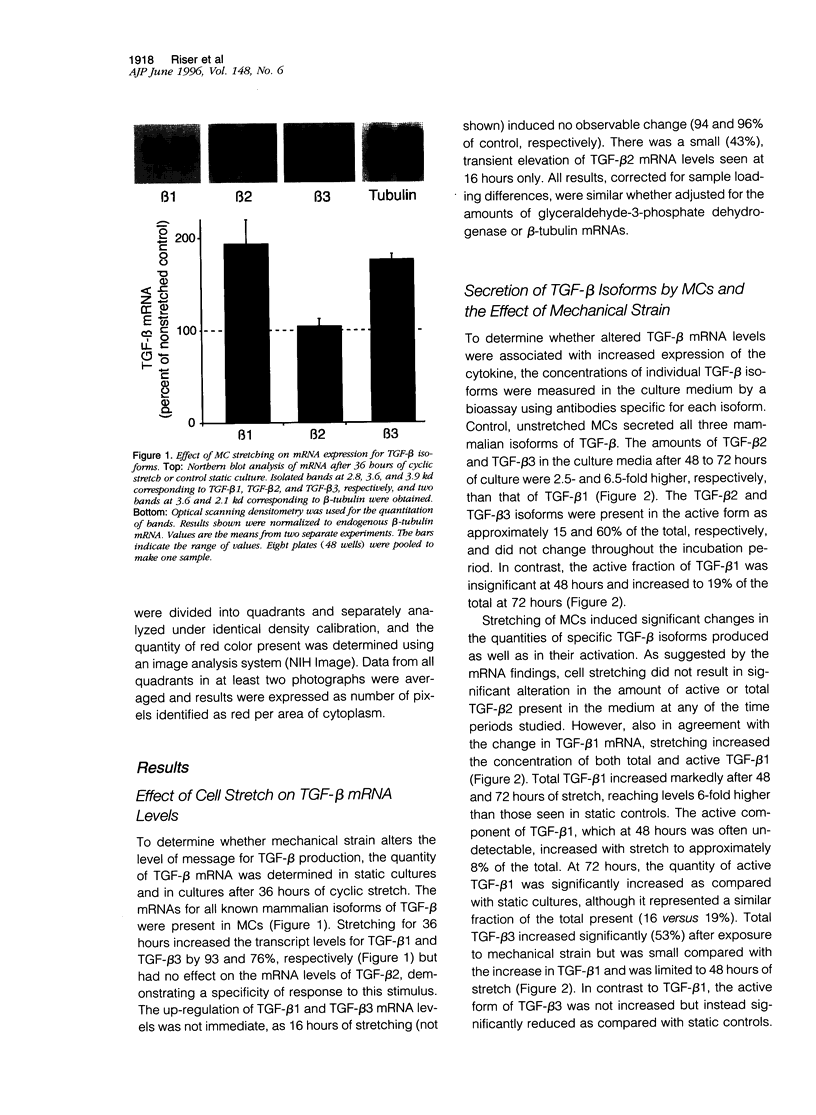
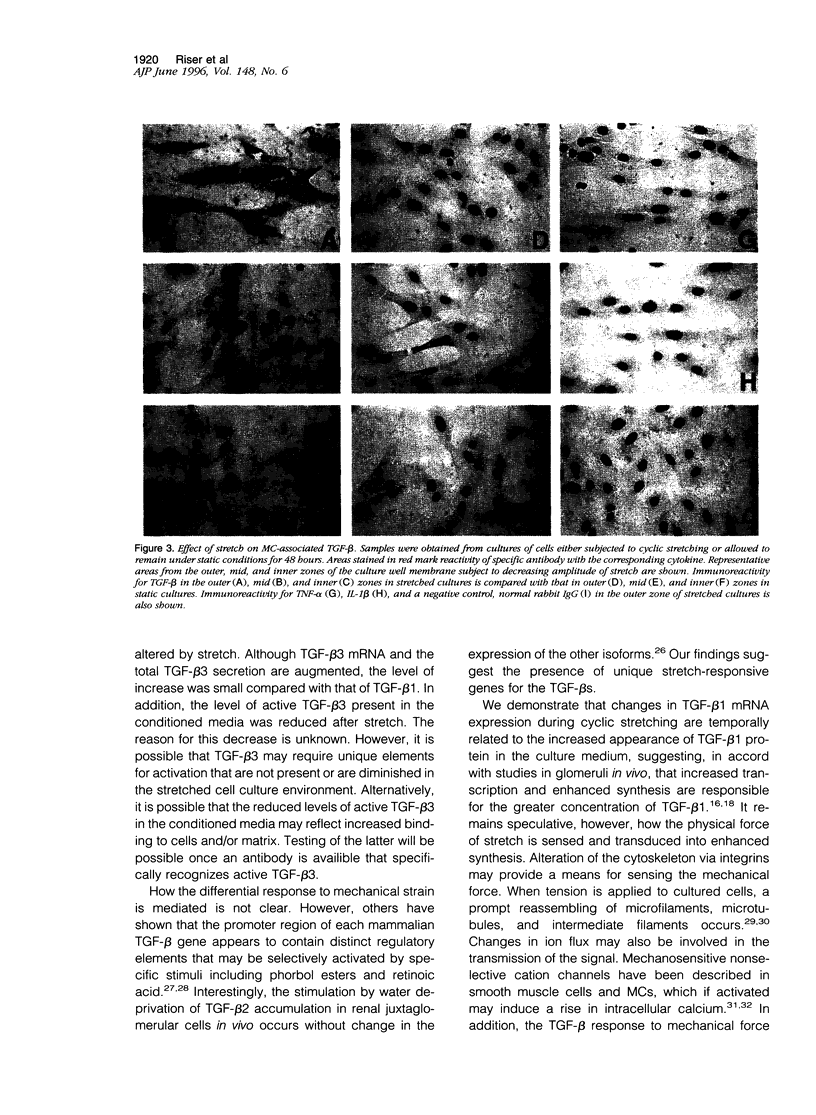
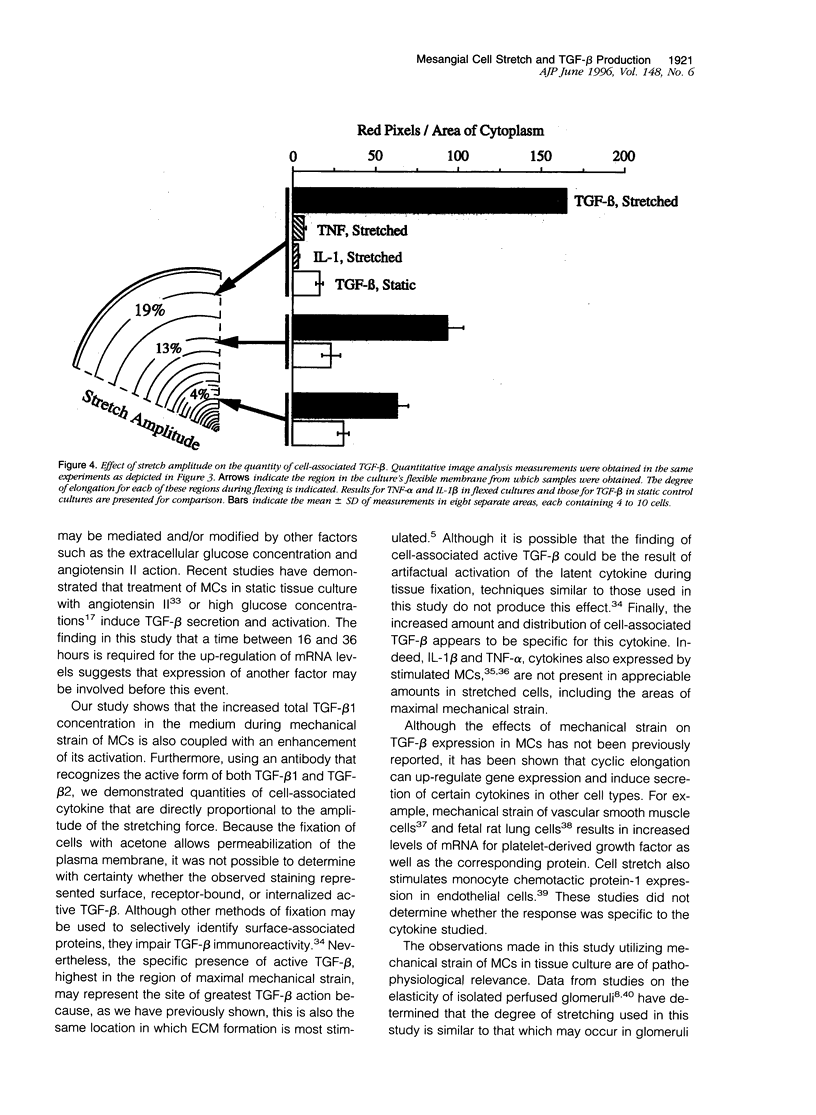
Glomerular distention from increased intraglomerular pressure stretches mesangial cells (MCs). Stretching MCs in culture stimulates extracellular matrix accumulation, suggesting that this may be a mechanism for glomerular hypertension-associated glomerulosclerosis. We examined whether mechanical stretching serves as a stimulus for the synthesis and activation of the prosclerotic molecule transforming growth factor (TGF)-beta, thus providing a potential system for auto-induction of extracellular matrix. Rat MCs cultured on flexible-bottom plates were subjected to cyclic stretching for up to 3 days and then assayed for TGF-beta mRNA, secretion of TGF-beta, and localization of active TGF-beta by immunostaining. MCs contained mRNA for all three mammalian isoforms of TGF-beta. Cyclic stretching for 36 hours increased TGF-beta1 and TGF-beta3 mRNA levels approximately twofold, without altering the levels of TGF-beta2 mRNA. This was followed at 48 to 72 hours by the increased secretion of both latent and active TGF-beta1. Latent, but not active, TGF-beta3 secretion also increased whereas the levels of TGF-beta2 were unaffected by mechanical force. The stretching force in this system is unequally distributed over the culture membrane. Localization of active TGF-beta by immunostaining demonstrated that the quantity of cell-associated cytokine across the culture was directly proportional to the zonal amplitude of the stretching force. These results demonstrate that stretching force stimulates MCs to selectively release and activate TGF-beta1. This mechanical induction of TGF-beta1 may help explain the increased extracellular matrix associated with intraglomerular hypertension.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Meyer T. W., Rennke H. G., Brenner B. M. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985 Aug;76(2):612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Derynck R., Tsang M. L., Weatherbee J. A. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994 Feb;93(2):892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Fouqueray B., Philippe C., Amrani A. Tumor necrosis factor alpha and mesangial cells. Kidney Int. 1992 Mar;41(3):600–603. doi: 10.1038/ki.1992.90. [DOI] [PubMed] [Google Scholar]
- Bidani A. K., Griffin K. A., Picken M., Lansky D. M. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993 Sep;265(3 Pt 2):F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990 Feb;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Coimbra T., Wiggins R., Noh J. W., Merritt S., Phan S. H. Transforming growth factor-beta production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol. 1991 Jan;138(1):223–234. [PMC free article] [PubMed] [Google Scholar]
- Cortes P., Riser B. L., Zhao X., Narins R. G. Glomerular volume expansion and mesangial cell mechanical strain: mediators of glomerular pressure injury. Kidney Int Suppl. 1994 Feb;45:S11–S16. [PubMed] [Google Scholar]
- Craelius W., Ross M. J., Harris D. R., Chen V. K., Palant C. E. Membrane currents controlled by physical forces in cultured mesangial cells. Kidney Int. 1993 Mar;43(3):535–543. doi: 10.1038/ki.1993.80. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Donovitz J. A., Hood J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Dumler F., Cortes P. Uracil ribonucleotide metabolism in rat and human glomerular epithelial and mesangial cells. Am J Physiol. 1988 Dec;255(6 Pt 1):C712–C718. doi: 10.1152/ajpcell.1988.255.6.C712. [DOI] [PubMed] [Google Scholar]
- Fukui M., Nakamura T., Ebihara I., Nagaoka I., Tomino Y., Koide H. Low-protein diet attenuates increased gene expression of platelet-derived growth factor and transforming growth factor-beta in experimental glomerular sclerosis. J Lab Clin Med. 1993 Feb;121(2):224–234. [PubMed] [Google Scholar]
- Harris R. C., Haralson M. A., Badr K. F. Continuous stretch-relaxation in culture alters rat mesangial cell morphology, growth characteristics, and metabolic activity. Lab Invest. 1992 May;66(5):548–554. [PubMed] [Google Scholar]
- Hayashi K., Epstein M., Loutzenhiser R., Forster H. Impaired myogenic responsiveness of the afferent arteriole in streptozotocin-induced diabetic rats: role of eicosanoid derangements. J Am Soc Nephrol. 1992 May;2(11):1578–1586. doi: 10.1681/ASN.V2111578. [DOI] [PubMed] [Google Scholar]
- Horikoshi S., McCune B. K., Ray P. E., Kopp J. B., Sporn M. B., Klotman P. E. Water deprivation stimulates transforming growth factor-beta 2 accumulation in the juxtaglomerular apparatus of mouse kidney. J Clin Invest. 1991 Dec;88(6):2117–2122. doi: 10.1172/JCI115541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest. 1991 Mar;99(3 Suppl):34S–40S. [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S., Border W. A., Miller D. E., Noble N. A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994 Jun;93(6):2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaname S., Uchida S., Ogata E., Kurokawa K. Autocrine secretion of transforming growth factor-beta in cultured rat mesangial cells. Kidney Int. 1992 Dec;42(6):1319–1327. doi: 10.1038/ki.1992.423. [DOI] [PubMed] [Google Scholar]
- Kriz W., Elger M., Lemley K., Sakai T. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int Suppl. 1990 Nov;30:S2–S9. [PubMed] [Google Scholar]
- Lafyatis R., Lechleider R., Kim S. J., Jakowlew S., Roberts A. B., Sporn M. B. Structural and functional characterization of the transforming growth factor beta 3 promoter. A cAMP-responsive element regulates basal and induced transcription. J Biol Chem. 1990 Nov 5;265(31):19128–19136. [PubMed] [Google Scholar]
- Liu M., Liu J., Buch S., Tanswell A. K., Post M. Antisense oligonucleotides for PDGF-B and its receptor inhibit mechanical strain-induced fetal lung cell growth. Am J Physiol. 1995 Aug;269(2 Pt 1):L178–L184. doi: 10.1152/ajplung.1995.269.2.L178. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Larsen A. Cell cycle-dependent interleukin 1 gene expression by cultured glomerular mesangial cells. J Clin Invest. 1988 Jul;82(1):115–122. doi: 10.1172/JCI113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay K., Kondaiah P., Danielpour D., Austin H. A., 3rd, Brown P. D. Expression of transforming growth factor-beta 1 and beta 2 in rat glomeruli. Kidney Int. 1990 Dec;38(6):1095–1100. doi: 10.1038/ki.1990.318. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Laiho M., Ralph D. A., Weis F. M., Zentella A. Transforming growth factor-beta. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Ellis E. N., Sutherland D. E., Brown D. M., Goetz F. C. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984 Oct;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S., Nakamura T., Yamamoto T., Ruoslahti E., Border W. A. Dietary protein restriction rapidly reduces transforming growth factor beta 1 expression in experimental glomerulonephritis. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9765–9769. doi: 10.1073/pnas.88.21.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelayo J. C., Westcott J. Y. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. J Clin Invest. 1991 Jul;88(1):101–105. doi: 10.1172/JCI115264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser B. L., Cortes P., Zhao X., Bernstein J., Dumler F., Narins R. G. Intraglomerular pressure and mesangial stretching stimulate extracellular matrix formation in the rat. J Clin Invest. 1992 Nov;90(5):1932–1943. doi: 10.1172/JCI116071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Kim S. J., Kondaiah P., Jakowlew S. B., Denhez F., Glick A. B., Geiser A. G., Watanabe S., Noma T., Lechleider R. Transcriptional control of expression of the TGF-betas. Ann N Y Acad Sci. 1990;593:43–50. doi: 10.1111/j.1749-6632.1990.tb16098.x. [DOI] [PubMed] [Google Scholar]
- Samuel J. L., Vandenburgh H. H. Mechanically induced orientation of adult rat cardiac myocytes in vitro. In Vitro Cell Dev Biol. 1990 Sep;26(9):905–914. doi: 10.1007/BF02624616. [DOI] [PubMed] [Google Scholar]
- Tamaki K., Okuda S., Ando T., Iwamoto T., Nakayama M., Fujishima M. TGF-beta 1 in glomerulosclerosis and interstitial fibrosis of adriamycin nephropathy. Kidney Int. 1994 Feb;45(2):525–536. doi: 10.1038/ki.1994.68. [DOI] [PubMed] [Google Scholar]
- Varani J., Taylor C. G., Riser B., Shumaker D. K., Yeh K. Y., Dame M., Gibbs D. F., Todd R. F., 3rd, Dumler F., Bromberg J. Mesangial cell killing by leukocytes: role of leukocyte oxidants and proteolytic enzymes. Kidney Int. 1992 Nov;42(5):1169–1177. doi: 10.1038/ki.1992.401. [DOI] [PubMed] [Google Scholar]
- Wang D. L., Wung B. S., Shyy Y. J., Lin C. F., Chao Y. J., Usami S., Chien S. Mechanical strain induces monocyte chemotactic protein-1 gene expression in endothelial cells. Effects of mechanical strain on monocyte adhesion to endothelial cells. Circ Res. 1995 Aug;77(2):294–302. doi: 10.1161/01.res.77.2.294. [DOI] [PubMed] [Google Scholar]
- Wilson E., Mai Q., Sudhir K., Weiss R. H., Ives H. E. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993 Nov;123(3):741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura T., Noble N. A., Ruoslahti E., Border W. A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz R., Dunn B. R., Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986 Jun;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz R., Meyer T. W., Rennke H. G., Brenner B. M. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5963–5967. doi: 10.1073/pnas.82.17.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh F. N., Sharma K., Ericksen M., Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994 Feb;93(2):536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]




