Abstract
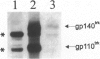
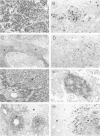
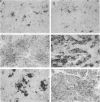
Primitive neuroectodermal tumors (PNETs) of the central nervous system (CNS) are poorly understood childhood neoplasms, and medulloblastomas are the most common pediatric PNETs. Neoplastic cells in medulloblastomas and other PNETs resemble progenitor cells of the developing central nervous system, but they also may exhibit the molecular phenotype of immature neurons or glia. As neurotrophins play a role in regulating differentiation, proliferation, and cell death in the normal developing central nervous system, and recent evidence suggests that neurotrophins may influence the behavior of medulloblastomas, we studied 29 PNET biopsy samples (27 of which were posterior fossa medulloblastomas) by immunobistochemistry using antibodies specific for each of the major high affinity neurotrophin receptor proteins, ie, TrkA, TrkB, and TrkC. A subset of these tumors also was examined by Western blot. Immunoreactive TrkA, TrkB, and TrkC were observed in neoplastic cells in 8 (27%), 18 (62%), and 14 (48%) of these PNETs, respectively. Additional immunohistochemical studies of a subset of these PNETs using antibodies to neurotrophins that primarily activate TrkB and TrkC, ie, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5, showed that immunoreactive brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 were detected in 22, 9, and 19% of these PNET biopsies, respectively. Finally, 19 pediatric brain tumors other than these PNETs also were studied here, and they expressed these neurotrophins and their receptors to a variable extent. The demonstration here that neurotrophins and their cognate receptor proteins are expressed in PNETs as well as in other pediatric brain tumors may imply that signal transduction pathways mediated by neurotrophins and/or their receptors influence the induction or progression of these common childhood neoplasms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allendoerfer K. L., Cabelli R. J., Escandón E., Kaplan D. R., Nikolics K., Shatz C. J. Regulation of neurotrophin receptors during the maturation of the mammalian visual system. J Neurosci. 1994 Mar;14(3 Pt 2):1795–1811. doi: 10.1523/JNEUROSCI.14-03-01795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. L., Molenaar W. M., Trojanowski J. Q., Evans A. E., Ross A. H., Rorke L. B., Packer R. J., Lee V. M., Pleasure D. Nerve growth factor receptor expression in peripheral and central neuroectodermal tumors, other pediatric brain tumors, and during development of the adrenal gland. Am J Pathol. 1991 Jul;139(1):115–122. [PMC free article] [PubMed] [Google Scholar]
- Baker D. L., Reddy U. R., Pleasure S., Hardy M., Williams M., Tartaglione M., Biegel J. A., Emanuel B. S., Lo Presti P., Kreider B. Human central nervous system primitive neuroectodermal tumor expressing nerve growth factor receptors: CHP707m. Ann Neurol. 1990 Aug;28(2):136–145. doi: 10.1002/ana.410280205. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994 Nov;25(11):1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Becker L. E., Hinton D. Primitive neuroectodermal tumors of the central nervous system. Hum Pathol. 1983 Jun;14(6):538–550. doi: 10.1016/s0046-8177(83)80006-9. [DOI] [PubMed] [Google Scholar]
- Berkemeier L. R., Winslow J. W., Kaplan D. R., Nikolics K., Goeddel D. V., Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991 Nov;7(5):857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Burger P. C., Grahmann F. C., Bliestle A., Kleihues P. Differentiation in the medulloblastoma. A histological and immunohistochemical study. Acta Neuropathol. 1987;73(2):115–123. doi: 10.1007/BF00693776. [DOI] [PubMed] [Google Scholar]
- Caputy A. J., McCullough D. C., Manz H. J., Patterson K., Hammock M. K. A review of the factors influencing the prognosis of medulloblastoma. The importance of cell differentiation. J Neurosurg. 1987 Jan;66(1):80–87. doi: 10.3171/jns.1987.66.1.0080. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Chao M. V., Hempstead B. L. p75 and Trk: a two-receptor system. Trends Neurosci. 1995 Jul;18(7):321–326. [PubMed] [Google Scholar]
- Davies A. M. The role of neurotrophins in the developing nervous system. J Neurobiol. 1994 Nov;25(11):1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- Donovan M. J., Hempstead B. L., Horvath C., Chao M. V., Schofield D. Immunohistochemical localization of Trk receptor protein in pediatric small round blue cell tumors. Am J Pathol. 1993 Dec;143(6):1560–1567. [PMC free article] [PubMed] [Google Scholar]
- Donovan M. J., Hempstead B., Huber L. J., Kaplan D., Tsoulfas P., Chao M., Parada L., Schofield D. Identification of the neurotrophin receptors p75 and trk in a series of Wilms' tumors. Am J Pathol. 1994 Oct;145(4):792–801. [PMC free article] [PubMed] [Google Scholar]
- Donovan M. J., Miranda R. C., Kraemer R., McCaffrey T. A., Tessarollo L., Mahadeo D., Sharif S., Kaplan D. R., Tsoulfas P., Parada L. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol. 1995 Aug;147(2):309–324. [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. D., Kaplan D. R., Price D. L., Koliatsos V. E. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995 Jul;130(1):149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung K. M., Trojanowski J. Q. Animal models of medulloblastomas and related primitive neuroectodermal tumors. A review. J Neuropathol Exp Neurol. 1995 May;54(3):285–296. doi: 10.1097/00005072-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Jansson D. S., Molenaar W. M., Rorke L. B., Trojanowski J. Q., Lee V. M., Packer R. J., Franke W. W. Primitive neuroectodermal tumors of the central nervous system. Patterns of expression of neuroendocrine markers, and all classes of intermediate filament proteins. Lab Invest. 1990 Apr;62(4):498–509. [PubMed] [Google Scholar]
- Götz R., Köster R., Winkler C., Raulf F., Lottspeich F., Schartl M., Thoenen H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature. 1994 Nov 17;372(6503):266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- Hart M. N., Earle K. M. Primitive neuroectodermal tumors of the brain in children. Cancer. 1973 Oct;32(4):890–897. doi: 10.1002/1097-0142(197310)32:4<890::aid-cncr2820320421>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hensler P. J., Pereira-Smith O. M. Human replicative senescence. A molecular study. Am J Pathol. 1995 Jul;147(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Matsumoto K., Lucarelli E., Thiele C. J. Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Eukaryotic Signal Transduction Group. Neuron. 1993 Aug;11(2):321–331. doi: 10.1016/0896-6273(93)90187-v. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Stephens R. M. Neurotrophin signal transduction by the Trk receptor. J Neurobiol. 1994 Nov;25(11):1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- Katsetos C. D., Herman M. M., Frankfurter A., Gass P., Collins V. P., Walker C. C., Rosemberg S., Barnard R. O., Rubinstein L. J. Cerebellar desmoplastic medulloblastomas. A further immunohistochemical characterization of the reticulin-free pale islands. Arch Pathol Lab Med. 1989 Sep;113(9):1019–1029. [PubMed] [Google Scholar]
- Katsetos C. D., Liu H. M., Zacks S. I. Immunohistochemical and ultrastructural observations on Homer Wright (neuroblastic) rosettes and the "pale islands" of human cerebellar medulloblastomas. Hum Pathol. 1988 Oct;19(10):1219–1227. doi: 10.1016/s0046-8177(88)80155-2. [DOI] [PubMed] [Google Scholar]
- Kleppner S. R., Robinson K. A., Trojanowski J. Q., Lee V. M. Transplanted human neurons derived from a teratocarcinoma cell line (NTera-2) mature, integrate, and survive for over 1 year in the nude mouse brain. J Comp Neurol. 1995 Jul 10;357(4):618–632. doi: 10.1002/cne.903570410. [DOI] [PubMed] [Google Scholar]
- Kogner P., Barbany G., Dominici C., Castello M. A., Raschellá G., Persson H. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res. 1993 May 1;53(9):2044–2050. [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993 Jul;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanishi T., Washiyama K., Watabe K., Sekiguchi K. Glial fibrillary acidic protein in medulloblastomas. Acta Neuropathol. 1985;67(1-2):1–5. doi: 10.1007/BF00688118. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Page C. D., Wu H. L., Schlaepfer W. W. Monoclonal antibodies to gel-excised glial filament protein and their reactivities with other intermediate filament proteins. J Neurochem. 1984 Jan;42(1):25–32. doi: 10.1111/j.1471-4159.1984.tb09692.x. [DOI] [PubMed] [Google Scholar]
- Mannoji H., Takeshita I., Fukui M., Ohta M., Kitamura K. Glial fibrillary acidic protein in medulloblastoma. Acta Neuropathol. 1981;55(1):63–69. doi: 10.1007/BF00691533. [DOI] [PubMed] [Google Scholar]
- Miller D. C., Koslow M., Budzilovich G. N., Burstein D. E. Synaptophysin: a sensitive and specific marker for ganglion cells in central nervous system neoplasms. Hum Pathol. 1990 Mar;21(3):271–276. doi: 10.1016/0046-8177(90)90226-u. [DOI] [PubMed] [Google Scholar]
- Miranda R. C., Sohrabji F., Toran-Allerand C. D. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar W. M., Jansson D. S., Gould V. E., Rorke L. B., Franke W. W., Lee V. M., Packer R. J., Trojanowski J. Q. Molecular markers of primitive neuroectodermal tumors and other pediatric central nervous system tumors. Monoclonal antibodies to neuronal and glial antigens distinguish subsets of primitive neuroectodermal tumors. Lab Invest. 1989 Dec;61(6):635–643. [PubMed] [Google Scholar]
- Nakagawara A., Arima-Nakagawara M., Scavarda N. J., Azar C. G., Cantor A. B., Brodeur G. M. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993 Mar 25;328(12):847–854. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- Packer R. J., Sutton L. N., Goldwein J. W., Perilongo G., Bunin G., Ryan J., Cohen B. H., D'Angio G., Kramer E. D., Zimmerman R. A. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg. 1991 Mar;74(3):433–440. doi: 10.3171/jns.1991.74.3.0433. [DOI] [PubMed] [Google Scholar]
- Packer R. J., Sutton L. N., Rorke L. B., Littman P. A., Sposto R., Rosenstock J. G., Bruce D. A., Schut L. Prognostic importance of cellular differentiation in medulloblastoma of childhood. J Neurosurg. 1984 Aug;61(2):296–301. doi: 10.3171/jns.1984.61.2.0296. [DOI] [PubMed] [Google Scholar]
- Pleasure S. J., Reddy U. R., Venkatakrishnan G., Roy A. K., Chen J., Ross A. H., Trojanowski J. Q., Pleasure D. E., Lee V. M. Introduction of nerve growth factor (NGF) receptors into a medulloblastoma cell line results in expression of high- and low-affinity NGF receptors but not NGF-mediated differentiation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8496–8500. doi: 10.1073/pnas.87.21.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabizadeh S., Oh J., Zhong L. T., Yang J., Bitler C. M., Butcher L. L., Bredesen D. E. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993 Jul 16;261(5119):345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Rorke L. B., Gilles F. H., Davis R. L., Becker L. E. Revision of the World Health Organization classification of brain tumors for childhood brain tumors. Cancer. 1985 Oct 1;56(7 Suppl):1869–1886. doi: 10.1002/1097-0142(19851001)56:7+<1869::aid-cncr2820561330>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rorke L. B. The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol. 1983 Jan;42(1):1–15. [PubMed] [Google Scholar]
- Ross A. H., Grob P., Bothwell M., Elder D. E., Ernst C. S., Marano N., Ghrist B. F., Slemp C. C., Herlyn M., Atkinson B. Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6681–6685. doi: 10.1073/pnas.81.21.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer D., Cavalla P., Chiò A., Giordana M. T., Marino S., Mauro A., Migheli A. Tumor cell proliferation and apoptosis in medulloblastoma. Acta Neuropathol. 1994;87(4):362–370. doi: 10.1007/BF00313605. [DOI] [PubMed] [Google Scholar]
- Schwechheimer K., Wiedenmann B., Franke W. W. Synaptophysin: a reliable marker for medulloblastomas. Virchows Arch A Pathol Anat Histopathol. 1987;411(1):53–59. doi: 10.1007/BF00734514. [DOI] [PubMed] [Google Scholar]
- Segal R. A., Goumnerova L. C., Kwon Y. K., Stiles C. D., Pomeroy S. L. Expression of the neurotrophin receptor TrkC is linked to a favorable outcome in medulloblastoma. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12867–12871. doi: 10.1073/pnas.91.26.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal R. A., Takahashi H., McKay R. D. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992 Dec;9(6):1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- Stiller C. A., Bunch K. J. Trends in survival for childhood cancer in Britain diagnosed 1971-85. Br J Cancer. 1990 Nov;62(5):806–815. doi: 10.1038/bjc.1990.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlhyama T., Lee V. M., Trojanowski J. Q. Co-expression of low molecular weight neurofilament protein and glial fibrillary acidic protein in established human glioma cell lines. Am J Pathol. 1993 Mar;142(3):883–892. [PMC free article] [PubMed] [Google Scholar]
- Tohyama T., Lee V. M., Rorke L. B., Marvin M., McKay R. D., Trojanowski J. Q. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992 Mar;66(3):303–313. [PubMed] [Google Scholar]
- Tremblay G. F., Lee V. M., Trojanowski J. Q. Expression of vimentin, glial filament, and neurofilament proteins in primitive childhood brain tumors. A comparative immunoblot and immunoperoxidase study. Acta Neuropathol. 1985;68(3):239–244. doi: 10.1007/BF00690201. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Tohyama T., Lee V. M. Medulloblastomas and related primitive neuroectodermal brain tumors of childhood recapitulate molecular milestones in the maturation of neuroblasts. Mol Chem Neuropathol. 1992 Oct;17(2):121–135. doi: 10.1007/BF03159987. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Yachnis A. T., Rorke L. B., Lee V. M., Trojanowski J. Q. Expression of neuronal and glial polypeptides during histogenesis of the human cerebellar cortex including observations on the dentate nucleus. J Comp Neurol. 1993 Aug 15;334(3):356–369. doi: 10.1002/cne.903340303. [DOI] [PubMed] [Google Scholar]
- Yachnis A. T., Rorke L. B., Trojanowski J. Q. Cerebellar dysplasias in humans: development and possible relationship to glial and primitive neuroectodermal tumors of the cerebellar vermis. J Neuropathol Exp Neurol. 1994 Jan;53(1):61–71. doi: 10.1097/00005072-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Yachnis A. T., Trojanowski J. Q. Studies of childhood brain tumors using immunohistochemistry and microwave technology: methodological considerations. J Neurosci Methods. 1994 Dec;55(2):191–200. doi: 10.1016/0165-0270(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Yan Q., Matheson C., Sun J., Radeke M. J., Feinstein S. C., Miller J. A. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp Neurol. 1994 May;127(1):23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]