Abstract
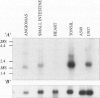
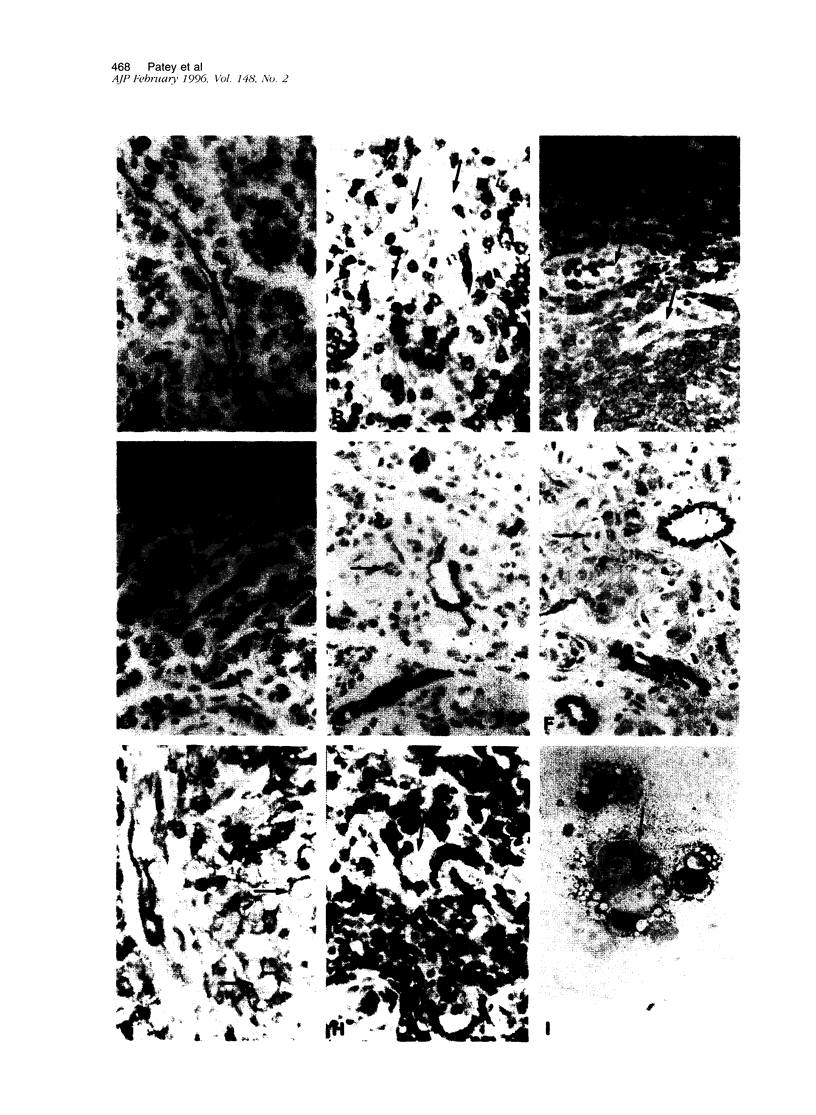
Intercellular adhesion molecule-3 (ICAM-3) was identified as the third counter-receptor for lymphocyte function-associated antigen-1. ICAM-3 is absent on endothelial cells in normal tissues but found on endothelial cells in lymphomas. Here, we examined ICAM-3 expression on vascular endothelial cells in lymphomas, nonlymphoid malignancies, benign tumors, and inflammatory diseases. We compared the expression of ICAM-3 on endothelial cells with the severity of inflammatory infiltrates and with the presence of E-selectin and VCAM-1. We found that ICAM-3 expression on endothelial cells was high on both benign and malignant tumors whereas it was low in inflammatory diseases. In contrast to E-selectin, ICAM-3 expression on endothelial cells was not correlated to the severity of inflammatory infiltrates. In hemangiomas, we showed by Northern blot analysis and immunocytochemistry that ICAM-3 expression was induced and that it was localized in immature areas that sustain the early stages of angiogenesis. Therefore, expression of ICAM-3 on blood vessels does not seem to play a role in the recruitment of leukocytes during inflammation but rather is correlated with angiogenesis and tumor development.
Full text
PDF

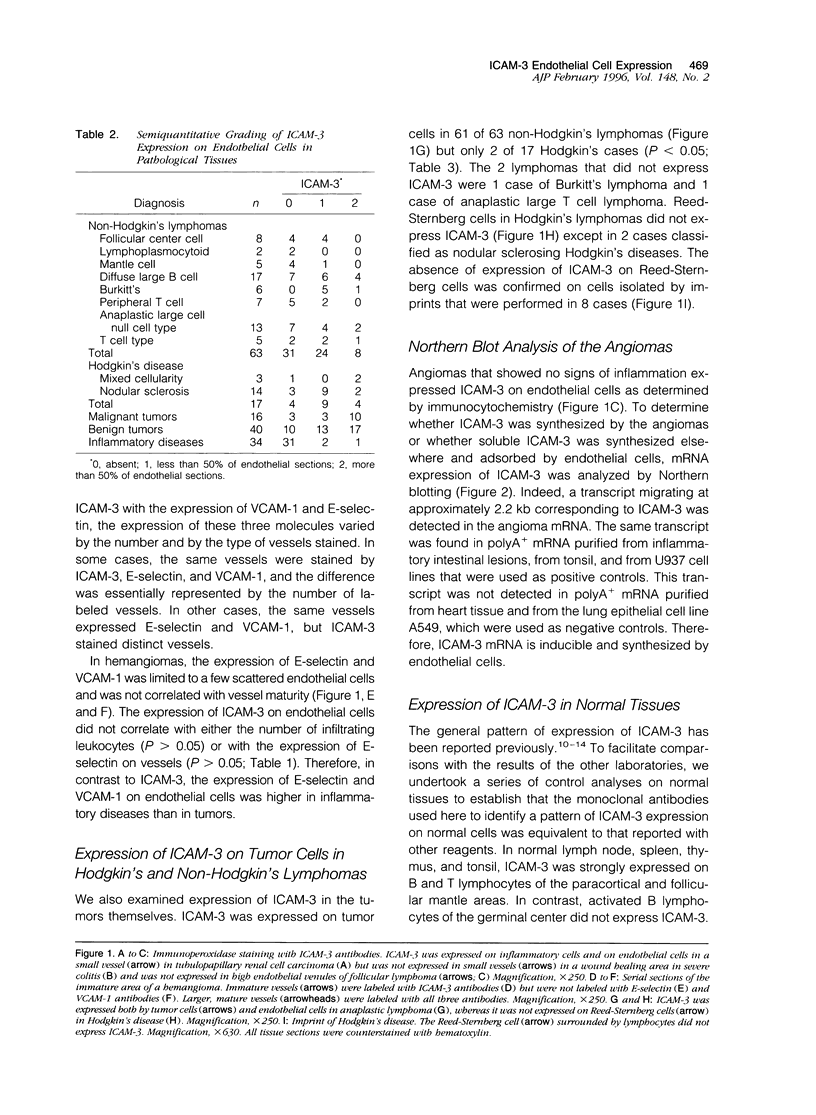
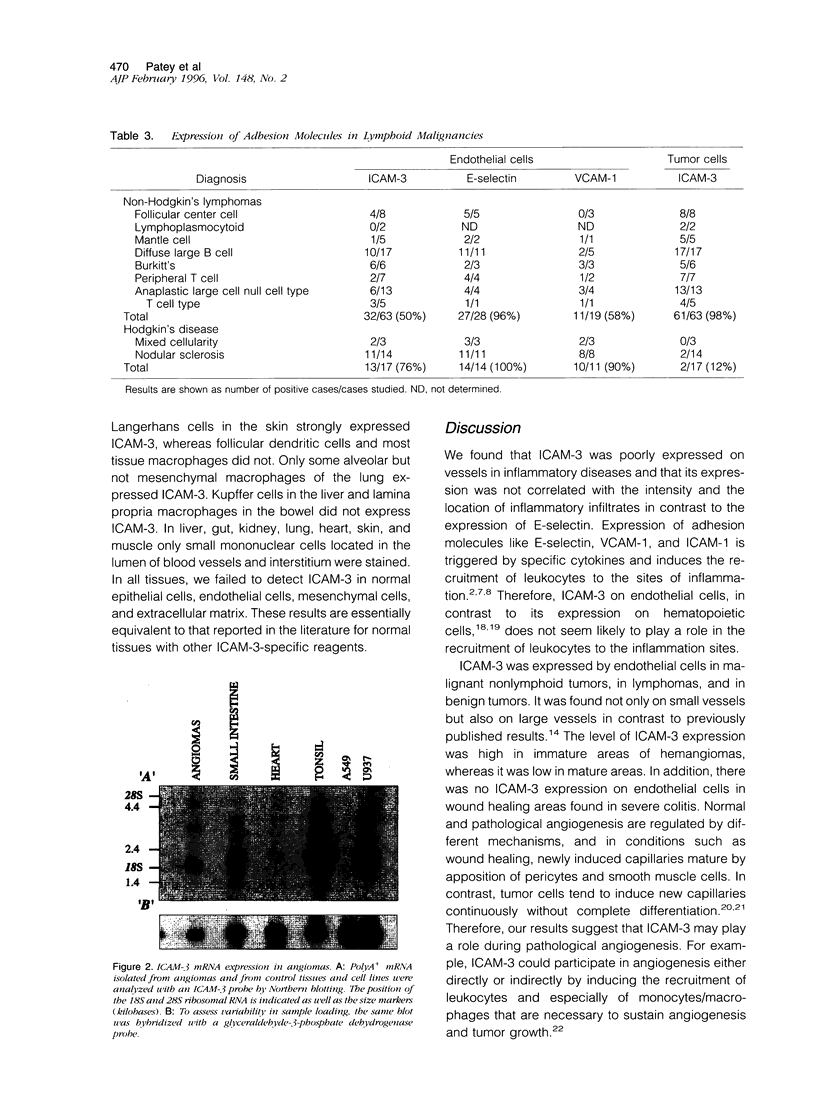


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo A., del Pozo M. A., Arroyo A. G., Sánchez-Mateos P., González-Amaro R., Sánchez-Madrid F. Distribution of ICAM-3-bearing cells in normal human tissues. Expression of a novel counter-receptor for LFA-1 in epidermal Langerhans cells. Am J Pathol. 1993 Sep;143(3):774–783. [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Campanero M. R., Sánchez-Mateos P., del Pozo M. A., Sánchez-Madrid F. ICAM-3 regulates lymphocyte morphology and integrin-mediated T cell interaction with endothelial cell and extracellular matrix ligands. J Cell Biol. 1994 Nov;127(3):867–878. doi: 10.1083/jcb.127.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanero M. R., del Pozo M. A., Arroyo A. G., Sánchez-Mateos P., Hernández-Caselles T., Craig A., Pulido R., Sánchez-Madrid F. ICAM-3 interacts with LFA-1 and regulates the LFA-1/ICAM-1 cell adhesion pathway. J Cell Biol. 1993 Nov;123(4):1007–1016. doi: 10.1083/jcb.123.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Chittal S. M., Caverivière P., Schwarting R., Gerdes J., Al Saati T., Rigal-Huguet F., Stein H., Delsol G. Monoclonal antibodies in the diagnosis of Hodgkin's disease. The search for a rational panel. Am J Surg Pathol. 1988 Jan;12(1):9–21. doi: 10.1097/00000478-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Pulford K., Turley H., Jones M., Micklem K., Doussis I. A., Tyler X., Mayne K., Gatter K. C., Mason D. Y. Cellular distribution of human leucocyte adhesion molecule ICAM-3. J Clin Pathol. 1994 Feb;47(2):143–147. doi: 10.1136/jcp.47.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussis-Anagnostopoulou I., Kaklamanis L., Cordell J., Jones M., Turley H., Pulford K., Simmons D., Mason D., Gatter K. ICAM-3 expression on endothelium in lymphoid malignancy. Am J Pathol. 1993 Oct;143(4):1040–1043. [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Flores L., Gutiérrez R., Varela H. Angiogenesis: an update. Histol Histopathol. 1994 Oct;9(4):807–843. [PubMed] [Google Scholar]
- Fawcett J., Holness C. L., Needham L. A., Turley H., Gatter K. C., Mason D. Y., Simmons D. L. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature. 1992 Dec 3;360(6403):481–484. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Hernandez-Caselles T., Rubio G., Campanero M. R., del Pozo M. A., Muro M., Sanchez-Madrid F., Aparicio P. ICAM-3, the third LFA-1 counterreceptor, is a co-stimulatory molecule for both resting and activated T lymphocytes. Eur J Immunol. 1993 Nov;23(11):2799–2806. doi: 10.1002/eji.1830231112. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Piali L., Fichtel A., Terpe H. J., Imhof B. A., Gisler R. H. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med. 1995 Feb 1;181(2):811–816. doi: 10.1084/jem.181.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J., Cotran R. S. What can be learned from the expression of endothelial adhesion molecules in tissues? Lab Invest. 1991 Mar;64(3):301–305. [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Sunderkötter C., Steinbrink K., Goebeler M., Bhardwaj R., Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994 Mar;55(3):410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Vazeux R., Hoffman P. A., Tomita J. K., Dickinson E. S., Jasman R. L., St John T., Gallatin W. M. Cloning and characterization of a new intercellular adhesion molecule ICAM-R. Nature. 1992 Dec 3;360(6403):485–488. doi: 10.1038/360485a0. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]