Abstract
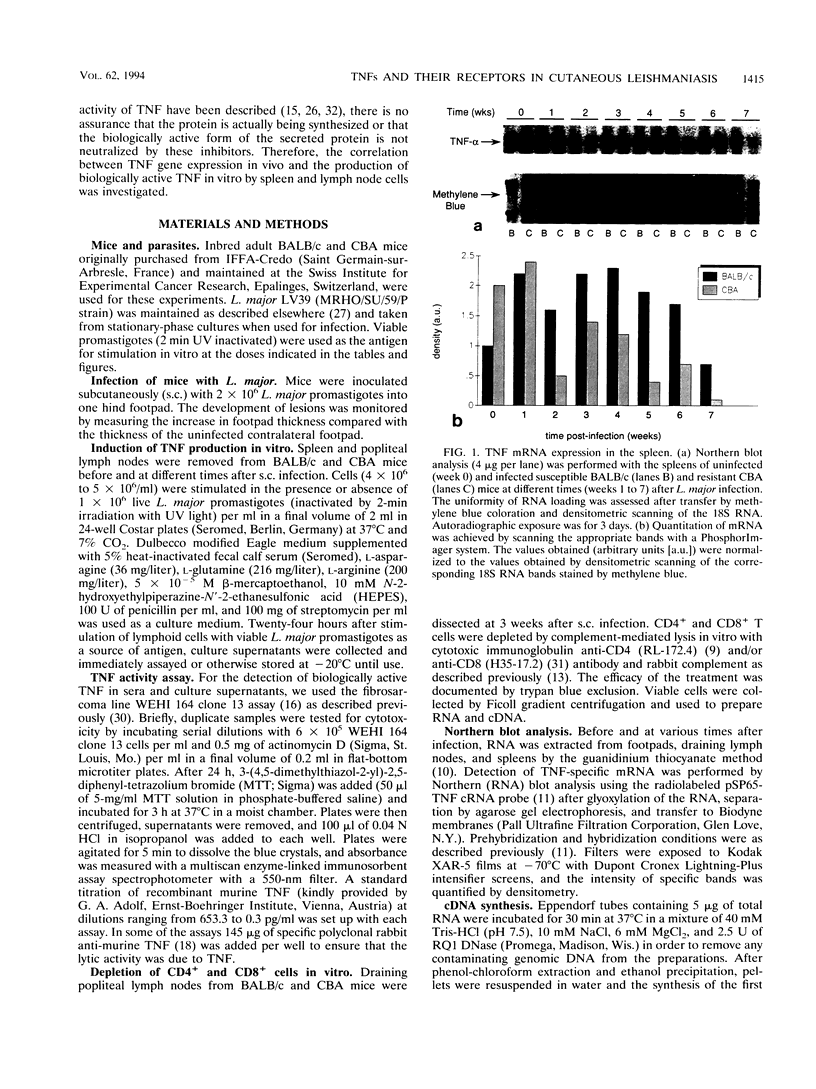
Experimental infection of BALB/c mice with Leishmania major leads to lesions which progress without healing and visceralization, reproducing the most severe forms of human leishmaniasis, while resistant mice like CBA spontaneously resolve lesions and develop protective immunity. Given the conflicting data pertaining to the role of tumor necrosis factor alpha (TNF) in Leishmania infection, we analyzed the expression of TNF, tumor necrosis factor beta (lymphotoxin), and TNF receptor type I (TNF-RI) and type II (TNF-RII) genes in vivo and correlated TNF gene expression in vivo with the production of biologically active TNF by lymphoid cells in vitro. No significant difference in the expression of TNF mRNA was found between susceptible and resistant strains of mice during the course of infection. The depletion of CD4+ T cells in vitro did not change the level of TNF mRNA in BALB/c lymph node cells but led to the total disappearance of TNF mRNA in CBA mice. Unprimed spleen cells did not produce detectable amounts of TNF, whereas 1 week after infection, TNF bioactivity was detected and increased in both strains of mice until 5 weeks of infection. While neutralization of TNF activity in vivo did not alter the course of infection in BALB/c mice, in CBA mice it led to an increase in lesion size and a delay in the healing process but did not interfere significantly with the outcome of infection. Finally, no significant difference in the levels of lymphotoxin, TNF-RI, or TNF RII mRNA expression was found between both strains. The information resulting from these investigations supports the notion that, in vivo, TNF is not the decisive factor responsible for the resistant versus susceptible phenotype in leishmania infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Engelmann H., Maor Y., Brakebusch C., Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992 Feb 1;175(2):323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behin R., Mauel J., Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979 Aug;48(1):81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Moll H., Solbach W., Röllinghoff M. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990 May;20(5):1131–1135. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Stenger S., Röllinghoff M., Solbach W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin 4 synergizes with interferon-gamma to activate murine macrophages for killing of Leishmania major amastigotes. Eur J Immunol. 1991 Feb;21(2):327–333. doi: 10.1002/eji.1830210213. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseh K., Beutler B. Alternative cleavage of the cachectin/tumor necrosis factor propeptide results in a larger, inactive form of secreted protein. J Biol Chem. 1989 Sep 25;264(27):16256–16260. [PubMed] [Google Scholar]
- Davignon J. L., Budd R. C., Ceredig R., Piguet P. F., MacDonald H. R., Cerottini J. C., Vassalli P., Izui S. Functional analysis of T cell subsets from mice bearing the lpr gene. J Immunol. 1985 Oct;135(4):2423–2428. [PubMed] [Google Scholar]
- Descoteaux A., Turco S. J., Sacks D. L., Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991 Apr 15;146(8):2747–2753. [PubMed] [Google Scholar]
- Engelmann H., Novick D., Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990 Jan 25;265(3):1531–1536. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Gehr G., Braun T., Lesslauer W. Cytokines, receptors, and inhibitors. Clin Investig. 1992 Jan;70(1):64–69. doi: 10.1007/BF00422944. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Parkinson C., Millott S., Severn A., Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990 Apr;69(4):570–573. [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Scott P. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol Today. 1991 Mar;12(3):A58–A61. doi: 10.1016/S0167-5699(05)80017-9. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Louis J., Moedder E., Behin R., Engers H. Recognition of protozoan parasite antigens by murine T lymphocytes. I. Induction of specific T lymphocyte-dependent proliferative response to Leishmania tropica. Eur J Immunol. 1979 Nov;9(11):841–847. doi: 10.1002/eji.1830091103. [DOI] [PubMed] [Google Scholar]
- Mirkovich A. M., Galelli A., Allison A. C., Modabber F. Z. Increased myelopoiesis during Leishmania major infection in mice: generation of 'safe targets', a possible way to evade the effector immune mechanism. Clin Exp Immunol. 1986 Apr;64(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Moll H., Binöder K., Bogdan C., Solbach W., Röllinghoff M. Production of tumour necrosis factor during murine cutaneous leishmaniasis. Parasite Immunol. 1990 Sep;12(5):483–494. doi: 10.1111/j.1365-3024.1990.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Müller I., Pedrazzini T., Kropf P., Louis J., Milon G. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite-specific CD8+ T cells. Int Immunol. 1991 Jun;3(6):587–597. doi: 10.1093/intimm/3.6.587. [DOI] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Stenger S., Solbach W., Röllinghoff M., Bogdan C. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-alpha production by macrophages is induced by the synergistic action of interferon (IFN)-gamma and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-gamma and IL 4. Eur J Immunol. 1991 Jul;21(7):1669–1675. doi: 10.1002/eji.1830210713. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Wyler D. J. Antileishmanial defense in macrophages triggered by tumor necrosis factor expressed on CD4+ T lymphocyte plasma membrane. J Exp Med. 1991 Oct 1;174(4):755–759. doi: 10.1084/jem.174.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Engelmann H., Nophar Y., Aderka D., Kemper O., Hornik V., Holtmann H., Brakebusch C. Soluble and cell surface receptors for tumor necrosis factor. Agents Actions Suppl. 1991;35:51–57. [PubMed] [Google Scholar]