Abstract
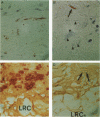
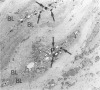
The present study analyzes the staining pattern of DNA in situ end-labeling techniques of human and rabbit atherosclerotic plaques. Both the terminal deoxynucleotidyl transferase end-labeling and the in situ nick translation technique detected, besides apoptotic nuclei, numerous round vesicles with diameters from 0.5 to 5 microns within the atherosclerotic plaques. These vesicles did not contain DNA but contained calcium. A pretreatment with EDTA or citric acid abolished the labeling of the vesicles but did not influence the detection of apoptotic nuclei. Ultrastructurally, the vesicles were of variable diameter and density, and their aspect was compatible with matrix vesicles, which are well known in epiphyses during bone formation. The larger vesicles contained cell organelles, and the small vesicles were very dense. X-ray microanalysis demonstrated high calcium and phosphorus levels within the most dense vesicles. Different stages of the process were present in the plaques. In this way we could demonstrate that cytoplasmic fragmentation of smooth muscle cells and subsequent formation of matrix vesicles are a frequent finding in atherosclerotic plaques. The association of apoptotic cell death and formation of matrix vesicles could be an interesting pathway in explaining calcification of atherosclerotic plaques. Both the terminal deoxynucleotidyl transferase end-labeling and the in situ nick translation technique detected simultaneously apoptotic nuclei and matrix vesicles if calcium is not removed from the sections.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969 Apr;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Bochaton-Piallat M. L., Gabbiani F., Redard M., Desmoulière A., Gabbiani G. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol. 1995 May;146(5):1059–1064. [PMC free article] [PubMed] [Google Scholar]
- Bonucci E. Fine structure and histochemistry of "calcifying globules" in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103(2):192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. J., Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995 Aug;147(2):251–266. [PMC free article] [PubMed] [Google Scholar]
- Greenhill N. S., Presland M. R., Rogers K. M., Stehbens W. E. X-ray microanalysis of mineralized matrix vesicles of experimental saccular aneurysms. Exp Mol Pathol. 1985 Oct;43(2):220–232. doi: 10.1016/0014-4800(85)90042-5. [DOI] [PubMed] [Google Scholar]
- Han D. K., Haudenschild C. C., Hong M. K., Tinkle B. T., Leon M. B., Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol. 1995 Aug;147(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- Isner J. M., Kearney M., Bortman S., Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995 Jun 1;91(11):2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., Cambier B. A., Bortier H. E., De Meyer G. R., Declercq S. C., van Cauwelaert P. A., Bultinck J. Foam cell replication and smooth muscle cell apoptosis in human saphenous vein grafts. Histopathology. 1994 Oct;25(4):365–371. doi: 10.1111/j.1365-2559.1994.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Kockx M. M., De Meyer G. R., Muhring J., Bult H., Bultinck J., Herman A. G. Distribution of cell replication and apoptosis in atherosclerotic plaques of cholesterol-fed rabbits. Atherosclerosis. 1996 Feb;120(1-2):115–124. doi: 10.1016/0021-9150(95)05691-2. [DOI] [PubMed] [Google Scholar]
- Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994 Sep 1;84(5):1415–1420. [PubMed] [Google Scholar]
- Puchtler H., Meloan S. N., Terry M. S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969 Feb;17(2):110–124. doi: 10.1177/17.2.110. [DOI] [PubMed] [Google Scholar]
- Rogers K. M., Stehbens W. E. The morphology of matrix vesicles produced in experimental arterial aneurysms of rabbits. Pathology. 1986 Jan;18(1):64–71. doi: 10.3109/00313028609090830. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Bennett M. R. Death by any other name. Am J Pathol. 1995 Aug;147(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Stehbens W. E. The ultrastructure of the anastomosed vein of experimental arteriovenous fistulae in sheep. Am J Pathol. 1974 Aug;76(2):377–400. [PMC free article] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]