Abstract
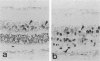
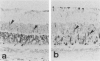
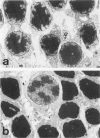
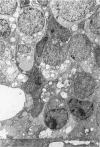
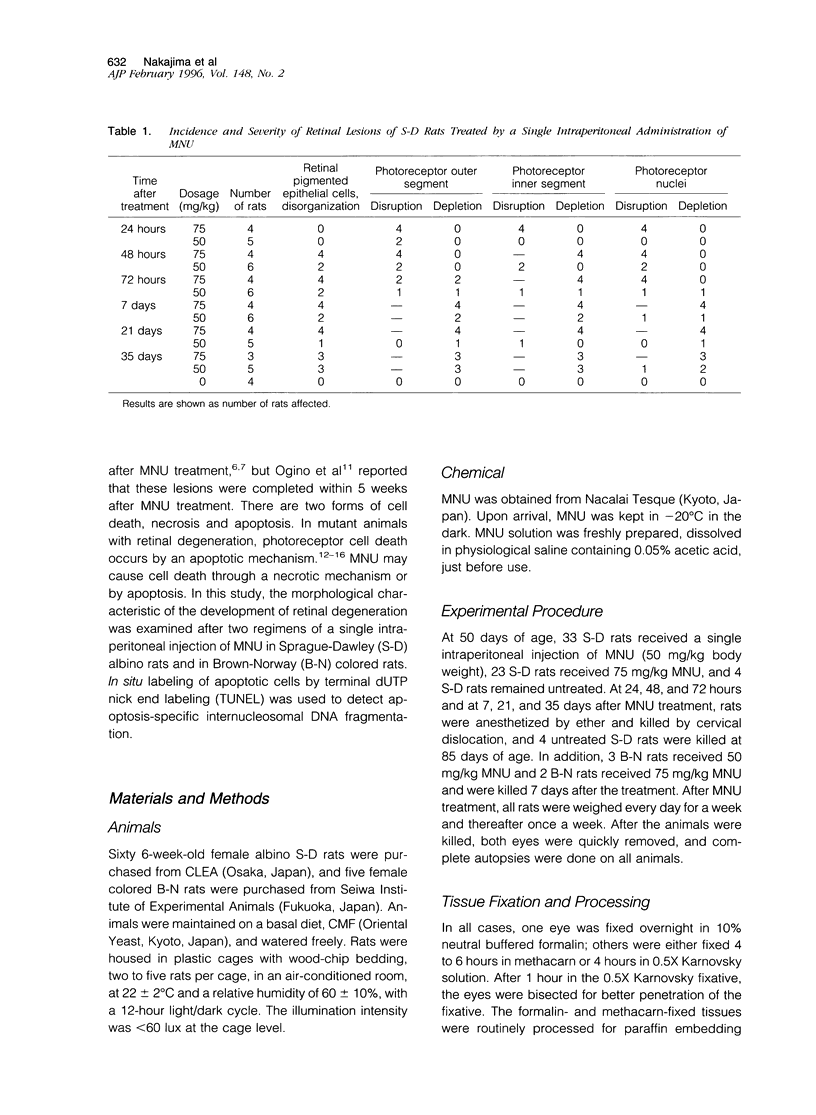
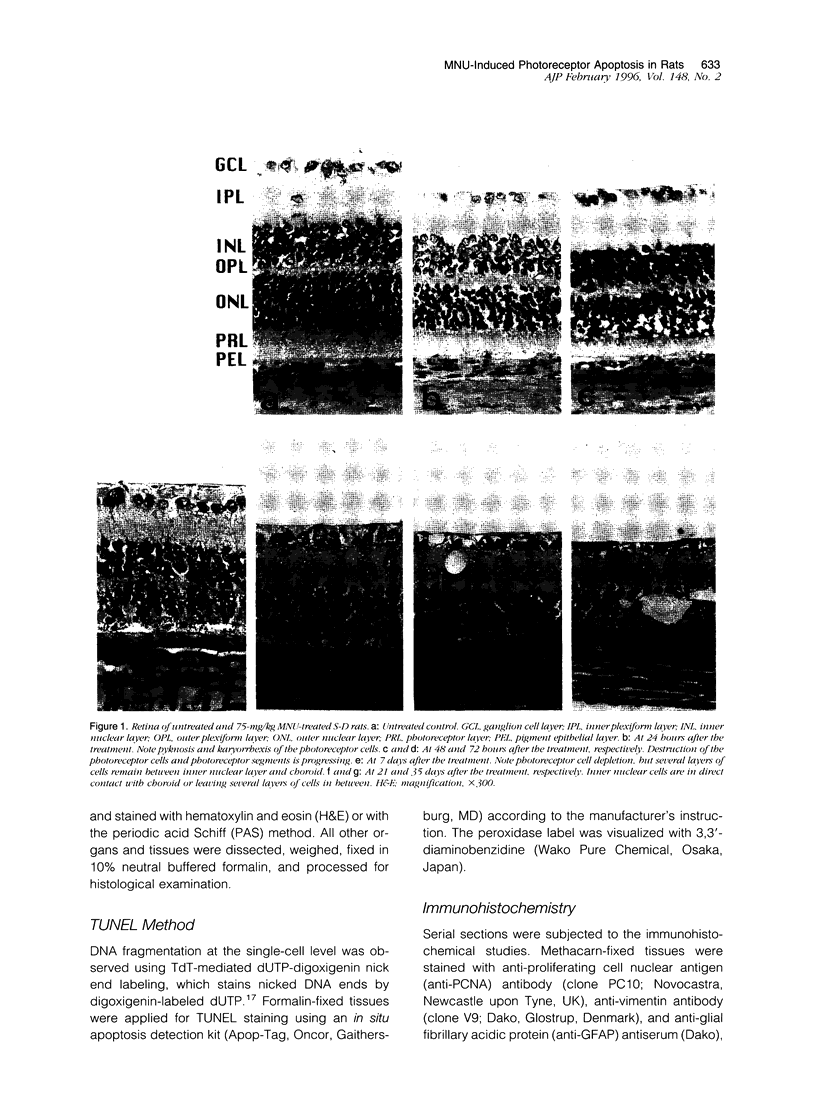
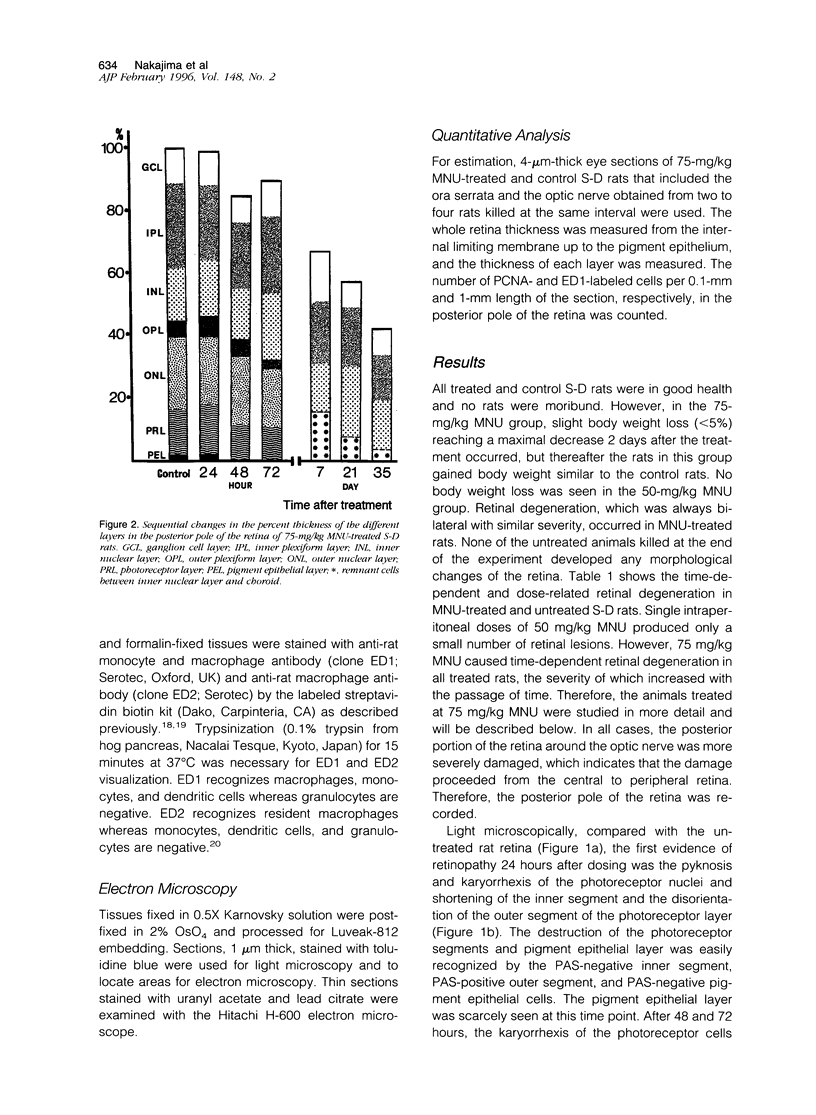
Retinal degeneration was induced by a single intraperitoneal injection of N-methyl-N-nitrosourea in female Sprague-Dawley albino rats at 50 days of age by two dose regimens, which were observed sequentially at 24, 48, and 72 hours and 7, 21, and 35 days after the treatment. After a dose of 75 mg/kg, methylnitrosourea evoked progressive retinal degeneration in all treated rats whereas a dose of 50 mg/kg was less effective. The 75-mg/kg-treated rats showed selective destruction of the photoreceptor cells by an apoptotic mechanism, as confirmed morphologically and by the terminal dUTP nick end labeling method. Apoptosis had already started at 24 hours after the treatment and was completed by day 7. During the photoreceptor degeneration, proliferation of glial fibrillary acidic protein and vimentin-positive Müller cells as detected by proliferating cell nuclear antigen labeling appeared at 48 hours and was prominent 72 hours after the treatment, and macrophage infiltration within the retina as recognized by ED1 positivity was maximal 7 and 21 days after the treatment. Retinal degeneration was also induced in female Brown-Norway colored rats in a similar dose-dependent manner. Pigment epithelium was discontinuous above Bruch's membrane, and migration of the swollen pigment epithelium toward the inner nuclear layer was seen 7 days after the treatment. Therefore, as also confirmed electron microscopically, the most striking change was the destruction of photoreceptor cells by the apoptotic process, followed by Müller cell proliferation, pigment epithelium migration, and macrophage infiltration for cell debris phagocytosis, resulting in a thin remnant of retina with attenuated inner nuclear cells in direct contact with Bruch's membrane or with the pigment epithelium and/or with the Müller cells 35 days after the treatment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. M., Puliafito C. A., Haluska F. G., Kimball G. P., Robinson N. L. Induction of ocular neoplasms in Wistar rat by N-methyl-N-nitrosourea. Exp Eye Res. 1986 Jan;42(1):83–86. doi: 10.1016/0014-4835(86)90019-9. [DOI] [PubMed] [Google Scholar]
- Bellhorn R. W., Bellhorn M., Friedman A. H., Henkind P. Urethan-induced retinopathy in pigmented rats. Invest Ophthalmol. 1973 Jan;12(1):65–76. [PubMed] [Google Scholar]
- Bignami A., Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979 Jan;28(1):63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- Björklund H., Bignami A., Dahl D. Immunohistochemical demonstration of glial fibrillary acidic protein in normal rat Müller glia and retinal astrocytes. Neurosci Lett. 1985 Mar 15;54(2-3):363–368. [PubMed] [Google Scholar]
- Bogovski P., Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27(4):471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- Bok D., Hall M. O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971 Jun;49(3):664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caley D. W., Johnson C., Liebelt R. A. The postnatal development of the retina in the normal and rodless CBA mouse: a light and electron microscopic study. Am J Anat. 1972 Feb;133(2):179–212. doi: 10.1002/aja.1001330205. [DOI] [PubMed] [Google Scholar]
- Chaitin M. H., Hall M. O. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci. 1983 Jul;24(7):812–820. [PubMed] [Google Scholar]
- DiLoreto D., Jr, Cox C., Grover D. A., Lazar E., del Cerro C., del Cerro M. The influences of age, retinal topography, and gender on retinal degeneration in the Fischer 344 rat. Brain Res. 1994 Jun 6;647(2):181–191. doi: 10.1016/0006-8993(94)91316-1. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Eisenfeld A. J., Bunt-Milam A. H., Sarthy P. V. Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984 Nov;25(11):1321–1328. [PubMed] [Google Scholar]
- Ekström P., Sanyal S., Narfström K., Chader G. J., van Veen T. Accumulation of glial fibrillary acidic protein in Müller radial glia during retinal degeneration. Invest Ophthalmol Vis Sci. 1988 Sep;29(9):1363–1371. [PubMed] [Google Scholar]
- Gartner S., Henkind P. Pathology of retinitis pigmentosa. Ophthalmology. 1982 Dec;89(12):1425–1432. doi: 10.1016/s0161-6420(82)34620-5. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M. H., Rutty D. A., Wood R. D. Differences in the retinotoxic action of chloroquine and phenothiazine derivatives. J Pathol. 1970 Nov;102(3):139–150. doi: 10.1002/path.1711020304. [DOI] [PubMed] [Google Scholar]
- Herrold K. M. Pigmentary degeneration of the retina induced by N-methyl-N-nitrosourea. An experimental study in syrian hamsters. Arch Ophthalmol. 1967 Nov;78(5):650–653. doi: 10.1001/archopht.1967.00980030652017. [DOI] [PubMed] [Google Scholar]
- Heywood R., Gopinath C. Morphological assessment of visual dysfunction. Toxicol Pathol. 1990;18(1 Pt 2):204–217. doi: 10.1177/019262339001800126. [DOI] [PubMed] [Google Scholar]
- LASANSKY A., DE ROBERTIS E. Submicroscopic changes in visual cells of the rabbit induced by iodoacetate. J Biophys Biochem Cytol. 1959 Mar 25;5(2):245–250. doi: 10.1083/jcb.5.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. P., Gibson J. R., Sherman H. Retinopathic effects of 2-aminooxy propionic acid derivatives in the rat. Toxicol Appl Pharmacol. 1979 Nov;51(2):219–232. doi: 10.1016/0041-008x(79)90464-2. [DOI] [PubMed] [Google Scholar]
- Lee K. P., Valentine R. Pathogenesis and reversibility of retinopathy induced by 1,4-bis (4-aminophenoxy)-2-phenylbenzene (2-phenyl-APB-144) in pigmented rats. Arch Toxicol. 1991;65(4):292–303. doi: 10.1007/BF01968963. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Rong H., Craft C. M. Linkage of photoreceptor degeneration by apoptosis with inherited defect in phototransduction. Invest Ophthalmol Vis Sci. 1994 Feb;35(2):358–362. [PubMed] [Google Scholar]
- Murthy A. S., Vawter G. F., Kopito L., Rossen E. Retinal atrophy and cataract in rats following administration of N-methyl-N-nitrosourea. Proc Soc Exp Biol Med. 1972 Jan;139(1):84–87. doi: 10.3181/00379727-139-36083. [DOI] [PubMed] [Google Scholar]
- Ogino H., Ito M., Matsumoto K., Yagyu S., Tsuda H., Hirono I., Wild C. P., Montesano R. Retinal degeneration induced by N-methyl-N-nitrosourea and detection of 7-methyldeoxyguanosine in the rat retina. Toxicol Pathol. 1993;21(1):21–25. doi: 10.1177/019262339302100103. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C., Sung C. H., Nathans J., Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., De Ruiter A., Hawkins R. K. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980 Nov 1;194(1):193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Yielding K. L. Retinal degeneration in the mouse. A model induced transplacentally by methylnitrosourea. Exp Eye Res. 1986 Nov;43(5):791–801. doi: 10.1016/s0014-4835(86)80010-0. [DOI] [PubMed] [Google Scholar]
- Spitznas M., Luciano L., Reale E. Occluding junctions surrounding cystoid spaces in the human peripheral retina. A thin-section and freeze-fracture study. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;217(3):155–165. doi: 10.1007/BF00411146. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Zhang C., Abler A. S., Chang C. J., Wong F., Chang G. Q., Lam T. T. Apoptosis leads to photoreceptor degeneration in inherited retinal dystrophy of RCS rats. Invest Ophthalmol Vis Sci. 1994 May;35(6):2693–2699. [PubMed] [Google Scholar]
- Tyler N. K., Burns M. S. Alterations in glial cell morphology and glial fibrillary acidic protein expression in urethane-induced retinopathy. Invest Ophthalmol Vis Sci. 1991 Feb;32(2):246–256. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Young R. W. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984 Nov 1;229(3):362–373. doi: 10.1002/cne.902290307. [DOI] [PubMed] [Google Scholar]
- Yuge K., Nakajima M., Uemura Y., Miki H., Uyama M., Tsubura A. Immunohistochemical features of the human retina and retinoblastoma. Virchows Arch. 1995;426(6):571–575. doi: 10.1007/BF00192111. [DOI] [PubMed] [Google Scholar]