Abstract
Establishment of the steroid-producing Leydig cell lineage is an event downstream of Sry that is critical for masculinization of mammalian embryos. Neither the origin of fetal Leydig cell precursors nor the signaling pathway that specifies the Leydig cell lineage is known. Based on the sex-specific expression patterns of Desert Hedgehog (Dhh) and its receptor Patched 1 (Ptch1) in XY gonads, we investigated the potential role of DHH/PTCH1 signaling in the origin and specification of fetal Leydig cells. Analysis of Dhh−/− XY gonads revealed that differentiation of fetal Leydig cells was severely defective. Defects in Leydig cell differentiation in Dhh−/− XY gonads did not result from failure of cell migration from the mesonephros, thought to be a possible source of Leydig cell precursors. Nor did DHH/PTCH1 signaling appear to be involved in the proliferation or survival of fetal Leydig precursors in the interstitium of the XY gonad. Instead, our results suggest that DHH/PTCH1 signaling triggers Leydig cell differentiation by up-regulating Steroidogenic Factor 1 and P450 Side Chain Cleavage enzyme expression in Ptch1-expressing precursor cells located outside testis cords.
Keywords: Desert Hedgehog, Patched 1, Leydig, mesonephros, testis, organogenesis
A critical event in testis organogenesis is the specification of somatic cell lineages including Sertoli cells, peritubular myoid cells, and Leydig cells. Specification of these lineages is crucial for the establishment of testis morphology and the production of hormones. A single gene on the Y chromosome, Sry (sex-determining region of the Y chromosome), is believed to induce a cascade of signaling pathways for the differentiation of these somatic cell lineages (Gubbay et al. 1990; Koopman et al. 1991). Autonomous expression of Sry in somatic cells in the XY gonad leads to differentiation of Sertoli cells (Albrecht and Eicher 2001). Differentiating gonadal cells induce migration of cells from the mesonephros into the gonad. The migrating cells contribute to precursors of the peritubular myoid and vascular cell lineages (Martineau et al. 1997; Capel et al. 1999; Tilmann and Capel 1999). Differentiation of peritubular myoid cells and the consequent formation of testis cords are regulated by Desert hedgehog (DHH), a signaling protein produced by Sertoli cells (Clark et al. 2000; Pierucci-Alves et al. 2001). Fetal Leydig cells are first identifiable within the interstitium of the XY gonad (between testis cords) when they express P450 Side Chain Cleavage (Scc) enzyme and other steroidogenic enzymes required for the production of androgens.
The specification of adult Leydig cells has been studied extensively (Habert et al. 2001). Adult Leydig cells are believed to be a separate population of steroidogenic cells that arise from adult peritubular mesenchymal cells (Ariyaratne et al. 2000). They are believed to be completely independent of the population of fetal Leydig cells responsible for initial masculinization of the embryo. The origin of fetal Leydig cells is unknown. During fetal life, Leydig cell precursors could arise from one or both of two possible sources: the mesonephros or the coelomic epithelium. When gonads from 11.5 days postcoitum (dpc) embryos were grafted to mesonephroi from mice carrying transgenic markers such as β-galactosidase (β-gal) or GFP, the markers were found in some of the peritubular myoid cells and other interstitial cells of the testis (Buehr et al. 1993; Merchant-Larios et al. 1993; Nishino et al. 2000). Some migratory mesonephric cells acquired ultrastructural features of steroidogenic Leydig cells (Merchant-Larios and Moreno-Mendoza 1998). A small population of these migrating cells differentiated into Leydig cells when cultured in vitro (Nishino et al. 2001). However, when the XY gonad was separated from the mesonephros at 11.5 dpc and cultured alone (Merchant-Larios et al. 1993) or when XY gonads were grafted to embryonic hind limbs at 11.5 dpc and subsequently cultured (Moreno-Mendoza et al. 1995), differentiation of Leydig cells proceeded normally. The results of these two experiments suggest that most Leydig precursors are already present in the gonad by 11.5 dpc. Another possible source of Leydig cell precursors is the coelomic epithelium that covers the entire coelomic surface of the gonad. Both proliferation studies (Schmahl et al. 2000) and DiI lineage tracing experiments (Karl and Capel 1998) revealed that coelomic epithelial cells in XY gonads proliferate rapidly between 11.5 and 12.5 dpc and contribute many interstitial cells to the developing testis. The fate of these cells has not been defined. The signals that induce differentiation of fetal Leydig cells are also unknown. At present only a negative regulator of Leydig cell differentiation (Wnt4) has been identified (Vainio et al. 1999). Expression of the hedgehog receptor, Patched 1 (Ptch1), throughout the cells of the interstitium in 12.5 dpc XY gonads suggested that DHH/PTCH1 signaling might function in Leydig cell differentiation in addition to its role in signaling between Sertoli and peritubular myoid cells (Bitgood et al. 1996). To determine the role of DHH/PTCH1 signaling in Leydig cell differentiation, we explored the temporal and spatial expression patterns of Dhh, Ptch1, and Scc, and analyzed gonads from Dhh−/− XY embryos. Here we show that disruption of DHH/PTCH1 signaling in Dhh−/− mice results in defects of fetal Leydig cell differentiation, whereas it has no effect on mesonephric cell migration or on the establishment of the interstitial cell population. These results suggest that DHH/PTCH1 signaling does not affect the origin of fetal Leydig precursors, but instead, operates later to specify the Leydig cell lineage by up-regulating Steroidogenic Factor 1 (Sf1) and Scc expression in Ptch1-expressing precursor cells located outside testis cords.
Results
Temporal and spatial expression of Dhh, Ptch1, and Scc in testis organogenesis
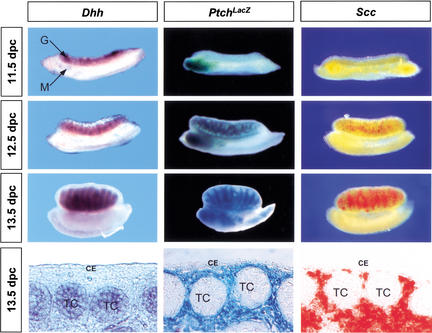
To determine whether fetal Leydig cells might be targets of DHH signaling, we first detailed the expression patterns of Dhh, its receptor, Ptch1, and a Leydig cell marker Scc (Rouiller et al. 1990) in XY gonads from 11.5 to 13.5 dpc, the period during which the differentiation of fetal Leydig cells occurs. Expression of Dhh began at 11.5 dpc and continued afterward in the Sertoli cell lineage as previously described (Fig. 1; Bitgood et al. 1996). Analyzing β-galactosidase activity in Ptchtm1Mps (PtchLacZ) XY gonads, we found that PtchLacZ was not expressed at 11.5 dpc XY gonads, but was prominently expressed in the interstitial space between testis cords in 12.5 and 13.5 dpc XY gonads (Fig. 1). PtchLacZ expression was also found around the mesonephric tubules in the anterior part of the mesonephros from 11.5 to 13.5 dpc. We compared PtchLacZ expression with Scc expression to determine whether PtchLacZ-expressing cells became Scc-positive. At 12.5 dpc, the majority of interstitial cells were PtchLacZ-positive and only a small population of them expressed Scc (Fig. 1). In 13.5 dpc XY gonads, a much larger percentage of PtchLacZ-expressing cells were also expressing Scc (Fig. 1, bottom panels). Neither PtchLacZ nor Scc was expressed in the coelomic epithelium of XY gonads (Fig. 1, bottom panels) or in endothelial cells of the vasculature (data not shown). Patched 2, another mammalian hedgehog receptor (Carpenter et al. 1998), was not expressed in XY gonads during this time period (data not shown). Other hedgehog genes such as Sonic Hedgehog and Indian Hedgehog are not expressed in the gonad (Bitgood and McMahon 1995).
Figure 1.
Expression patterns of Dhh (dark purple), Ptch1 (blue), and the Leydig cell marker Scc (red) in XY gonads from 11.5 dpc to 13.5 dpc. Expression of Dhh and Scc were detected by whole-mount in situ hybridization. Ptch1 expression was detected by analyzing β-galactosidase activity in the Ptchtm1Mps XY gonads. Sections of 13.5 dpc whole-mount samples are shown at the bottom to confirm the cell-specific expression patterns of Dhh, Ptch1, and Scc in the gonads. CE, coelomic epithelium; G, gonad; M, mesonephros; TC, testis cords.
Defects in differentiation of fetal Leydig cells in Dhh−/− XY gonads
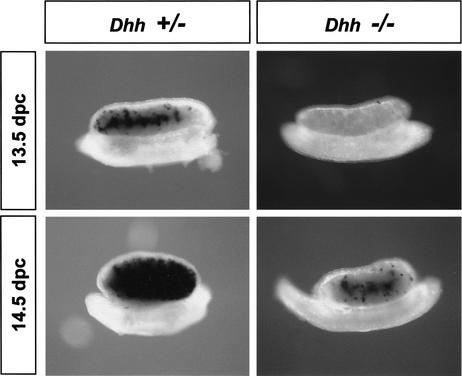
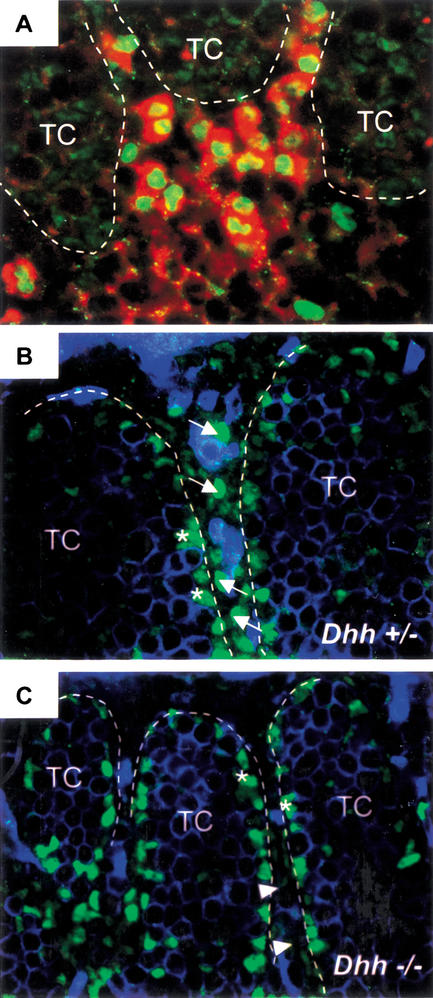
The expression patterns of Dhh and its receptor, Ptch1, indicated that DHH signaling could be involved in the early development of Leydig cells. To investigate whether differentiation of fetal Leydig cells was affected by loss of DHH signaling, we analyzed the expression of Scc in 13.5–14.5 dpc Dhh+/+, Dhh+/−, and Dhh−/− XY gonads (Clark et al. 2000). No differences were noted between Dhh+/+ and Dhh+/− samples. Representative Dhh+/− samples are shown in Figures 2 and 3. At 13.5 dpc, expression of Scc appeared in the center of all Dhh+/+ and Dhh+/− gonads, whereas Scc expression was completely absent in 70% (7/10) of Dhh−/− gonads (Fig. 2). By 14.5 dpc, Scc expression reached its peak in interstitial cells in Dhh+/+ and Dhh+/− gonads. However, only sparse staining for Scc was seen in the majority of 14.5 dpc Dhh−/− gonads (Fig. 2). It is known that the expression of Scc is under the regulation of SF1 (Clemens et al. 1994; Hatano et al. 1994). We performed immunocytochemistry for SF1 on 13.5 dpc XY gonads after in situ hybridization for Scc to verify that Scc-expressing cells were also SF1-positive. We found that all Scc-expressing cells (Fig. 3A, red cells outside of testis cords) showed strong nuclear staining for SF1 (Fig. 3A, green stain). In Dhh−/− gonads, the number of interstitial Leydig cells with strong nuclear SF1 staining was dramatically decreased compared to Dhh+/+ and Dhh+/− gonads (Fig. 3B,C, arrows). However, interstitial cells with weak nuclear SF1 staining were still present in Dhh−/− gonads in normal numbers (Fig. 3C, arrowheads). Expression of SF1 in Sertoli cells in testis cords was not affected by disruption of DHH signaling (Fig. 3B,C, asterisks).
Figure 2.
Expression of Scc in Dhh+/− and Dhh−/− XY gonads. The mRNA for the Leydig cell marker Scc (black stain) is present at 13.5 and 14.5 dpc in Dhh+/+ (data not shown) and Dhh+/− but is reduced or absent in Dhh−/− XY gonads.
Figure 3.
Expression of SF1 and Scc in 13.5 dpc Dhh+/− and Dhh−/− XY gonads. (A) Colocalization of SF1 (green nuclear staining) and Scc (red cytoplasmic staining) in Leydig cells in normal 13.5 dpc XY gonads by immunocytochemistry for SF1 and in situ hybridization for Scc. (B) Strong nuclear SF1 staining (arrows) in Leydig cells in Dhh+/− gonads. (C) Absence of strong nuclear SF1 staining in Leydig cells in Dhh−/− gonads. Weak nuclear SF1 staining was still present (arrowheads). SF1 staining was also detected in Sertoli cells (asterisks) in testis cords (TC, outlined by dotted lines). Germ cells and endothelial cells were stained with an anti-PECAM antibody (blue staining in B and C).
Normal mesonephric cell migration in Dhh−/− XY gonads
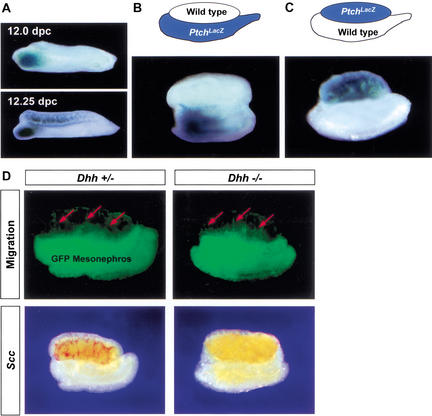
One of the cellular events downstream of Sry is migration of interstitial cells from the mesonephros into the gonad between 11.5 and 12.5 dpc (Capel et al. 1999; Tilmann and Capel 1999). Because most interstitial cells express PtchLacZ at 12.5 dpc (Fig. 1), we investigated whether Dhh signaling regulates mesonephric cell migration. PtchLacZ expression showed a unique pattern during the period when mesonephric cell migration occurs. At 11.5 dpc, PtchLacZ expression was observed only around the mesonephric tubules at the anterior part of the mesonephros but not in gonads of either sex (Fig. 1). As the development of gonads proceeded to 12.0 dpc, PtchLacZ expression appeared in the interstitium in the anterior part the XY gonad close to the mesonephric tubules (Fig. 4A). At 12.25 dpc, PtchLacZ expression in the XY gonad extended anteriorly and posteriorly (Fig. 4A). By 12.5 dpc, the entire interstitium of the XY gonad expressed PtchLacZ, except for the most posterior tip of the gonad (Fig. 1). No PtchLacZ expression was found in XX gonads at any stage examined (data not shown).
Figure 4.
DHH/PTCH1 signaling is not responsible for induction of mesonephric cell migration into XY gonads. (A) Analysis of PtchLacZ expression in 12.0 and 12.25 dpc XY gonads. (B) Recombinant organ culture using a wild-type gonad apposed to a PtchLacZ mesonephros. (C) Reciprocal organ culture with a PtchLacZ gonad apposed to a wild-type mesonephros. Recombinant organ cultures in both B and C were assembled at 11.25 dpc, cultured for 48 h, and assayed for PtchLacZ expression. (D) Mesonephric cell migration and Scc expression in Dhh+/− and Dhh−/− gonads: Recombinant gonad cultures were assembled with an 11.5 dpc Dhh+/− or Dhh−/− XY gonad apposed to an 11.5 dpc mesonephros expressing GFP. Cell migration (green cell, red arrows) was detected 48 h after culture. Samples were fixed, and then expression of Scc was detected by in situ hybridization (red staining in gonads).
This unique pattern of PtchLacZ expression (Fig. 4A) suggested that the DHH/PTCH1 signaling pathway might induce migration of Ptch1-expressing cells from the mesonephros into the interstitium of the XY gonad, beginning near the anterior end of the gonad. To test this hypothesis, we assembled two different recombinant organ cultures at 11.25 dpc. In the first recombinant culture (Fig. 4B), we assembled a wild-type gonad with a PtchLacZ mesonephros. We reasoned that if PtchLacZ-expressing cells derive from the mesonephros, we should observe β-gal-positive cells in the wild-type gonad after migration has taken place. In the second recombinant culture (Fig. 4C), we assembled the reciprocal combination with a PtchLacZ gonad apposed to a wild-type mesonephros. After culture for 30 h (corresponding to ∼12.5 dpc in vivo), samples were stained for β-gal. We found no β-gal staining in the interstitium of the wild-type gonad in the first recombinant culture (Fig. 4B), suggesting that few if any cells that have migrated from the mesonephros during this period of culture express PtchLacZ. In the second recombinant culture with a PtchLacZ gonad and a wild-type mesonephros, β-gal staining appeared in the interstitium of all PtchLacZ gonads (Fig. 4C), suggesting that PtchLacZ expression is induced in cells already present in the gonad by 11.25 dpc.
To further test the possibility that DHH/PTCH1 signaling was involved in mesonephric cell migration, we assembled an 11.5 dpc Dhh+/+, Dhh+/−, or Dhh−/− XY gonad apposed to an 11.5 dpc mesonephros expressing GFP and compared the migration of GFP-expressing cells in the presence and absence of DHH signaling. We found that GFP-expressing cells migrated from the mesonephros into the XY gonad in a similar pattern in Dhh+/+ (data not shown), Dhh+/−, and Dhh−/− gonads (Fig. 4D, red arrows). Analysis of Scc expression in these samples revealed that despite normal mesonephric cell migration, expression of Scc is completely absent in Dhh−/− XY gonads compared to Dhh+/+ and Dhh+/− gonads (Fig. 4D, red staining).
Stage-specific effects of the hedgehog inhibitor cyclopamine on Leydig cell differentiation
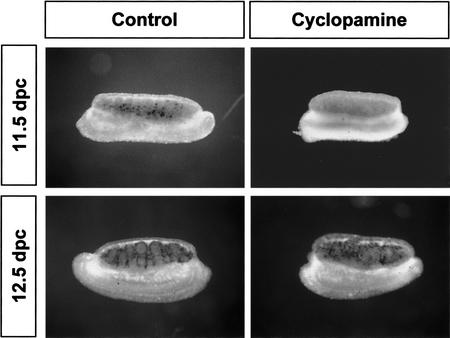
To determine whether DHH/PTCH1 signaling regulates the earliest stages of Leydig cell differentiation or later maintenance or expansion of the Leydig cell population, we examined Scc expression in gonad organ cultures in the presence and absence of a DHH signaling inhibitor, cyclopamine, introduced at 11.5 dpc or 12.5 dpc. Cyclopamine inhibits hedgehog signaling by inactivating Smoothened, the first downstream signaling molecule after binding of hedgehog protein to its receptor, PTCH1 (Taipale et al. 2000). Scc was expressed normally in both 11.5 and 12.5 dpc gonads after 24-h culture in the absence of cyclopamine. When cyclopamine was added at 11.5 dpc, the expression of Scc in Leydig cells was completely inhibited. In contrast, addition of cyclopamine to cultures at 12.5 dpc or 13.5 dpc had no effect on Scc expression in Leydig cells (Fig. 5, black stain; 13.5 dpc data not shown).
Figure 5.
Stage-specific effects of the hedgehog inhibitor cyclopamine on expression of Scc mRNA in Leydig cells. XY gonads (11.5 or 12.5 dpc) were cultured in the presence or absence of cyclopamine (25 μM) for 24 h followed by whole-mount in situ hybridization for Scc (black staining in gonads).
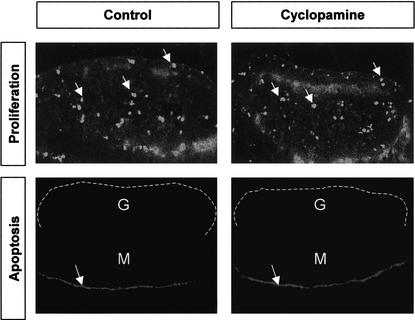
To determine whether the loss of DHH signaling affected proliferation or maintenance of Leydig precursors, we examined cell proliferation using an antibody against phosphorylated Histone H3 (pHH3; Paulson and Taylor 1982; Hendzel et al. 1997; Saka and Smith 2001), and apoptosis, using LysoTracker reagent (Zucker et al. 1998, 1999), in 11.5 dpc gonad explants cultured for 40 h in the presence or absence of cyclopamine. We found a similar total number of pHH3-positive cells (cell counts from 10 serial sections) in gonads cultured in the absence or presence of cyclopamine (Fig. 6, arrows). Although normal apoptotic cells were detected in the Müllerian duct in the mesonephros at this stage (Roberts et al. 1999), no apoptotic cells were found in the gonadal region of samples cultured in the presence or absence of cyclopamine (Fig. 6, the gonad is outlined by a dotted line).
Figure 6.
Effects of the hedgehog inhibitor cyclopamine on proliferation and apoptosis in 11.5 dpc gonads. Gonads (11.5 dpc) were cultured for 24 h in the presence or absence of cyclopamine (25 μM) followed by immunocytochemistry for phosphorylated Histone H3 (arrows) or LysoTracker staining for apoptosis (arrows indicate position of the Müllerian duct). G, gonad (outlined by a dotted line); M, mesonephros.
Discussion
It has been more than five decades since Jost first discovered that testosterone synthesized by the fetal testis is essential for differentiation of the Wölffian duct and development of male secondary sex characteristics (Jost 1947). Here we report that DHH/PTCH1 signaling is a positive regulator of the differentiation of steroid-producing Leydig cells in the fetal testis. Dhh is expressed downstream of Sry, specifically in Sertoli cells inside testis cords (Bitgood et al. 1996), and is the only known mammalian hedgehog protein expressed in the gonad between 11.5 and 13.5 dpc. One of the hedgehog receptors, Ptch1, was known to be expressed in interstitial cell populations (Bitgood et al. 1996). Original generation of Dhh-null mice on a 129/Sv genetic background resulted in defects in spermatogenesis but no defects in testis organogenesis and Leydig cell differentiation despite down-regulation of Ptch1 (Bitgood et al. 1996). However, transfer of the Dhh mutation to another genetic background resulted in discrete defects in development of the peritubular myoid cell lineage, leading to abnormal cord organization and loss of adult Leydig cells (Clark et al. 2000; Pierucci-Alves et al. 2001). We show here that it also results in a defect in differentiation of fetal Leydig cells.
Ptch1 is first expressed around the mesonephric tubules at the anterior end of the mesonephros. By 12.0 dpc, interstitial cells toward the anterior end of the gonad begin to express Ptch1 under the positive regulation of DHH. Expression of Ptch1 gradually extends toward both anterior and posterior ends of the gonad. Despite the implications of this expression pattern, we find no evidence that DHH is involved in signaling for mesonephric cell migration. Nor does loss of Dhh appear to exert a detrimental effect on Sertoli differentiation, as MIS and Sox9 expression in Dhh−/− gonads and in cyclopamine treated gonads (Yao and Capel 2002) are normal.
Instead, this and previous data suggest that DHH is involved in signaling proximal cells to differentiate along specific pathways. For example, it has been shown that DHH influences the differentiation of peritubular myoid cells in Ptch1-expressing cells most proximal to the DHH signal (Clark et al. 2000; Pierucci-Alves et al. 2001). Here we show that DHH signals the Ptch1-expressing cells located slightly further away from the DHH-producing Sertoli cells to differentiate as Leydig cells. Although it appears that all Leydig cells express Ptch1, not all Ptch1-expressing cells differentiate as Leydig cells. This likely means that other signals combine with DHH signals to specify Leydig cell fate.
Leydig precursors responsive to the DHH signal may be set aside earlier by their lineage origin, or they may be specified among cells of the interstitium by the intersection of multiple signals. Some evidence suggests that Leydig cells and steroid cells of the adrenal share a common origin at 10.5 dpc near the anterior end of the mesonephros (Hatano et al. 1996). If this is true, they must move into the gonad prior to 11.25 dpc under the control of signals other than DHH or they would have been detected in our recombinant organ culture system. Another possibility is that Leydig cells do not have a discrete lineage origin: pluripotent cells may derive from the coelomic epithelium between 11.5 and 12.5 dpc whose differentiation is under the control of combinatorial signals that intersect in the field of the gonad. This type of paradigm could suggest that the interstitial cells of the gonad are equivalent and plastic in the sense that, regardless of where they originate, they may follow one of several cell fates in the gonad. This decision could depend not on their lineage origin, but on their distance from other signaling cells or their spatial relationship to the vasculature or to other structural features of the gonad. Hedgehog signaling effects related to distance from the signal have been noted in many systems (Bumcrot and McMahon 1996; Neumann and Cohen 1997; Strigini and Cohen 1999; Vervoort 2000).
DHH does not regulate the size of the precursor population. We found that interstitial cells with low SF1 expression were still present in the Dhh−/− gonads, which may account for morphological identification of fetal Leydig cells in electron micrographs in Dhh−/− gonads (Clark et al. 2000). In previous work, we showed that low SF1-expressing cells derived from a second wave of proliferation in the coelomic epithelium (Schmahl et al. 2000). No difference in proliferation or apoptosis was observed in gonads cultured with the hedgehog inhibitor cyclopamine, suggesting that DHH/PTCH1 signaling does not regulate proliferation or survival of fetal Leydig cell precursors as has been shown to occur in other systems (Cann et al. 1999; Oppenheim et al. 1999; Charrier et al. 2001). The time at which DHH affects Leydig differentiation, based on in vitro experiments using cyclopamine to block hedgehog signals, suggests that DHH/PTCH1 signaling specifies Leydig cell fate by early up-regulation of SF1 and its target, Scc.
The failure of fetal Leydig cell differentiation provides an explanation for the feminized external genitalia phenotype of Dhh−/− XY mice (Clark et al. 2000) and a 46,XY partial gonad dysgenesis patient with a Dhh mutation (Umehara et al. 2000). Both cases developed premature female external genitalia with a blind vagina. The internal accessory sex glands and ducts, whose development depends upon the proper amount of testosterone from fetal Leydig cells, are decreased in size, and the testes were undescended. The appearance of a few Leydig cells in Dhh−/− gonads at later stages is not sufficient to rescue differentiation of secondary sex characteristics in Dhh−/− mice; however, it does suggest that other signaling pathways may partially compensate for loss of the DHH/PTCH1 signaling pathway. Alternatively, a subpopulation of Leydig cells may derive independent of DHH/PTCH1 signaling. We are conducting more experiments to explore the origin of Leydig cell precursors and the interaction between DHH/PTCH1 and other signaling pathways.
Materials and methods
Mouse strains
The generation of Dhh-null mice was described previously, and original breeding mice for the Curis colony were kindly provided by Dr. Andrew McMahon (Harvard University, Cambridge, MA). Mice were bred on a mixed background of 129/Sv, C57BL/6, and Swiss Webster. The Dhh genotype was determined by polymerase chain reaction (PCR) of tail DNA. CD1 random-bred mouse strains (Charles River) were used for organ culture, immunocytochemistry, and in situ hybridization. GFP transgenic mice (Hadjantonakis et al. 1998) were used for migration studies. The Ptchtm1Mps mice were generated as described by Goodrich et al. (1997) and were kindly provided by Dr. Matthew Scott of Stanford University.
Organ culture
Genital ridges (gonad plus mesonephros) from 11.25–11.5 dpc embryos (0.5 dpc represents noon of the day when the vaginal plug was detected) were obtained for organ culture. To determine the sex of 11.25–12.5 dpc embryos, we used a staining method (Palmer and Burgoyne 1991) to detect the presence of XX-specific Barr bodies in the amnion of individual embryos. Genital ridges were cultured at 37°C with 5% CO2/95% air on a 1.5% agar block for 48 h in Dulbecco's Minimal Eagle Medium (DMEM), supplemented with 10% fetal calf serum (Hyclone), and 50 μg/mL ampicillin. Cyclopamine (25 μM, TRC Biomedical Research Chemicals) was added to the culture medium to inhibit the hedgehog signaling pathway. This concentration of cyclopamine represented the minimal concentration resulting in disruption of testis cord formation as determined previously (Yao and Capel 2002). An equivalent volume of methanol (solvent for cyclopamine) was added to other organ cultures as controls.
Whole-mount in situ hybridization
Samples were fixed overnight in 4% paraformaldehyde in PBS at 4°C and processed according to the method of Henrique et al. (1995). We used alkaline phosphatase-conjugated digoxigenin-labeled RNA probes for Dhh and Scc. Two different alkaline phosphatase substrates (NBT/BCIP for Dhh, Fast Red for Scc, Boehringer Mannheim) were used for color development.
Double whole-mount in situ hybridization and immunocytochemistry
To double-label Scc (mRNA) and SF1 (protein) in the gonads, whole-mount in situ hybridization was performed as described above using Fast Red as the substrate for alkaline phosphate followed by immunocytochemistry against SF1. After fast red color development (∼5 h at room temperature), samples were washed in PBS for 10 min and blocked in the blocking solution (10% heat-inactivated goat serum and 0.1% Triton X-100 in PBS) for 1 h at room temperature. A rabbit polyclonal antibody against SF1 (1:200) was added to the blocking solution and samples were incubated overnight at 4°C. Samples were then washed 3 times for 10 min each in washing solution (1% heat-inactivated goat serum and 0.1% Triton X-100 in PBS) followed by incubation in the blocking solution with the secondary antibody (FITC-conjugated goat anti-rabbit antibody, 1:1000, Jackson Immunochemicals). Samples were washed 3 times for 10 min each in washing solution and mounted for confocal microscopy.
Migration assay
Gonads and mesonephroi from 11.5 dpc CD1 or GFP or PtchLacZ embryos were separated. A CD1 XY gonad was assembled with a GFP or a PtchLacZ mesonephros and cultured on an agar block for 48 h as described (Martineau et al. 1997). Images were obtained using a Leica MZFLIII dissecting microscope with a GFP filter.
β-gal stain
Samples were washed in PBS and fixed in 2% paraformaldehyde for 20 min at room temperature. Samples were then rinsed in washing solution (2 mM MgCl2, 0.02% Nonidet P-40 in PBS), incubated overnight at 37°C in β-gal stain (1 mg/mL X-gal, 200 mM K3Fe(CN)6, 200 mM K4Fe(CN)6), washed, and postfixed in 4% paraformaldehyde.
Assay for proliferation and apoptosis
To assay proliferation, gonad explants were fixed overnight in 4% paraformaldehyde in PBS at 4°C immediately after culture. Samples were processed and cut into 10-μm frozen serial sections as described (Karl and Capel 1998) and stained immunocytochemically for a proliferation marker, phosphorylated Histone H3 (pHH3). The primary antibody was a rabbit polyclonal antibody against pHH3 (1:1000; Upstate Biotechnology) and the secondary was an FITC-conjugated goat anti-rabbit antibody (1:500, Jackson Immunochemicals). pHH3-positive cells from 10 serial sections of each gonad (n = 5) were counted and subjected to statistical analysis. To assay apoptosis, gonad explants were cultured in 1 mL medium with 2 μL of LysoTracker Red DND-99 (Molecular Probes) for an additional 30 min at the end of 24-h of culture. Gonad explants were washed 3 times in PBS, fixed overnight in 4% paraformaldehyde in PBS at 4°C, and mounted for confocal imaging.
Acknowledgments
We sincerely thank Dr. Ann Clark, who initiated this collaboration; Christopher Tilmann, Jennifer Brennan, Jennifer Schmahl, Andrea Ross, Jordan Bachvarov, and Leo DiNapoli, who all contributed to useful discussions. For their generous gifts of materials, we thank Harold Erickson (laminin antibody), Ken-ichirou Morohashi (SF1 antibody), Keith Parker (Scc probe), and Matthew Scott (Ptchtm1Mps mice). This work was supported by grants to B.C. from the NIH (HD39963-04) and a postdoctoral fellowship from the Lalor Foundation to H.Y.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL b.capel@cellbio.duke.edu; FAX (919) 684-5481.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.981202.
References
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Ariyaratne SHB, Mendis-Handagama CS, Hales BD, Mason IJ. Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol Reprod. 2000;63:165–171. doi: 10.1095/biolreprod63.1.165. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert Hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Bumcrot DA, McMahon AP. Sonic hedgehog: Making the gradient. Chem Biol. 1996;3:13–16. doi: 10.1016/s1074-5521(96)90077-0. [DOI] [PubMed] [Google Scholar]
- Cann GM, Lee JW, Stockdale FE. Sonic hedgehog enhances somite cell viability and formation of primary slow muscle fibers in avian segmented mesoderm. Anat Embryol (Berl) 1999;200:239–252. doi: 10.1007/s004290050276. [DOI] [PubMed] [Google Scholar]
- Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev. 1999;84:127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two Patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci. 1998;95:13630–13634. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier JB, Lapointe F, Douarin NM, Teillet MA. Anti-apoptotic role of Sonic hedgehog protein at the early stages of nervous system organogenesis. Development. 2001;128:4011–4020. doi: 10.1242/dev.128.20.4011. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Clemens JW, Lala DS, Parker KL, Richards JS. Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in rat granulosa cells. Endocrinology. 1994;134:1499–1508. doi: 10.1210/endo.134.3.8119192. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse Patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- Hatano O, Takayama K, Imai T, Waterman MR, Takakusu A, Omura T, Morohashi K. Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P450 genes in the gonads during prenatal and postnatal rat development. Development. 1994;120:2787–2797. doi: 10.1242/dev.120.10.2787. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Jost A. Recherches sur la differenciation sexuelle de l'embryon de lapin. Arch Anat Microsc Morphol Exp. 1947;36:271–315. [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N. Mesonephric stromal cells differentiate into Leydig cells in the mouse fetal testis. Exp Cell Res. 1998;244:230–238. doi: 10.1006/excr.1998.4215. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N, Buehr M. The role of the mesonephros in cell differentiation and morphogenesis of the mouse fetal testis. Int J Dev Biol. 1993;37:407–415. [PubMed] [Google Scholar]
- Moreno-Mendoza N, Herrera-Munoz J, Merchant-Larios H. Limb bud mesenchyme permits seminiferous cord formation in the mouse fetal testis but subsequent testosterone output is markedly affected by the sex of the donor stromal tissue. Dev Biol. 1995;169:51–56. doi: 10.1006/dbio.1995.1125. [DOI] [PubMed] [Google Scholar]
- Neumann C, Cohen S. Morphogens and pattern formation. BioEssays. 1997;19:721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- Nishino K, Kato M, Yokouchi K, Yamanouchi K, Naito K, Tojo H. Establishment of fetal gonad/mesonephros coculture system using EGFP transgenic mice. J Exp Zool. 2000;286:320–327. [PubMed] [Google Scholar]
- Nishino K, Yamanouchi K, Naito K, Tojo H. Characterization of mesonephric cells that migrate into the XY gonad during testis differentiation. Exp Cell Res. 2001;267:225–232. doi: 10.1006/excr.2001.5238. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Homma S, Marti E, Prevette D, Wang S, Yaginuma H, McMahon AP. Modulation of early but not later stages of programmed cell death in embryonic avian spinal cord by sonic hedgehog. Mol Cell Neurosci. 1999;13:348–361. doi: 10.1006/mcne.1999.0755. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepuberal and adult XX-XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Paulson JR, Taylor SS. Phosphorylation of histones 1 and 3 and nonhistone high mobility group 14 by an endogenous kinase in HeLa metaphase chromosomes. J Biol Chem. 1982;257:6064–6072. [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the desert hedgehog-null mouse testis. Biol Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Hirokawa Y, Nachtigal MW, Ingraham HA. Paracrine-mediated apoptosis in reproductive tract development. Dev Biol. 1999;208:110–122. doi: 10.1006/dbio.1998.9190. [DOI] [PubMed] [Google Scholar]
- Rouiller V, Gangnerau MN, Vayssiere JL, Picon R. Cholesterol side-chain cleavage activity in rat fetal gonads: A limiting step for ovarian steroidogenesis. Mol Cell Endocrinol. 1990;72:111–120. doi: 10.1016/0303-7207(90)90101-d. [DOI] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev Biol. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Formation of morphogen gradients in the Drosophila wing. Semin Cell Dev Biol. 1999;10:335–344. doi: 10.1006/scdb.1999.0293. [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Tilmann K, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet. 2000;67:1302–1305. doi: 10.1016/s0002-9297(07)62958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon A. Female development in mammals is regulated by Wnt-4 signaling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vervoort M. hedgehog and wing development in Drosophila: A morphogen at work? BioEssays. 2000;22:460–468. doi: 10.1002/(SICI)1521-1878(200005)22:5<460::AID-BIES8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Yao, H.H.C. and Capel, B. 2002. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev. Biol. (in press). [DOI] [PMC free article] [PubMed]
- Zucker RM, Hunter ES, Rogers JM. Apoptosis and morphology in mouse embryos by confocal laser scanning microscopy. Methods. 1999;18:473–480. doi: 10.1006/meth.1999.0815. [DOI] [PubMed] [Google Scholar]
- Zucker RM, Hunter S, Rogers JM. Confocal laser scanning microscopy of apoptosis in organogenesis-stage mouse embryos. Cytometry. 1998;33:348–354. doi: 10.1002/(sici)1097-0320(19981101)33:3<348::aid-cyto9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]