Abstract
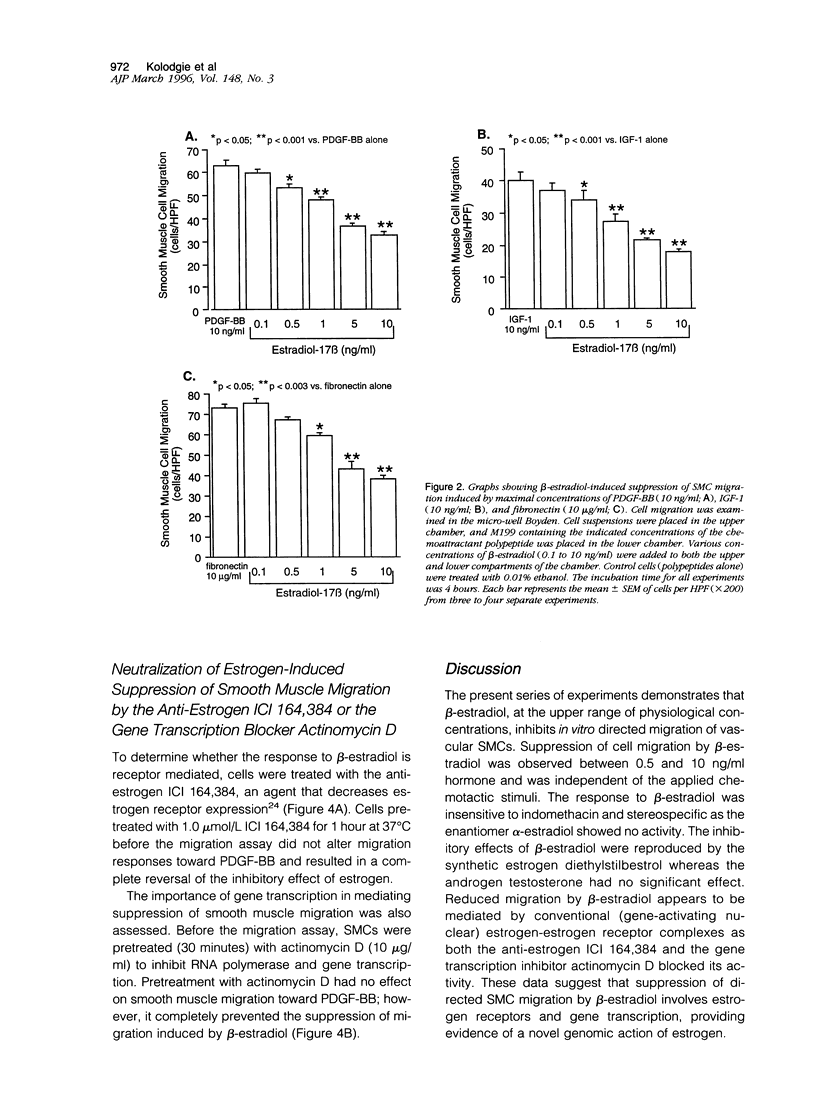
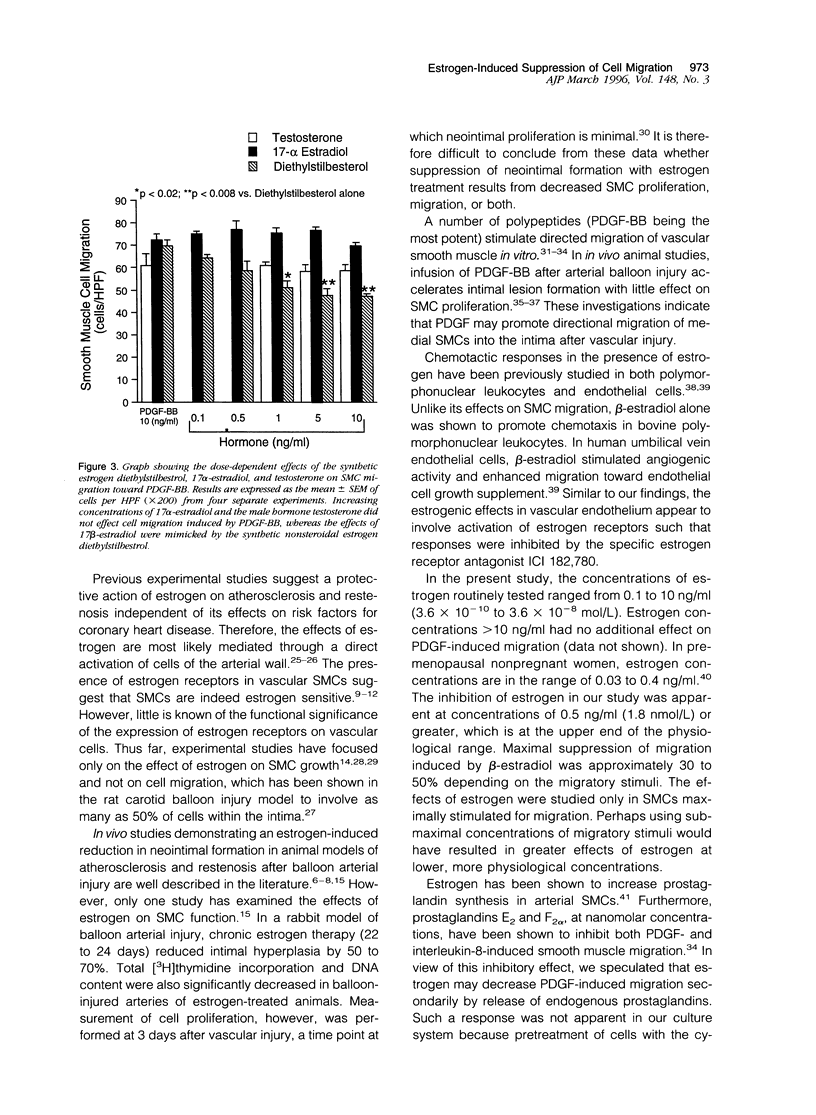
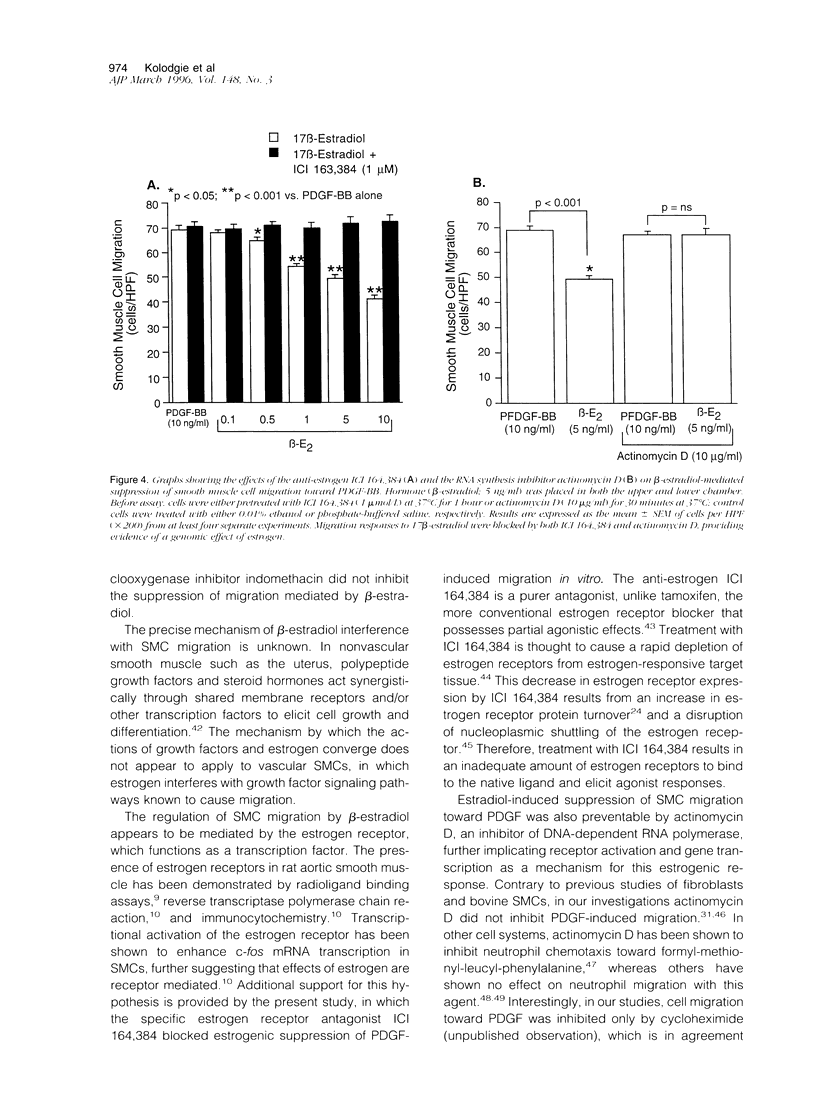
Although the cardiovascular benefits of the hormone estrogen are at least, in part, mediated by its antiproliferative effect on vascular smooth muscle, its action on the migration of these cells is unknown. To explore this relationship, female rat aortic smooth muscle cells were grown in hormone-free medium, and the effect of various concentrations of beta-estradiol on directed cellular migration was measured in vitro using a microwell Boyden chamber apparatus. Migration of smooth muscle cells to the known chemoattractants platelet-derived growth factor, insulin-like growth factor-1, and fibronectin (all at peak doses for migratory activity) was attenuated by beta-estradiol (0.5 to 10 ng/ml) in a concentration-dependent manner relative to control cells treated with vehicle (0.01% ethanol). This response was insensitive to pretreatment with indomethacin and was stereospecific (17 alpha-estradiol lacked response). Like beta-estradiol, the synthetic estrogen diethylstilbestrol attenuated directed smooth muscle cell migration whereas the male hormone testosterone was ineffective. Additional studies showed that beta-estradiol-mediated suppression of migration was inhibited by the anti-estrogen ICI 164,384 and the gene transcription inhibitor actinomycin D. These are the first results demonstrating a reduction in directed smooth muscle cell migration by beta-estradiol. The mechanism of this estrogen-mediated response appears to involve conventional estrogen receptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. R., Kaplan J. R., Manuck S. B., Koritnik D. R., Parks J. S., Wolfe M. S., Clarkson T. B. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990 Nov-Dec;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E., Bush T. L. Estrogen and coronary heart disease in women. JAMA. 1991 Apr 10;265(14):1861–1867. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Graves L. M., Raines E. W., Igarashi Y., Wayman G., Yamamura S., Yatomi Y., Sidhu J. S., Krebs E. G., Hakomori S. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995 Jul;130(1):193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Raines E. W., Nakano T., Graves L. M., Krebs E. G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994 Mar;93(3):1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M. S., Mallett C., VandeVen C., Kempert P., Bennetts G. A., Katz J. Impaired in vitro polymorphonuclear function secondary to the chemotherapeutic effects of vincristine, adriamycin, cyclophosphamide, and actinomycin D. J Clin Oncol. 1986 May;4(5):798–804. doi: 10.1200/JCO.1986.4.5.798. [DOI] [PubMed] [Google Scholar]
- Chang F. Y., Shaio M. F. In vitro effect of actinomycin D on human neutrophil function. Microbiol Immunol. 1990;34(3):311–321. doi: 10.1111/j.1348-0421.1990.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Chang W. C., Nakao J., Orimo H., Murota S. Stimulation of prostacyclin biosynthetic activity by estradiol in rat aortic smooth muscle cells in culture. Biochim Biophys Acta. 1980 Jul 14;619(1):107–118. [PubMed] [Google Scholar]
- Clowes A. W., Schwartz S. M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985 Jan;56(1):139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- Colburn P., Buonassisi V. Estrogen-binding sites in endothelial cell cultures. Science. 1978 Sep 1;201(4358):817–819. doi: 10.1126/science.684408. [DOI] [PubMed] [Google Scholar]
- Colditz G. A., Willett W. C., Stampfer M. J., Rosner B., Speizer F. E., Hennekens C. H. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987 Apr 30;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- Cole O. F., Fan T. P., Lewis G. P. Isolation, characterisation, growth and culture of endothelial cells from the rat aorta. Cell Biol Int Rep. 1986 Jun;10(6):399–405. doi: 10.1016/0309-1651(86)90034-2. [DOI] [PubMed] [Google Scholar]
- Dauvois S., Danielian P. S., White R., Parker M. G. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S., White R., Parker M. G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993 Dec;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Farhat M. Y., Vargas R., Dingaan B., Ramwell P. W. In vitro effect of oestradiol on thymidine uptake in pulmonary vascular smooth muscle cell: role of the endothelium. Br J Pharmacol. 1992 Nov;107(3):679–683. doi: 10.1111/j.1476-5381.1992.tb14506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Dzoga K., Wissler R. W., Vesselinovitch D. The effect of estradiol on the proliferation of rabbit aortic medial tissue culture cells induced by hyperlipemic serum. Exp Mol Pathol. 1983 Dec;39(3):355–363. doi: 10.1016/0014-4800(83)90064-3. [DOI] [PubMed] [Google Scholar]
- Foegh M. L., Asotra S., Howell M. H., Ramwell P. W. Estradiol inhibition of arterial neointimal hyperplasia after balloon injury. J Vasc Surg. 1994 Apr;19(4):722–726. doi: 10.1016/s0741-5214(94)70047-8. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Haarbo J., Leth-Espensen P., Stender S., Christiansen C. Estrogen monotherapy and combined estrogen-progestogen replacement therapy attenuate aortic accumulation of cholesterol in ovariectomized cholesterol-fed rabbits. J Clin Invest. 1991 Apr;87(4):1274–1279. doi: 10.1172/JCI115129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedemaker M., Lund L. A., Wagner W. C. Influence of arachidonic acid metabolites and steroids on function of bovine polymorphonuclear neutrophils. Am J Vet Res. 1992 Sep;53(9):1534–1539. [PubMed] [Google Scholar]
- Ignar-Trowbridge D. M., Teng C. T., Ross K. A., Parker M. G., Korach K. S., McLachlan J. A. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol Endocrinol. 1993 Aug;7(8):992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Raines E. W., Ross R., Reidy M. A. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993 Aug;13(8):1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas R. H., Patterson B. L., Mendelsohn M. E. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994 May;89(5):1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- Koyama N., Koshikawa T., Morisaki N., Saito Y., Yoshida S. Bifunctional effects of transforming growth factor-beta on migration of cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1990 Jun 15;169(2):725–729. doi: 10.1016/0006-291x(90)90391-y. [DOI] [PubMed] [Google Scholar]
- Koyama N., Morisaki N., Saito Y., Yoshida S. Regulatory effects of platelet-derived growth factor-AA homodimer on migration of vascular smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22806–22812. [PubMed] [Google Scholar]
- Kushwaha R. S., Lewis D. S., Carey K. D., McGill H. C., Jr Effects of estrogen and progesterone on plasma lipoproteins and experimental atherosclerosis in the baboon (Papio sp.). Arterioscler Thromb. 1991 Jan-Feb;11(1):23–31. doi: 10.1161/01.atv.11.1.23. [DOI] [PubMed] [Google Scholar]
- Losordo D. W., Kearney M., Kim E. A., Jekanowski J., Isner J. M. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994 Apr;89(4):1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- Mendelsohn M. E., Karas R. H. Estrogen and the blood vessel wall. Curr Opin Cardiol. 1994 Sep;9(5):619–626. doi: 10.1097/00001573-199409000-00018. [DOI] [PubMed] [Google Scholar]
- Morales D. E., McGowan K. A., Grant D. S., Maheshwari S., Bhartiya D., Cid M. C., Kleinman H. K., Schnaper H. W. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995 Feb 1;91(3):755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- Nakao J., Chang W. C., Murota S. I., Orimo H. Estradiol-binding sites in rat aortic smooth muscle cells in culture. Atherosclerosis. 1981 Jan-Feb;38(1-2):75–80. doi: 10.1016/0021-9150(81)90105-2. [DOI] [PubMed] [Google Scholar]
- Orimo A., Inoue S., Ikegami A., Hosoi T., Akishita M., Ouchi Y., Muramatsu M., Orimo H. Vascular smooth muscle cells as target for estrogen. Biochem Biophys Res Commun. 1993 Sep 15;195(2):730–736. doi: 10.1006/bbrc.1993.2106. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J. 1993 Jan;69(1 Suppl):S30–S37. doi: 10.1136/hrt.69.1_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano G. M., Sarrel P. M. Ovarian hormones and the cardiovascular system: recent findings. Cardiologia. 1994 Apr;39(4):275–279. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaet T. H., Stemerman M. B., Veith F. J., Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975 Jan;36(1):58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Colditz G. A. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991 Jan;20(1):47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Willett W. C., Colditz G. A., Rosner B., Speizer F. E., Hennekens C. H. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985 Oct 24;313(17):1044–1049. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Katz D., Shima T. B., Wakeling A. E., Lippman M. E., Dickson R. B. ICI 164,384, a pure antagonist of estrogen-stimulated MCF-7 cell proliferation and invasiveness. Cancer Res. 1989 Dec 15;49(24 Pt 1):6929–6934. [PubMed] [Google Scholar]
- Vargas R., Wroblewska B., Rego A., Hatch J., Ramwell P. W. Oestradiol inhibits smooth muscle cell proliferation of pig coronary artery. Br J Pharmacol. 1993 Jul;109(3):612–617. doi: 10.1111/j.1476-5381.1993.tb13616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling A. E. Use of pure antioestrogens to elucidate the mode of action of oestrogens. Biochem Pharmacol. 1995 May 26;49(11):1545–1549. doi: 10.1016/0006-2952(94)00528-t. [DOI] [PubMed] [Google Scholar]
- Wren B. G. The effect of oestrogen on the female cardiovascular system. Med J Aust. 1992 Aug 3;157(3):204–208. doi: 10.5694/j.1326-5377.1992.tb137091.x. [DOI] [PubMed] [Google Scholar]
- Yue T. L., Wang X., Sung C. P., Olson B., McKenna P. J., Gu J. L., Feuerstein G. Z. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994 Jul;75(1):1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]


