Abstract
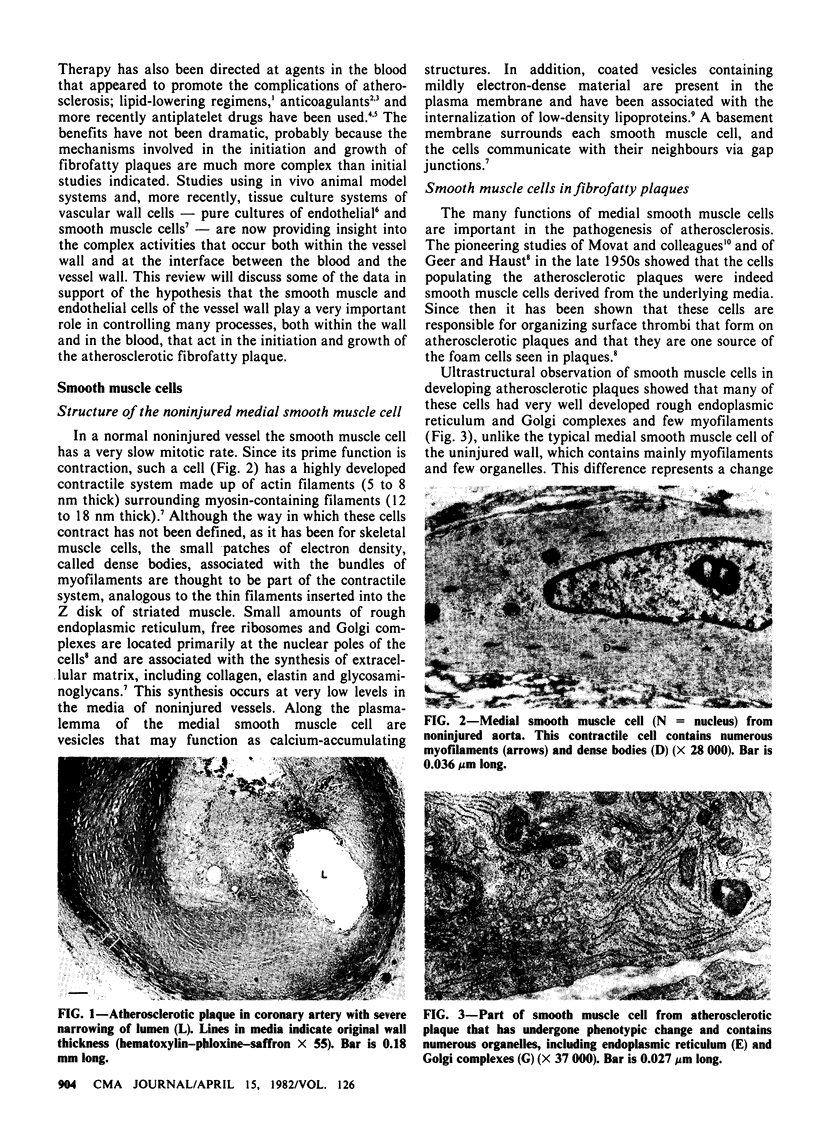
Although clinical studies have been very useful in identifying factors that accelerate the development of atherosclerotic vascular disease, the pathogenesis of the vascular lesions remains unknown. Studies carried out in the last 10 years have shown that smooth muscle and endothelial cells of the vascular wall play a very important role in atherogenesis. It has become apparent that these cells are very active metabolically during the initiation and subsequent growth of the plaques, and that the materials that these cells produce and secrete are important in the composition and growth of the plaques. In addition, there are important interactions at the vessel wall-blood interface that involve endothelial cells, hemodynamic forces and many constituents of the blood, including platelets, lipoproteins, coagulation factors, fibrinolytic agents and leukocytes. In this article the numerous functions of both smooth muscle and endothelial cells are discussed and the effects of known atherogenic agents on these cellular functions are reviewed. Emphasis is placed on the important interactions that take place both within the vessel wall and at the vessel wall-blood interface. Understanding of the regulation of smooth muscle and endothelial cell function during the development and subsequent growth of fibrofatty plaques may be useful in designing appropriate therapeutic interventions to control atherosclerotic disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker C. G., Harpel P. C. alpha2-Macroglobulin on human vascular endothelium. J Exp Med. 1976 Jul 1;144(1):1–9. doi: 10.1084/jem.144.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy B., Srinivasan S. R., Dalferes E. R., Jr, Malinow M. R. Chemical changes in the arterial wall during regression of atherosclerosis in monkeys. Artery. 1981;9(1):44–58. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Danon D., Skutelsky E. Endothelial surface charge and its possible relationship to thrombogenesis. Ann N Y Acad Sci. 1976;275:47–63. doi: 10.1111/j.1749-6632.1976.tb43337.x. [DOI] [PubMed] [Google Scholar]
- Falcone D. J., Hajjar D. P., Minick C. R. Enhancement of cholesterol and cholesteryl ester accumulation in re-endothelialized aorta. Am J Pathol. 1980 Apr;99(1):81–104. [PMC free article] [PubMed] [Google Scholar]
- Fischer-Dzoga K., Fraser R., Wissler R. W. Stimulation of proliferation in stationary primary cultures of monkey and rabbit aortic smooth muscle cells. I. Effects of lipoprotein fractions of hyperlipemic serum and lymph. Exp Mol Pathol. 1976 Jun;24(3):346–359. doi: 10.1016/0014-4800(76)90070-8. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Gajdusek C., DiCorleto P., Ross R., Schwartz S. M. An endothelial cell-derived growth factor. J Cell Biol. 1980 May;85(2):467–472. doi: 10.1083/jcb.85.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W. Migration into an in vitro experimental wound: a comparison of porcine aortic endothelial and smooth muscle cells and the effect of culture irradiation. Am J Pathol. 1981 May;103(2):271–282. [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., Wong M. K. The effect of platelet poor plasma serum on endothelial cell spreading. Artery. 1981;9(1):59–68. [PubMed] [Google Scholar]
- Hoak J. C., Czervionke R. L., Fry G. L., Smith J. B. Interaction of thrombin and platelets with the vascular endothelium. Fed Proc. 1980 Jul;39(9):2606–2609. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins V. I., Subrahmanyan L., Gotlieb A. I. The reorganization of cytoskeletal fibre systems in spreading porcine endothelial cells in cultures. Eur J Cell Biol. 1981 Apr;24(1):36–44. [PubMed] [Google Scholar]
- Kramsch D. M., Franzblau C., Hollander W. The protein and lipid composition of arterial elastin and its relationship to lipid accumulation in the atherosclerotic plaque. J Clin Invest. 1971 Aug;50(8):1666–1677. doi: 10.1172/JCI106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledet T. Growth hormone antiserum suppresses the growth effect of diabetic serum. Studies on rabbit aortic medical cell cultures. Diabetes. 1977 Aug;26(8):798–803. doi: 10.2337/diab.26.8.798. [DOI] [PubMed] [Google Scholar]
- Levy R. I. Cholesterol, lipoproteins, apoproteins, and heart disease: present status and future prospects. Clin Chem. 1981 May;27(5):653–662. [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., HAUST M. D., MORE R. H. The morphologic elements in the early lesions of arteriosclerosis. Am J Pathol. 1959 Jan-Feb;35(1):93–101. [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. The role of blood and platelets in atherosclerosis and the complications of atherosclerosis. Thromb Diath Haemorrh. 1975 Jun 30;33(3):444–456. [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1981 Jun 10;256(11):5532–5535. [PubMed] [Google Scholar]
- Richardson M., Ihnatowycz I., Moore S. Glycosaminoglycan distribution in rabbit aortic wall following balloon catheter deendothelialization. An ultrastructural study. Lab Invest. 1980 Dec;43(6):509–516. [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Aortic endothelial cell replication. I. Effects of age and hypertension in the rat. Circ Res. 1977 Aug;41(2):248–255. doi: 10.1161/01.res.41.2.248. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Reidy M. A., Selden S. C., 3rd, Haudenschild C. C. Maintenance of integrity in aortic endothelium. Fed Proc. 1980 Jul;39(9):2618–2625. [PubMed] [Google Scholar]
- Seaman A. J., Griswold H. E., Reaume R. B., Ritzmann L. Long-term anticoagulant prophylaxis after myocardial infarction. N Engl J Med. 1969 Jul 17;281(3):115–119. doi: 10.1056/NEJM196907172810301. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Colombo M., Gonzales J. J., Hollander W., Schmid K. The glycosaminoglycans of the human artery and their changes in atherosclerosis. J Clin Invest. 1976 Aug;58(2):470–481. doi: 10.1172/JCI108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. W., Bierman E. L., Ross R. Effect of insulin on the proliferation of cultured primate arterial smooth muscle cells. Circ Res. 1975 Feb;36(2):319–327. doi: 10.1161/01.res.36.2.319. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson G., Robertson A. L., Jr, Cowan D. H. Migration of human vascular endothelial and smooth muscle cells. Lab Invest. 1979 Jul;41(1):51–62. [PubMed] [Google Scholar]
- Thorgeirsson G., Robertson A. L., Jr The vascular endothelium-pathobiologic significance. Am J Pathol. 1978 Dec;93(3):803–848. [PMC free article] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Striker G. E. Human endothelial cell migration: stimulation by a released platelet factor. Lab Invest. 1978 Nov;39(5):523–529. [PubMed] [Google Scholar]






