Abstract
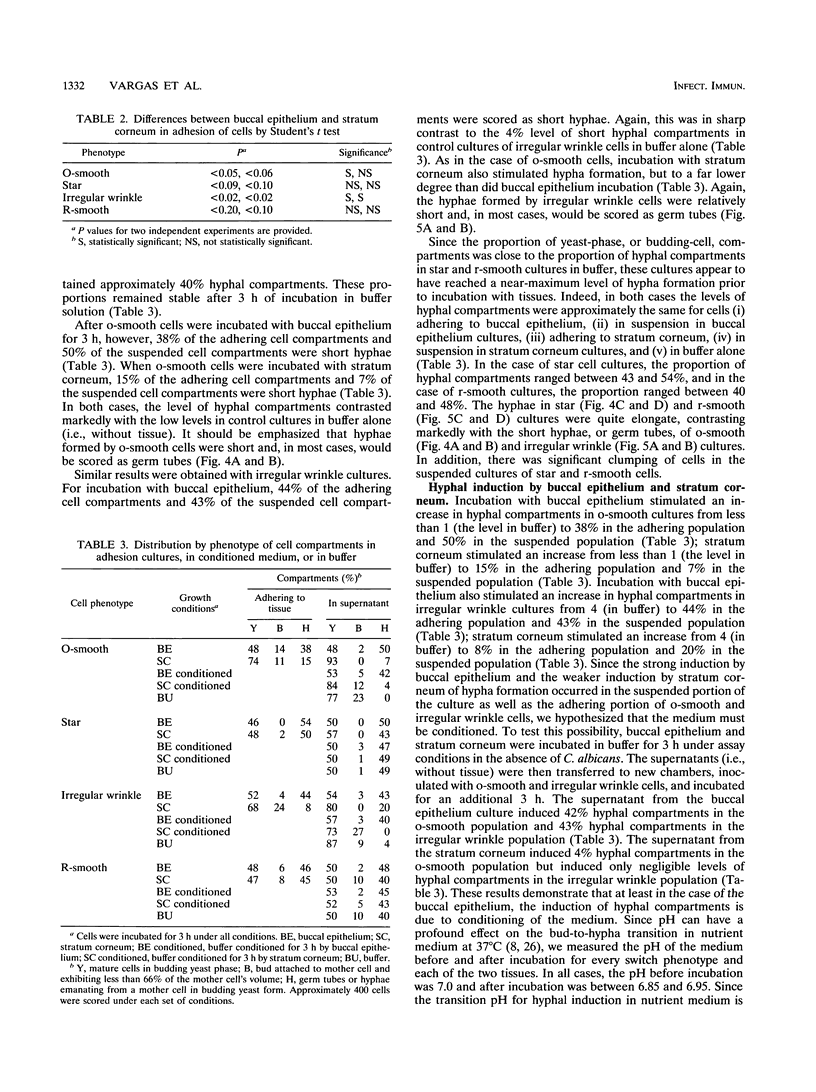
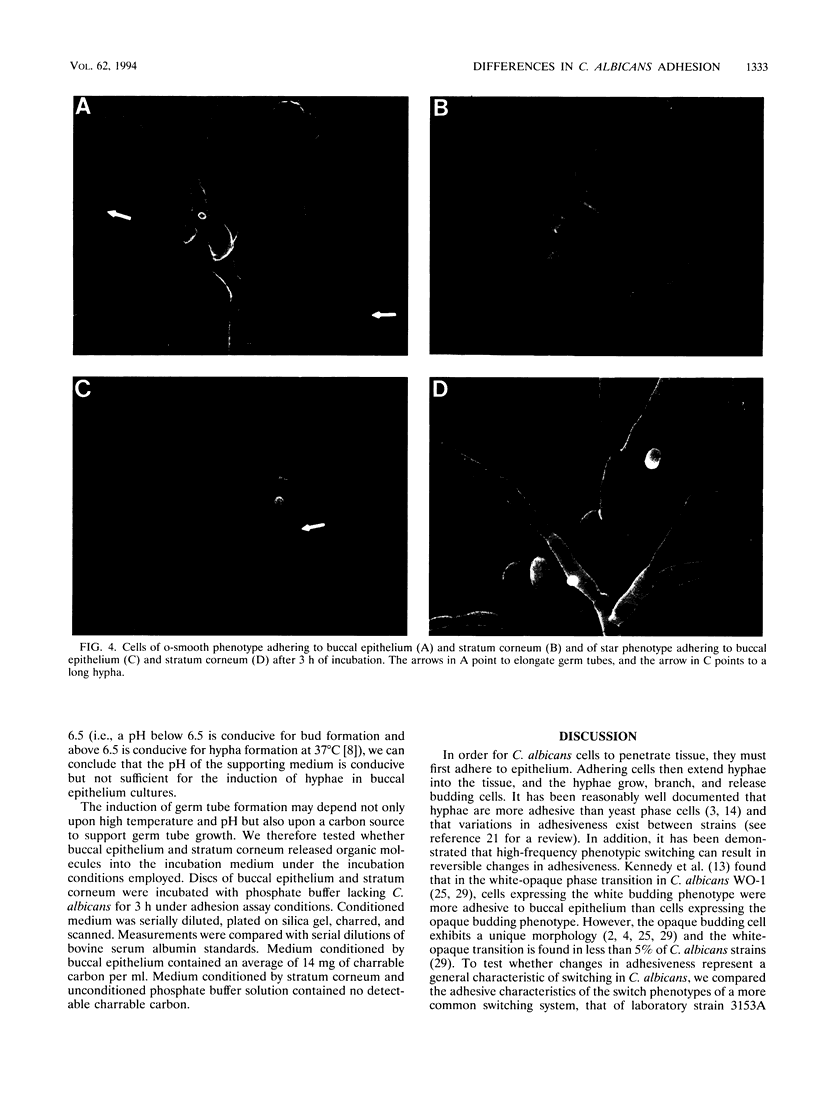
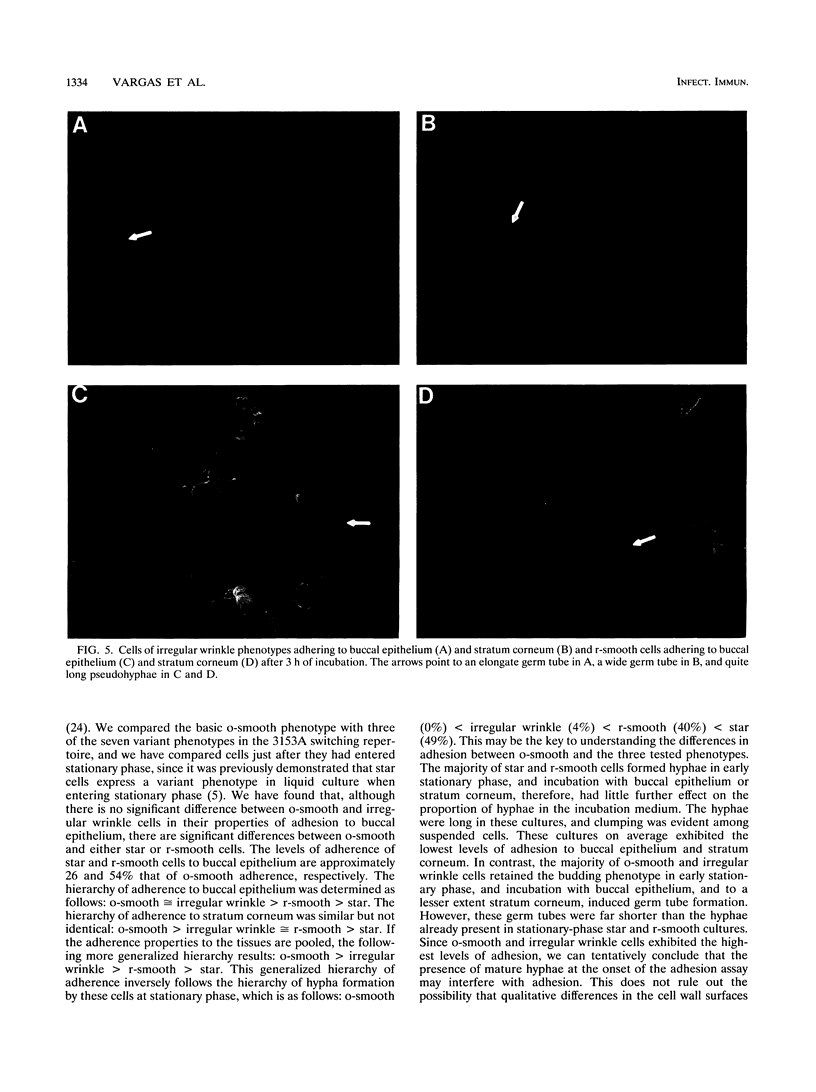
Cells of the laboratory strain 3153A of Candida albicans can be stimulated to undergo high-frequency phenotypic switching by a low dose of UV. We have compared the adhesive properties of cells exhibiting the basic original smooth (o-smooth) phenotype and three switch phenotypes (star, irregular wrinkle, and revertant smooth) to buccal epithelium and stratum corneum. The generalized hierarchy of adhesion is as follows: o-smooth > irregular wrinkle > revertant smooth > star. This is the inverse of the hierarchy of the proportions of elongate hyphae formed by these phenotypes in culture. These results suggest that the differences in adhesion between o-smooth and the three switch phenotypes of strain 3153A reflect, at least in part, the level of interference due to the formation of elongate hyphae, which tend to cause clumping in suspension. No major differences in the levels of adhesion of cells of the different phenotypes between buccal epithelium and stratum corneum were observed. Results which demonstrate that buccal epithelium induces germination (hypha formation) by conditioning the medium are also presented.
Full text
PDF

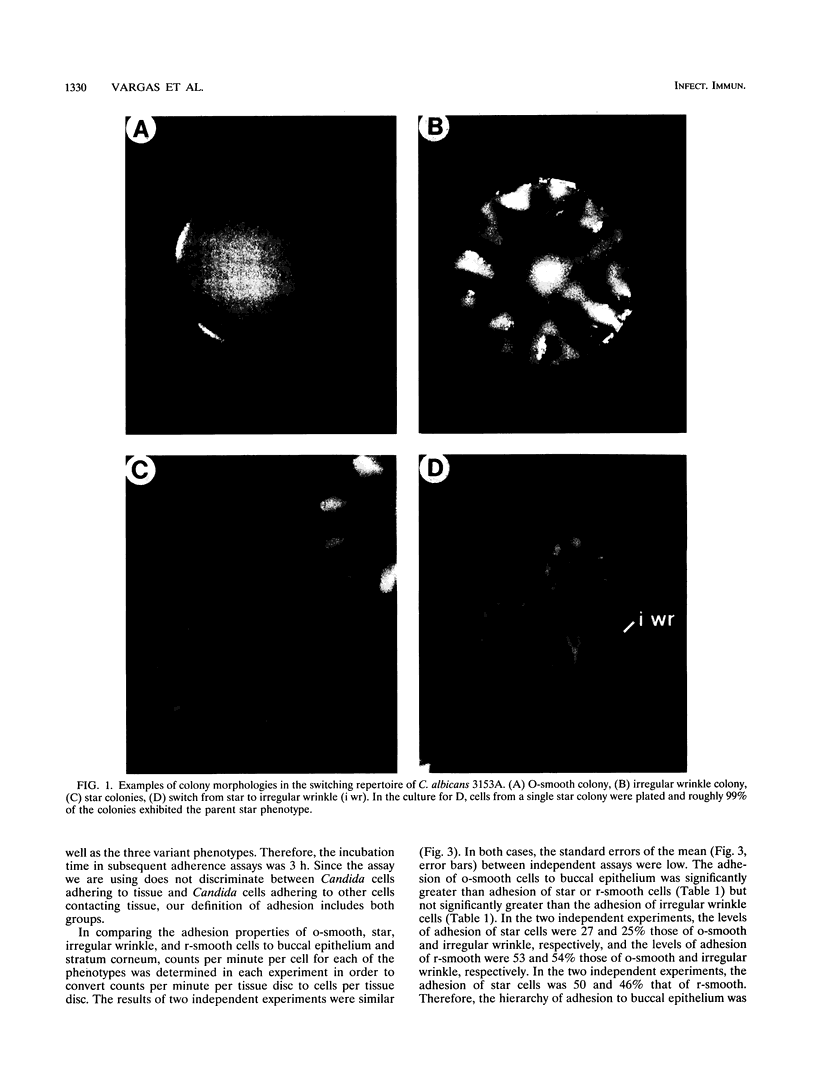
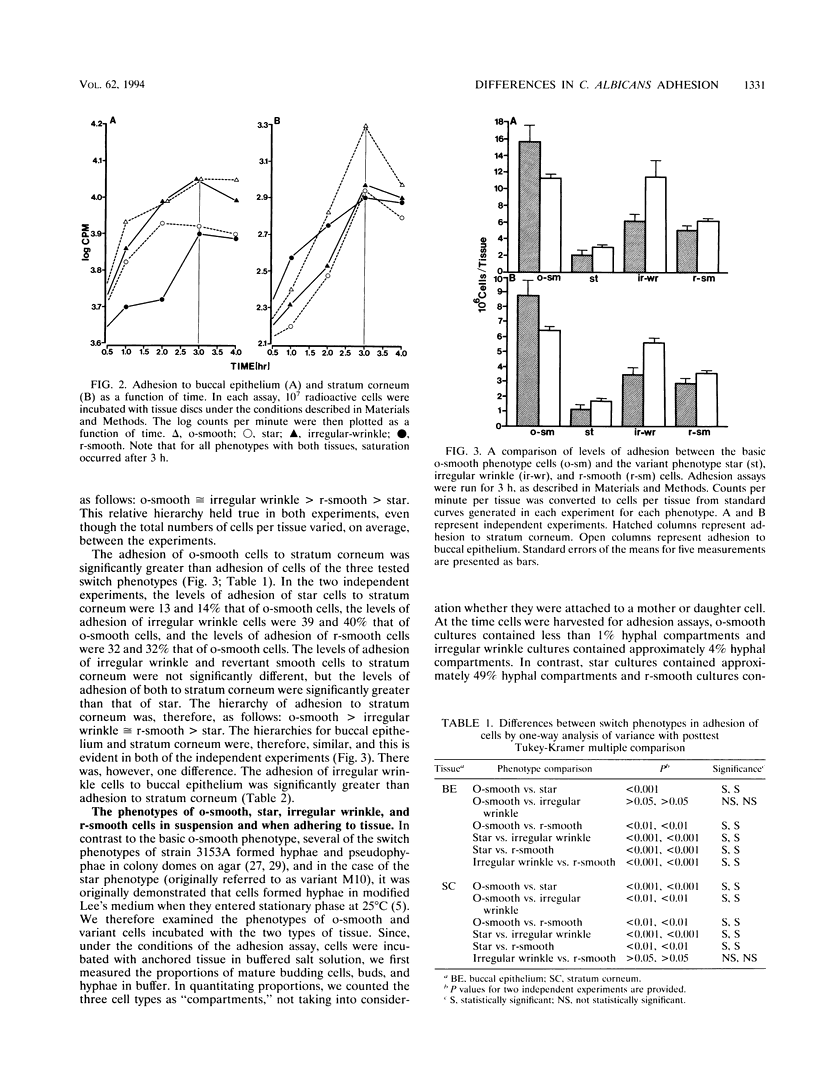

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Soll D. R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987 Dec;169(12):5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Cundiff L., Schnars B., Gao M. X., Mackenzie I., Soll D. R. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989 Feb;57(2):458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Mihalik R., Soll D. R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990 Jan;172(1):224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. L., Odds F. C. Adherence of Candida albicans to vaginal epithelia: significance of morphological form and effect of ketoconazole. Mykosen. 1985 Nov;28(11):531–540. doi: 10.1111/j.1439-0507.1985.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo J., Herman M. A., Soll D. R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984 Mar 15;85(1-2):21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Cihlar R. L., Lee D. D., Hoberg K., Scheld W. M. Yeast adhesion in the pathogenesis of endocarditis due to Candida albicans: studies with adherence-negative mutants. J Infect Dis. 1985 Oct;152(4):710–715. doi: 10.1093/infdis/152.4.710. [DOI] [PubMed] [Google Scholar]
- Cawson R. A., Rajasingham K. C. Ultrastructural features of the invasive phase of Candida albicans. Br J Dermatol. 1972 Nov;87(5):435–443. doi: 10.1111/j.1365-2133.1972.tb01591.x. [DOI] [PubMed] [Google Scholar]
- Howlett J. A., Squier C. A. Candida albicans ultrastructure: colonization and invasion of oral epithelium. Infect Immun. 1980 Jul;29(1):252–260. doi: 10.1128/iai.29.1.252-260.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Rogers A. L., Hanselmen L. R., Soll D. R., Yancey R. J., Jr Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988 Jun;102(3):149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Cihlar R. L., Calderone R. A. Pathogenesis of vaginal candidiasis: studies with a mutant which has reduced ability to adhere in vitro. J Med Vet Mycol. 1986 Apr;24(2):127–131. doi: 10.1080/02681218680000191. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect Immun. 1973 Nov;8(5):846–849. doi: 10.1128/iai.8.5.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow B., Anderson J., Wilson J., Soll D. R. Bidirectional stimulation of the white-opaque transition of Candida albicans by ultraviolet irradiation. J Gen Microbiol. 1989 May;135(5):1201–1208. doi: 10.1099/00221287-135-5-1201. [DOI] [PubMed] [Google Scholar]
- Morrow B., Srikantha T., Soll D. R. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol Cell Biol. 1992 Jul;12(7):2997–3005. doi: 10.1128/mcb.12.7.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomés R., Gil C., Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985 Aug;131(8):2107–2113. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T. Comparative studies of gastrointestinal colonization and systemic spread by Candida albicans and nonlethal yeast in the infant mouse. Scan Electron Microsc. 1982;(Pt 4):1667–1676. [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992 Apr;5(2):183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Morrow B., Srikantha T. High-frequency phenotypic switching in Candida albicans. Trends Genet. 1993 Feb;9(2):61–65. doi: 10.1016/0168-9525(93)90189-O. [DOI] [PubMed] [Google Scholar]
- Soll D. R. The regulation of cellular differentiation in the dimorphic yeast Candida albicans. Bioessays. 1986 Jul;5(1):5–11. doi: 10.1002/bies.950050103. [DOI] [PubMed] [Google Scholar]