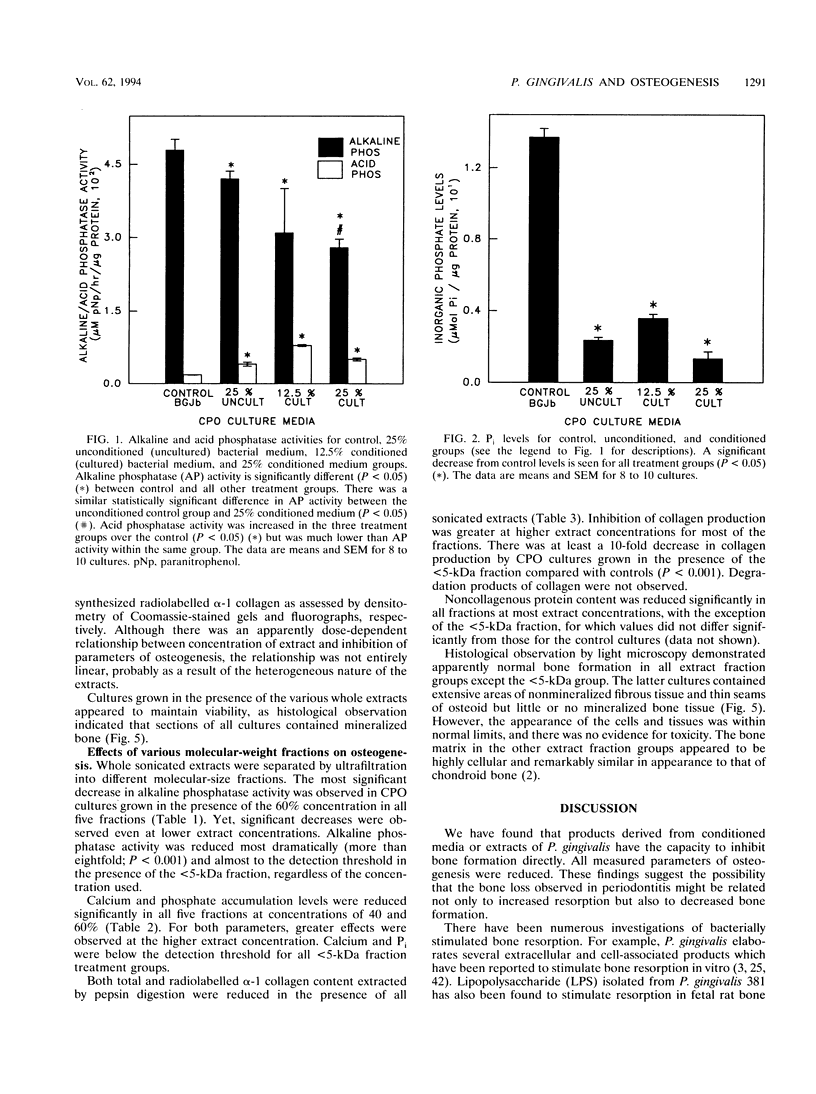
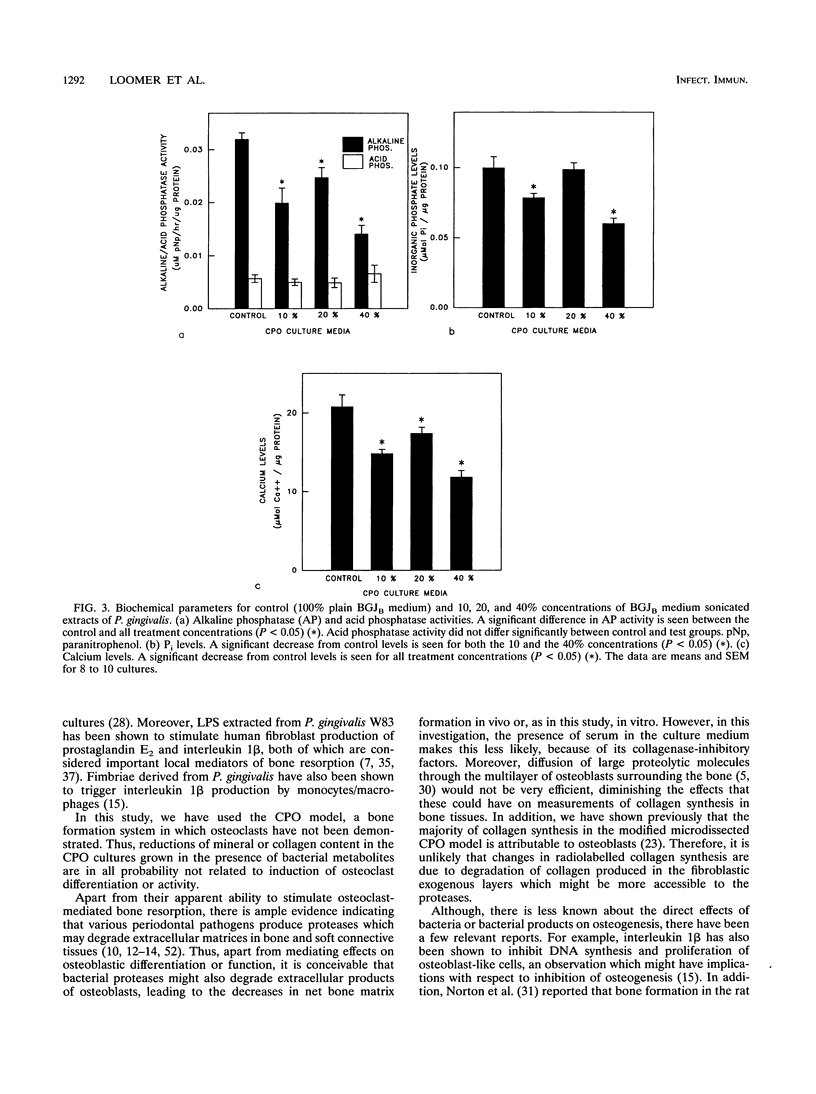
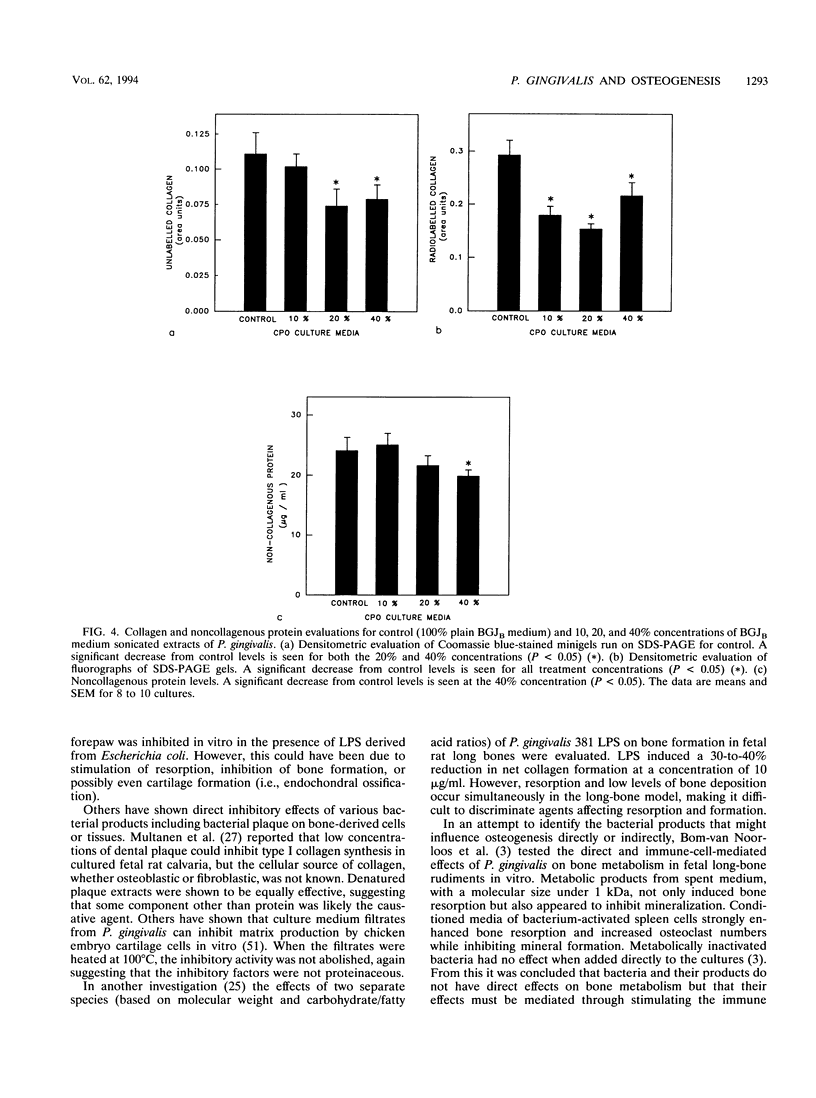
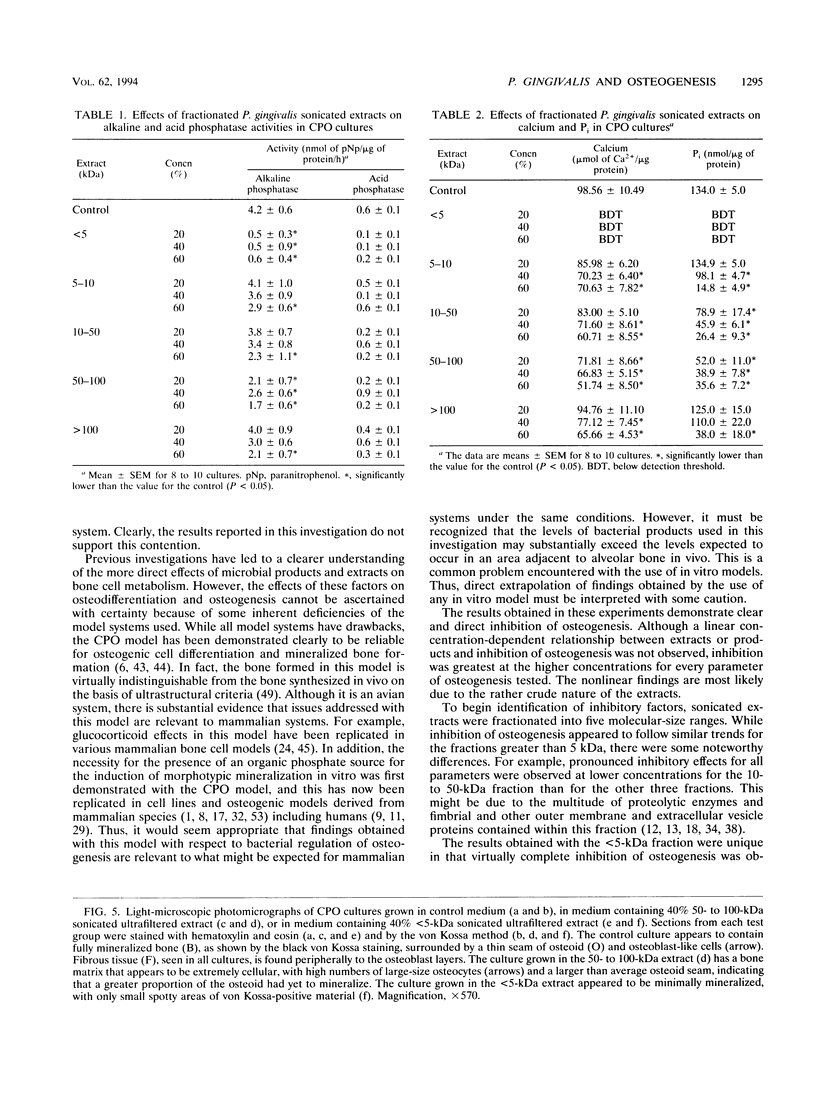
Abstract
It is well documented that oral microorganisms play a significant role in the initiation and progression of periodontal disease. By using various in vitro models, it has been shown that some bacteria considered periodontal pathogens or their products can stimulate bone resorption and some other parameters of osteoblast-like cell activity. However, the effects of these organisms and their products on osteogenesis itself are not known. This study was undertaken to determine the direct effects of metabolic products and sonicated extracts of Porphyromonas gingivalis on bone formation in the chick periosteal osteogenesis model. Cultures of P. gingivalis 2561 were grown under standard anaerobic culture conditions. The spent medium was collected, and following centrifugation, sonicated bacterial extracts were prepared from the bacterial pellet. These were added in various proportions to the chick periosteal osteogenesis cultures. Sonicated extracts were further fractionated into five molecular-size ranges and similarly tested. Parameters of osteogenesis, including alkaline phosphatase activity, calcium and Pi accumulation, and collagen synthesis, were measured on 6-day-old cultures. Compared with controls devoid of bacterial products, osteogenesis was inhibited significantly in cultures treated with either conditioned medium or extracts obtained from P. gingivalis. Various amounts of inhibitory activity were observed in the different ultrafiltration molecular-size fractions, with very profound inhibitory effects observed in the < 5-kDa range. Histological observations indicated the presence of cells, some bone, and/or new fibrous connective tissue at all concentrations, indicating that toxicity was not a factor. These results suggest that periodontal pathogens such as P. gingivalis might contribute to the bone loss in periodontal diseases not only by stimulating resorption but, possibly, by inhibiting bone formation directly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellows C. G., Aubin J. E., Heersche J. N., Antosz M. E. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986 Mar;38(3):143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Bom-van Noorloos A. A., van Steenbergen T. J., Burger E. H. Direct and immune-cell-mediated effects of Bacteroides gingivalis on bone metabolism in vitro. J Clin Periodontol. 1989 Aug;16(7):412–418. doi: 10.1111/j.1600-051x.1989.tb01669.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brommage R., Neuman W. F. Passive accumulation of magnesium, sodium, and potassium by chick calvaria. Calcif Tissue Int. 1979 Aug 24;28(1):57–63. doi: 10.1007/BF02441218. [DOI] [PubMed] [Google Scholar]
- Bruder S. P., Caplan A. I. Osteogenic cell lineage analysis is facilitated by organ cultures of embryonic chick periosteum. Dev Biol. 1990 Oct;141(2):319–329. doi: 10.1016/0012-1606(90)90388-y. [DOI] [PubMed] [Google Scholar]
- Canalis E. Interleukin-1 has independent effects on deoxyribonucleic acid and collagen synthesis in cultures of rat calvariae. Endocrinology. 1986 Jan;118(1):74–81. doi: 10.1210/endo-118-1-74. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Glorieux F. H., van der Rest M., Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol. 1983 Mar;96(3):639–643. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of a protease from Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):716–720. doi: 10.1128/iai.55.3.716-720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D. Inactivation of human serum bactericidal activity by a trypsinlike protease isolated from Porphyromonas gingivalis. Infect Immun. 1992 May;60(5):1854–1857. doi: 10.1128/iai.60.5.1854-1857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., McBride B. C. Isolation of a membrane-associated Bacteroides gingivalis glycylprolyl protease. Infect Immun. 1987 Dec;55(12):3131–3136. doi: 10.1128/iai.55.12.3131-3136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Hirose K., Amano S., Ohmori Y., Higuchi H., Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991 Jun;59(6):1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki K., Termine J. D., Whitson S. W., Young M. F. Bone matrix mRNA expression in differentiating fetal bovine osteoblasts. J Bone Miner Res. 1992 Jul;7(7):743–754. doi: 10.1002/jbmr.5650070704. [DOI] [PubMed] [Google Scholar]
- Lawson D. A., Meyer T. F. Biochemical characterization of Porphyromonas (Bacteroides) gingivalis collagenase. Infect Immun. 1992 Apr;60(4):1524–1529. doi: 10.1128/iai.60.4.1524-1529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Bourgeau G. Production of phenylacetic acid by anaerobes. J Clin Microbiol. 1982 Oct;16(4):747–750. doi: 10.1128/jcm.16.4.747-750.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D. Identification of clinical isolates of selected species of Bacteroides: production of phenylacetic acid. Can J Microbiol. 1979 Aug;25(8):927–928. doi: 10.1139/m79-138. [DOI] [PubMed] [Google Scholar]
- McCulloch C. A., Tenenbaum H. C. Dexamethasone induces proliferation and terminal differentiation of osteogenic cells in tissue culture. Anat Rec. 1986 Aug;215(4):397–402. doi: 10.1002/ar.1092150410. [DOI] [PubMed] [Google Scholar]
- McCulloch C. A., Tenenbaum H. C., Fair C. A., Birek C. Site-specific regulation of osteogenesis: maintenance of discrete levels of phenotypic expression in vitro. Anat Rec. 1989 Jan;223(1):27–34. doi: 10.1002/ar.1092230105. [DOI] [PubMed] [Google Scholar]
- Meghji S., Henderson B., Nair S., Wilson M. Inhibition of bone DNA and collagen production by surface-associated material from bacteria implicated in the pathology of periodontal disease. J Periodontol. 1992 Sep;63(9):736–742. doi: 10.1902/jop.1992.63.9.736. [DOI] [PubMed] [Google Scholar]
- Millar S. J., Goldstein E. G., Levine M. J., Hausmann E. Modulation of bone metabolism by two chemically distinct lipopolysaccharide fractions from Bacteroides gingivalis. Infect Immun. 1986 Jan;51(1):302–306. doi: 10.1128/iai.51.1.302-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Moore L. H., Ranney R. R., Smibert R. M., Burmeister J. A., Schenkein H. A. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991 Nov;18(10):729–739. doi: 10.1111/j.1600-051x.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Multanen V. M., Paunio K., Larjava H. Inhibition of bone collagen synthesis by dental plaque extract. J Periodontal Res. 1985 Nov;20(6):637–643. doi: 10.1111/j.1600-0765.1985.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Nair B. C., Mayberry W. R., Dziak R., Chen P. B., Levine M. J., Hausmann E. Biological effects of a purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983 Jan;18(1):40–49. doi: 10.1111/j.1600-0765.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Nefussi J. R., Boy-Lefevre M. L., Boulekbache H., Forest N. Mineralization in vitro of matrix formed by osteoblasts isolated by collagenase digestion. Differentiation. 1985;29(2):160–168. doi: 10.1111/j.1432-0436.1985.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Neuman W. F., Neuman M. W. Studies of diffusion in calvaria. Calcif Tissue Int. 1981;33(4):441–444. doi: 10.1007/BF02409468. [DOI] [PubMed] [Google Scholar]
- Norton L. A., Proffit W. R., Moore R. R. In vitro bone growth inhibition in the presence of histamine and endotoxins. J Periodontol. 1970 Mar;41(3):153–157. doi: 10.1902/jop.1970.41.3.153. [DOI] [PubMed] [Google Scholar]
- Notoya K., Tsukuda R., Yoshida K., Taketomi S. Stimulatory effect of ipriflavone on formation of bone-like tissue in rat bone marrow stromal cell culture. Calcif Tissue Int. 1992;51 (Suppl 1):S16–S20. doi: 10.1007/BF02180244. [DOI] [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Current status of the host response in chronic marginal periodontitis. J Periodontol. 1981 Sep;52(9):477–491. doi: 10.1902/jop.1981.52.9.477. [DOI] [PubMed] [Google Scholar]
- Papaioannou S., Marsh P. D., Ivanyi L. The immunogenicity of outer membrane proteins of haemin-depleted Porphyromonas (Bacteroides) gingivalis W50 in periodontal disease. Oral Microbiol Immunol. 1991 Dec;6(6):327–331. doi: 10.1111/j.1399-302x.1991.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Kream B. E. Regulation of bone formation (second of two parts). N Engl J Med. 1983 Jul 14;309(2):83–89. doi: 10.1056/NEJM198307143090206. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Sismey-Durrant H. J., Atkinson S. J., Hopps R. M., Heath J. K. The effect of lipopolysaccharide from bacteroides gingivalis and muramyl dipeptide on osteoblast collagenase release. Calcif Tissue Int. 1989 May;44(5):361–363. doi: 10.1007/BF02556318. [DOI] [PubMed] [Google Scholar]
- Sismey-Durrant H. J., Hopps R. M. Effect of lipopolysaccharide from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1-beta release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991 Dec;6(6):378–380. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J. Extracellular vesicle-associated and soluble trypsin-like enzyme fractions of Porphyromonas gingivalis W50. Oral Microbiol Immunol. 1991 Aug;6(4):202–208. doi: 10.1111/j.1399-302x.1991.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Haffajee A. D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992 Apr;63(4 Suppl):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- Sveen K., Skaug N. Bone resorption stimulated by lipopolysaccharides from Bacteroides, Fusobacterium and Veillonella, and by the lipid A and the polysaccharide part of Fusobacterium lipopolysaccharide. Scand J Dent Res. 1980 Dec;88(6):535–542. doi: 10.1111/j.1600-0722.1980.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., Heersche J. N. Dexamethasone stimulates osteogenesis in chick periosteum in vitro. Endocrinology. 1985 Nov;117(5):2211–2217. doi: 10.1210/endo-117-5-2211. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., Heersche J. N. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int. 1982 Jan;34(1):76–79. doi: 10.1007/BF02411212. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., Limeback H., McCulloch C. A., Mamujee H., Sukhu B., Torontali M. Osteogenic phase-specific co-regulation of collagen synthesis and mineralization by beta-glycerophosphate in chick periosteal cultures. Bone. 1992;13(2):129–138. doi: 10.1016/8756-3282(92)90002-e. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., McCulloch C. A., Fair C., Birek C. The regulatory effect of phosphates on bone metabolism in vitro. Cell Tissue Res. 1989 Sep;257(3):555–563. doi: 10.1007/BF00221466. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., Palangio K. G., Holmyard D. P., Pritzker K. P. An ultrastructural study of osteogenesis in chick periosteum in vitro. Bone. 1986;7(4):295–302. doi: 10.1016/8756-3282(86)90211-5. [DOI] [PubMed] [Google Scholar]
- Tenenbaum H. C., Palangio K. Phosphoethanolamine- and fructose 1,6-diphosphate-induced calcium uptake in bone formed in vitro. Bone Miner. 1987 May;2(3):201–210. [PubMed] [Google Scholar]
- Touw J. J., van Kampen G. P., van Steenbergen T. J., Veldhuijzen J. P., de Graaff J. The effect of culture filtrates of oral strains of black-pigmented Bacteroides on the matrix production of chick embryo cartilage cells in vitro. J Periodontal Res. 1982 Jul;17(4):351–357. doi: 10.1111/j.1600-0765.1982.tb01164.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson S. W., Harrison W., Dunlap M. K., Bowers D. E., Jr, Fisher L. W., Robey P. G., Termine J. D. Fetal bovine bone cells synthesize bone-specific matrix proteins. J Cell Biol. 1984 Aug;99(2):607–614. doi: 10.1083/jcb.99.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]



