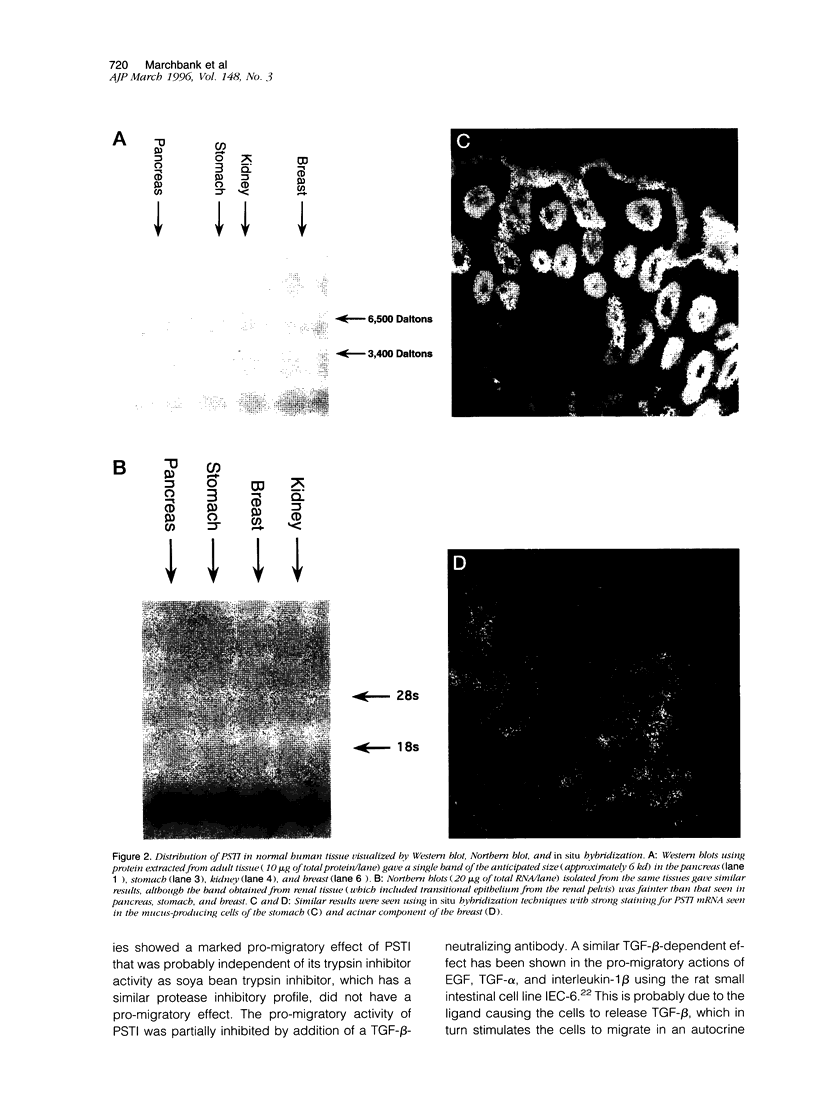
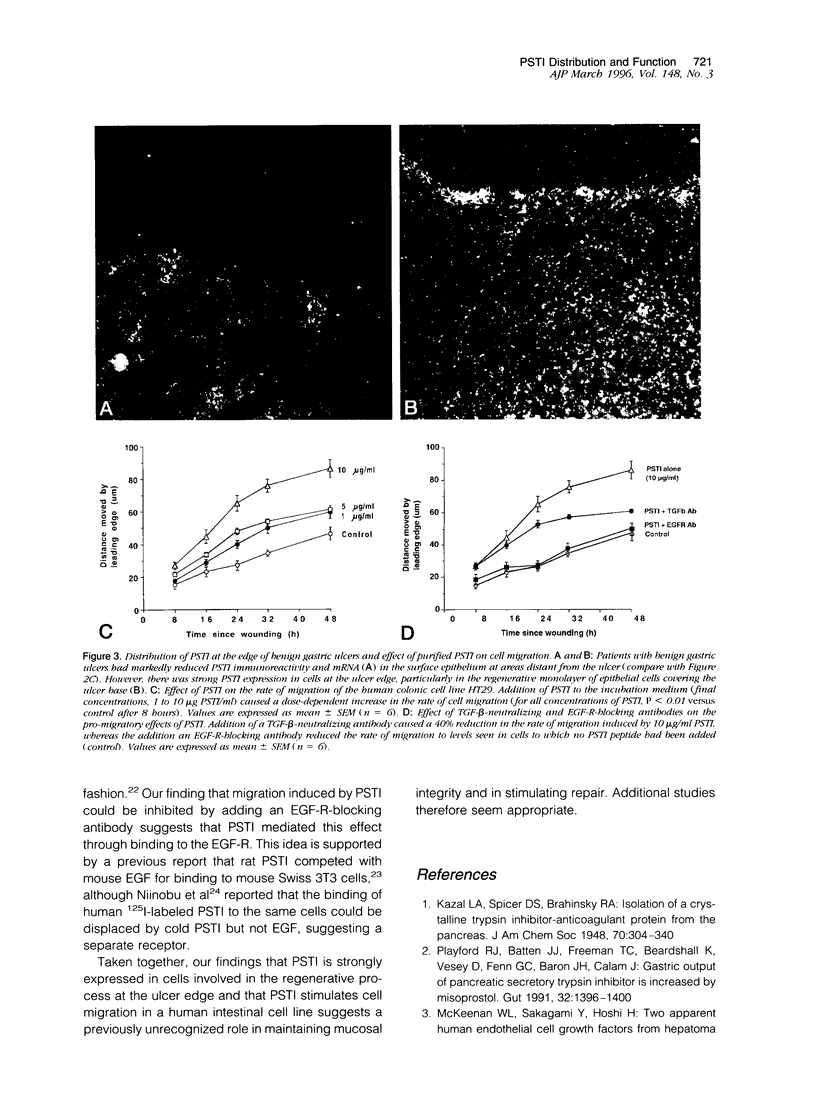
Abstract
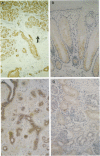
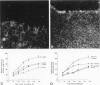
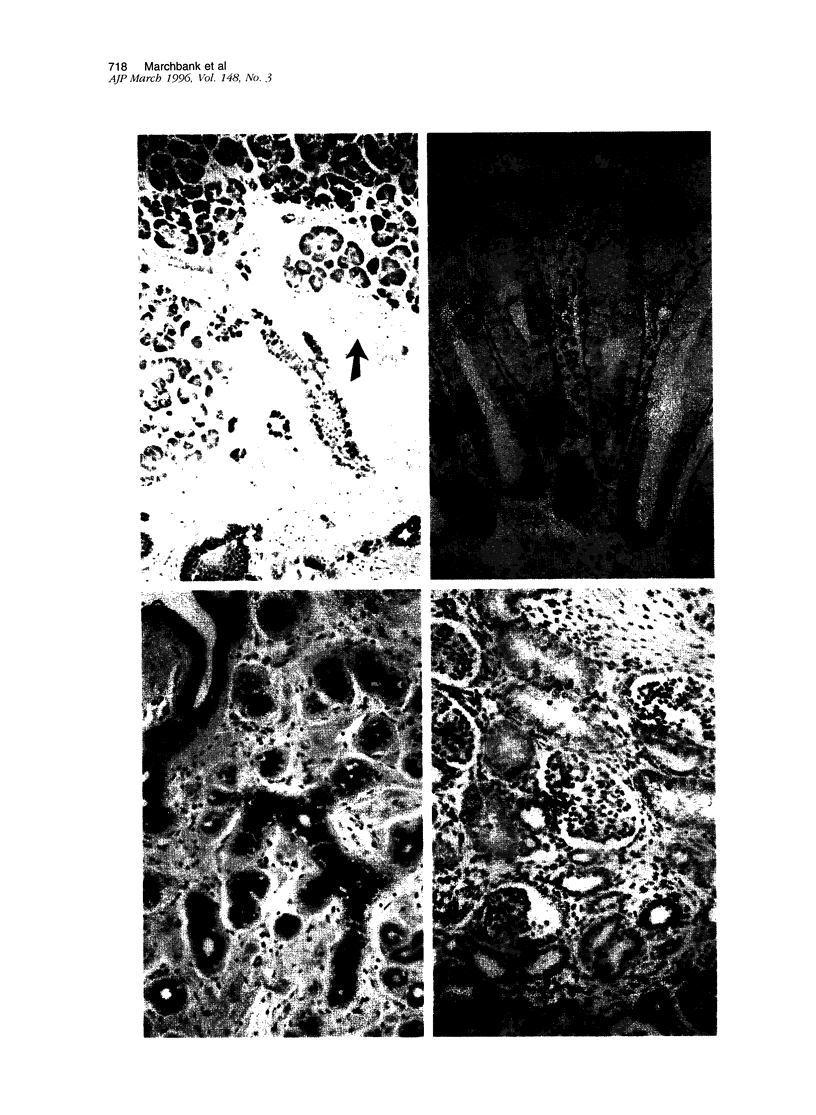
Pancreatic secretory trypsin inhibitor (PSTI) is a potent serine protease inhibitor that prevents excessive digestion of the gastrointestinal mucus but may also directly affect epithelial function. We therefore examined the distribution of PSTI in the human adult and fetus using immunohistochemistry and in situ hybridization and examined its effects on cell proliferation and migration in vitro. PSTI peptide and mRNA were found in the exocrine pancreas, mucus-producing cells of the normal gastrointestinal tract, acinar component of the normal breast, and surface epithelial cells at the edge of benign gastric ulcers. Peptide, but not message, was identified in the renal proximal tubule, probably reflecting reabsorption of filtered peptide. Purified human PSTI did not affect proliferation of the human colonic cell line HT-29 but caused a threefold increase in the rate of migration in an in vitro wounding model of restitution. This effect was inhibited by co-administering a PSTI-neutralizing antibody, a transforming growth factor-beta-neutralizing antibody, or an epidermal growth factor receptor-blocking antibody. As PSTI is widely distributed in several human organ systems and stimulates cell migration in vitro, we conclude that PSTI is likely to have additional roles to that of preserving the gastrointestinal mucous layer from excessive digestion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinery R., Playford R. J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995 Apr;88(4):401–403. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dignass A. U., Podolsky D. K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993 Nov;105(5):1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Freeman T. C., Curry B. J., Calam J., Woodburn J. R. Pancreatic secretory trypsin inhibitor stimulates the growth of rat pancreatic carcinoma cells. Gastroenterology. 1990 Nov;99(5):1414–1420. doi: 10.1016/0016-5085(90)91170-b. [DOI] [PubMed] [Google Scholar]
- Freeman T. C., Playford R. J., Quinn C., Beardshall K., Poulter L., Young J., Calam J. Pancreatic secretory trypsin inhibitor in gastrointestinal mucosa and gastric juice. Gut. 1990 Nov;31(11):1318–1323. doi: 10.1136/gut.31.11.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama M., Hayashi Y., Koike M., Ogawa M., Kosaki G. Immunohistochemical localization of pancreatic secretory trypsin inhibitor in fetal and adult pancreatic and extrapancreatic tissues. J Histochem Cytochem. 1986 Feb;34(2):227–235. doi: 10.1177/34.2.3511141. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Fushiki T., Kitagawa Y., Sugimoto E., Iwai K. Competition of a growth stimulating-/cholecystokinin (CCK) releasing-peptide (monitor peptide) with epidermal growth factor for binding to 3T3 fibroblasts. Biochem Biophys Res Commun. 1987 Jun 15;145(2):646–650. doi: 10.1016/0006-291x(87)91013-8. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Hughes C. M., Mellon K., Neal D. E., Lemoine N. R. Immunohistochemical detection of the epidermal growth factor receptor in paraffin-embedded human tissues. J Pathol. 1991 Aug;164(4):285–289. doi: 10.1002/path.1711640403. [DOI] [PubMed] [Google Scholar]
- Marks W. H., Ohlsson K. Elimination of pancreatic secretory trypsin inhibitor from the circulation. A study in man. Scand J Gastroenterol. 1983 Oct;18(7):955–959. doi: 10.3109/00365528309182122. [DOI] [PubMed] [Google Scholar]
- Niinobu T., Ogawa M., Murata A., Nishijima J., Mori T. Identification and characterization of receptors specific for human pancreatic secretory trypsin inhibitor. J Exp Med. 1990 Oct 1;172(4):1133–1142. doi: 10.1084/jem.172.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Batten J. J., Freeman T. C., Beardshall K., Vesey D. A., Fenn G. C., Baron J. H., Calam J. Gastric output of pancreatic secretory trypsin inhibitor is increased by misoprostol. Gut. 1991 Nov;32(11):1396–1400. doi: 10.1136/gut.32.11.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Batten J. J., Freeman T. C., Beardshall K., Vesey D. A., Fenn G. C., Baron J. H., Calam J. Gastric output of pancreatic secretory trypsin inhibitor is increased by misoprostol. Gut. 1991 Nov;32(11):1396–1400. doi: 10.1136/gut.32.11.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Hanby A. M., Quinn C., Calam J. Influence of inflammation and atrophy on pancreatic secretory trypsin inhibitor levels within the gastric mucosa. Gastroenterology. 1994 Mar;106(3):735–741. doi: 10.1016/0016-5085(94)90709-9. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheving L. A. Primary amino acid sequence similarity between human epidermal growth factor-urogastrone, human pancreatic secretory trypsin inhibitor, and members of porcine secretin family. Arch Biochem Biophys. 1983 Oct 15;226(2):411–413. doi: 10.1016/0003-9861(83)90309-0. [DOI] [PubMed] [Google Scholar]
- Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. The localization of laminin mRNA and protein in the postimplantation embryo and placenta of the mouse: an in situ hybridization and immunocytochemical study. Development. 1988 Nov;104(3):431–446. doi: 10.1242/dev.104.3.431. [DOI] [PubMed] [Google Scholar]
- Shibata T., Ogawa M., Takata N., Matsuda K., Niinobu T., Uda K., Wakasugi C., Mori T. Distribution of pancreatic secretory trypsin inhibitor in various human tissues and its inactivation in the gastric mucosa. Res Commun Chem Pathol Pharmacol. 1987 Feb;55(2):243–248. [PubMed] [Google Scholar]
- Williamson K. E., Hamilton P. W., Grimes J., Gilliland R. Advances in cell kinetics. BMJ. 1991 Oct 5;303(6806):855–856. doi: 10.1136/bmj.303.6806.855-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura Y., Nishide J., Emi M., Ogawa M., Mori T., Matsubara K. Molecular cloning and nucleotide sequence of human pancreatic secretory trypsin inhibitor (PSTI) cDNA. Biochem Biophys Res Commun. 1985 Oct 30;132(2):605–612. doi: 10.1016/0006-291x(85)91176-3. [DOI] [PubMed] [Google Scholar]