Abstract
The central nervous system of altricial infants is specialized for optimizing attachments to their caregiver. During the first postnatal days, infant rats show a sensitive period for learning and are particularly susceptible to learning an attraction to their mother’s odor. Classical conditioning appears to underlie this learning that is expressed behaviorally as an increased ability to acquire odor preferences and a decreased ability to acquire odor aversions. Specifically, in neonatal rats, pairing an odor with moderately painful shock (0.5mA) or milk produces a subsequent relative preference for that odor. The neural circuitry supporting the increased ability to acquire odor preferences appears to be the heightened functioning of the noradrenergic pontine nucleus locus coeruleus. Indeed, norepinephrine from the locus coeruleus appears to be both necessary and sufficient for learning during the sensitive period. On the other hand, the decreased ability to acquire odor aversions seems to be due to the lack of participation of the amygdala in at least some aversive learning situations. The site of plasticity in the pup’s brain appears to be limited to the olfactory bulb. This neonatal sensitive period for learning ends around postnatal day 9-10, at which time pups make the transition from crawling to walking and classical conditioning becomes “adultlike.” The neonatal behavioral and neural induced changes are retained into adulthood where it modifies sexual behavior.
Keywords: classical conditioning, olfactory learning, imprinting, sensitive period, neonatal learning, odor conditioning, attachment, abuse, amygdala, fear conditioning, locus coeruleus, norepinephrine
IN ALTRICIAL SPECIES, such as humans and rats, rapid formation of attachment is critical for survival. While some prenatal experiences contribute to attachment formation, most species dependent on parental care exhibit some form of rapid, specialized postnatal learning, such as imprinting in avian species (Salzen, 1970). We have recently developed a mammalian model of imprinting in the rat which has enabled us to explore the neurobiology of attachment using a classical conditioning paradigm (for a review of other maternalinfant attachment models see Brennen & Keverne, 1997; Hudson, 1993).
According to Bowlby (1965), attachment is characterized by the infant seeking proximity to the caregiver and the infant’s endurance of considerable abuse to maintain contact with the caregiver. These attachment characteristics are not unique to mammals and appears to occur throughout the animal kingdom. Specifically, attachment to an abusive caregiver has been clinically documented in both human infants (review, Helfer, Kempe & Krugman, 1997), and nonhuman infant primates (Harlow & Harlow, 1965). These results are consistent with experimental manipulations; chicks shocked during imprinting show enhanced following (Hess, 1962) and abused infant dogs form attachments to an abusive handler (Fisher, 1955 cited in Rajecki, Lamb & Obmascher, 1978). Bowlby (1965) & others (Hofer, 1981) have speculated that unique infant learning abilities may have evolved to ensure that altricial animals easily learn the proximity seeking behaviors necessary for survival, regardless of the quality of the care they receive from their caregiver.
Observations of naturalistic interactions between rat pups and their mother shows that it is not uncommon for the mother rat to hurt her pups. Indeed, pain elicited vocalizations from pups can be heard when the rat mother steps on pups while entering and leaving the nest or when the mother retrieves pups. The benefits of preventing neonatal pups from learning an aversion to their mother’s odor ensures pups will learn only approach responses to the mother and are therefore critical for pups’ survival. Indeed, without pups’ approach responses to the mother, pups can not obtain the mother’s milk, warmth and protection. Again, from an evolutionary perspective, it may be better for an altricial infant to have a bad caretaker than no caretaker. Although a learning based attachment system may seem precarious, the ecological niche of the neonatal pup, along with the motoric limitation of the pup, ensures neonatal rat pups will not venture from the nest. Therefore, the range of possible attachment figures is greatly limited. Furthermore, characteristics of neonatal rat learning greatly enhance the probability of pups learning the odor preference which underlies their attachment to the mother. This learning is temporally limited to a sensitive period during the first week and a half of the neonatal rat’s life.
I. During the sensitive period for learning, neonatal rat pup classical conditioning has unique characteristics
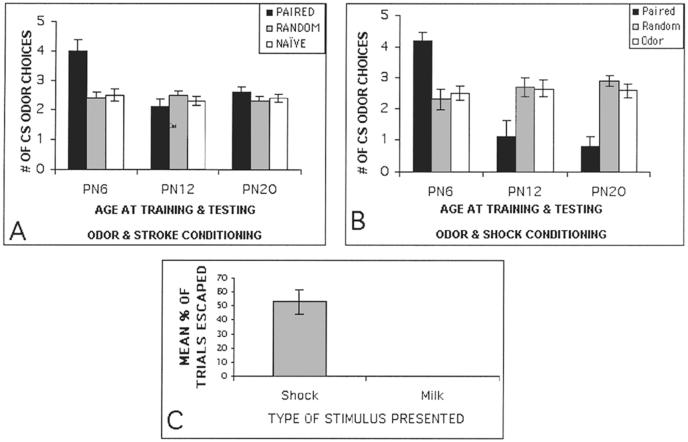
Pup classical conditioning is very similar to adult learning and has been demonstrated within the natural nest, as well as in more controlled situations outside the nest (Brunjes & Alberts, 1979; Campbell, 1984; Cornwell-Jones & Sobrian, 1977; Galef & Kaner, 1980; Leon, 1975; Miller, Jagielo & Spear,1989; Pedersen, Williams & Blass, 1982, Rudy & Cheatle, 1977; Sullivan, Hofer, Brake & Williams,1986a, Sullivan, Hofer & Brake, 1986b; Sullivan, Wilson, Wong, Correa & Leon, 1990; Terry & Johanson, 1996). However, there are unique characteristics of neonatal learning which greatly enhance the likelihood of pups developing odor preferences necessary for forming maternal attachment. First, inhibitory conditioning and passive avoidance do not appear to emerge until after postnatal days (PN) 10-11 (Blowovski & Cudennec, 1980; Collier, Mast, Meyer & Jacobs, 1979; Goldman & Tobach, 1967; Martin & Alberts, 1979; Stehower & Campbell, 1978; review Myslivecek, 1997). Second, pre-exposure to a conditioned stimulus (CS) facilitates conditioning in infant rats, whereas it retards conditioning in weanling and adult rats (Hoffmann & Spear, 1989). Thus, exposure to maternal odors without a reward would appear to enhance pups learning a preference to maternal odors. Third, when pups do learn, they appear to be less selective learners as compared to adult rats (Spear et al., 1989). That is, whereas an adult will focus on only a few environmental stimuli during leaning, pups will acquire information about a broad range of stimuli, thus, facilitating acquisition of a myriad of maternal features. Fourth, many stimuli, even those with aversive qualities, can function as a positive reward and when paired with an odor, produce a subsequent preference to that odor (Figure 1; Alberts & May, 1984, Brake, 1981; Camp & Rudy, 1988; Dominguez, Lopez & Molina, 1999; Johanson & Teicher, 1980; Johanson & Hall, 1982; McLean, Darby-King, Sullivan & King, 1993; Pedersen et al., 1982; Sullivan, Hofer & Brake, 1986b; Sullivan, Brake, Hofer & Williams,1986a; Sullivan & Leon, 1986; Sullivan & Hall, 1988; Sullivan, Landers, Yeaman & Wilson, 2000a; Sullivan, Stackenwalt, Nasr, Lemon & Wilson, 2000b; Weldon, Travis & Kennedy, 1991). The ability of stimuli such as shock (0.5mA) and tailpinch to support odor preference conditioning is not due to pups’ inability to detect pain, and pain threshold does not change between PN4 and PN12 (Camp & Rudy, 1988; Haroutunian & Campbell, 1979; Emerich, Scalzo, Enters, Spear & Spear, 1985).
FIG. 1.
Odor conditioning in PN6, PN12 and PN21 pups. (A) Odor-stroking (tactile stimulation) produces an odor preference in PN6 pups, but fails to support learning in older pups (B) Odor-shock conditioning produces a subsequent relative odor preference in PN6 pups, while older pups expressed an aversion to the conditioned odor. (C) Comparison of PN9 and PN12 pain responses to 0.5mA tail shock illustrates that shock is painful to pups during the time it supports odor preference conditioning.
It should be noted that pups can learn an odor aversion if that odor is paired with malaise. Specially, pairing an odor with either LiCl or very strong shock (1.2mA-1.5mA), both of which produce illness in pups, results in a subsequent aversion for that odor. It should be noted that this high shock level is above that typically used in adult fear conditioning experiments (Ader & Peck, 1977; Gemberling & Domjan, 1982; Rudy & Cheatle 1977, 1978; Sullivan & Wilson, 1995). Moreover, according to work from Jerry Rudy’s lab and Byron Campbell’s lab, until about PN9-10, pups easily learn aversions about interoceptive cues (malaise or internal shock) but not exteroceptive cues (moderate 0.5mA tail or paw shock (Camp & Rudy, 1988; Haroutunian & Campbell, 1979). Indeed, Camp and Rudy have suggested that changes in categorization of appetitive and aversive events occur in pups sometime between PN8 and PN12. However, it should be noted that even Odor-LiCl learning has additional constraints during the neonatal period: pups presented with odor-LiCl pairings while nursing have a difficult time learning to avoid that odor (Martin & Alberts, 1982).
At PN9 to PN10, pups mature and begin to venture outside the nest (Bolles & Woods,1965), the sensitive period ends (Sullivan et al., 2000a) and learning begins to more closely resemble learning in adults. Thus, the broad odor preference learning which underlies early attachment appears to end when pups are no longer confined to the nest. Indeed, pup learning appears to become more selective at a time in development when pups begin leaving the nest and encounter odors not associated with the safety of the nest.
The odor preferences acquired during the sensitive period are retained into adulthood
In rats, early attachment-related odors are retained into adulthood, where they take on a role in modulating reproductive behaviors (Fillion & Blass, 1986), albeit a limited role (Moore, Jordan & Wong, 1996). Thus, as has been documented in imprinting in other species, stimuli associated with early experience and attachment influence adult mate preference and enhance sexual performance.
Good memories of bad events
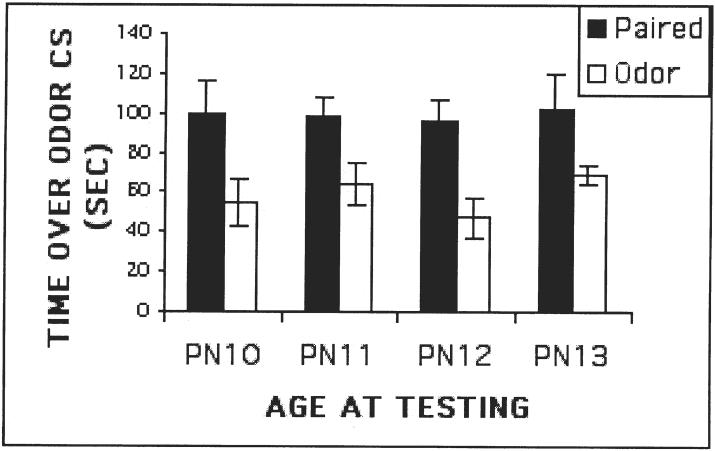
Recent work from our laboratory has further characterized the paradoxical odor-shock preference conditioning of neonatal rats. First, we found that the odor-shock induced odor-preference was very resilient and persisted even after 4 days of continued odor-shock conditioning after the sensitive period (Sullivan, Roth, Moriceau, Dikes & Holman, submitted, Figure 2). This memory seems persistent since it is still expressed as an odor preference at weanling. This suggests that once a neonatal memory is formed, the original memory trace continues to be strengthened by subsequent experience. In the present case, it is possible that the neonatal learning circuitry continues to be used, even after a more complex neural circuitry of adult learning emerges.
FIG. 2.
Pain-induced odor preference is maintained despite odor-shock training following the termination of the sensitive period. Pups received odor-shock conditioning during (PN8-PN9) and after (PN10, 11, 12, 13) the sensitive period until testing. Pups were tested on a 2-odor choice test at either PN10, PN11, PN12 or PN13. All experimental pups continued to exhibit a conditioned relative odor preference to the conditioned odor.
II. Sensitive period learning is characterized by learning-dependent olfactory bulb plasticity
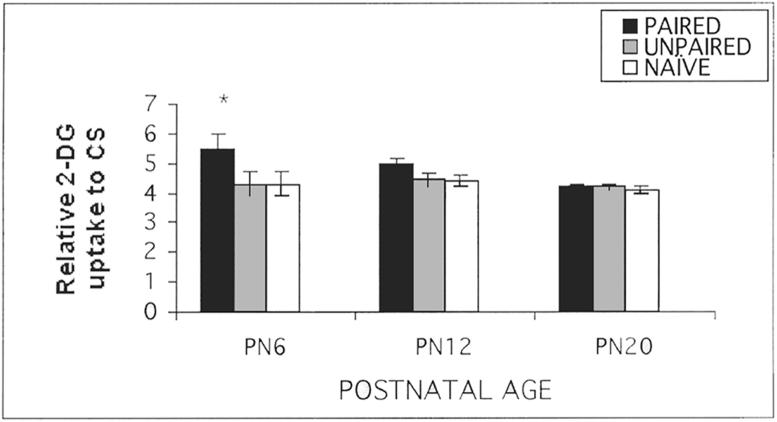
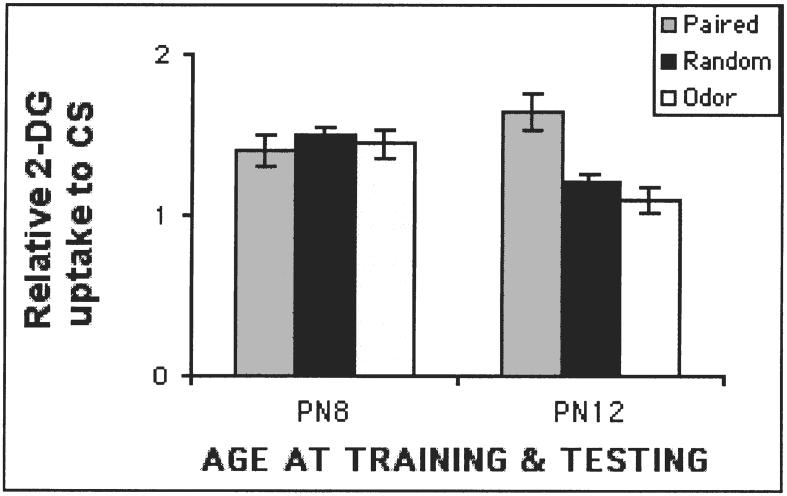
The development of a specific olfactory-based attachment system in the neonatal rat pup is associated with the acquisition of odor-specific olfactory bulb neural changes. Similarly to the acquisition of the attachment based learning, these induced olfactory bulb changes are only acquired during the sensitive period (Figure 3). We found that rat pups express this modified olfactory bulb response to both natural maternal and artificial odors experienced in the nest, as well as to odors in controlled learning experiments (Sullivan & Leon, 1986; Johnson, Woo, Duong, Nguyen & Leon, 1995; Wilson & Sullivan, 1991; Wilson, Sullivan & Leon, 1987). The modified olfactory bulb response is characterized by enhanced immediate-early gene activity (c-fos), and enhanced 2-deoxyglucose (2-DG) uptake in focal, odor-specific glomerular regions in response to the conditioned odor. In addition, modified single-unit response patterns of mitral/tufted cells near the enhanced glomerular foci were found (Wilson et al., 1987; Wilson & Leon, 1988a,b; Wilson & Sullivan, 1990), olfactory bulb anatomical changes reflected in enlarged glomeruli were found within these foci (Woo, Coopersmith & Leon, 1987), as well as an increase in pCREB (Mclean, Harley, Darby-King & Yuan, 1999). As with the behavioral changes in attachment, these neural changes are retained into adulthood, but their acquisition is dependent upon experiences during infancy (Woo & Leon, 1988; Pager, 1974).
FIG. 3.
Neonatal (PN6) odor conditioning produces learning associated changes in the olfactory bulb as measured by 14C 2-DG autoradiography. These changes are only acquired during the sensitive period. PN6, 12 or 20 pups were conditioned with odor-shock. The next day, the olfactory bulbs were assessed for learning associated changes using 14C 2-deoxyglucose autoradiography (Sullivan & Wilson, 1995).
III. Without the neural structures involved in adult learning circuitry, the neonate relies on a unique neural circuitry for learning
A pre and early postnatal rat can be classically conditioned prior to maturation of brain areas important in adult learning, such as the hippocampus and amygdala. Specifically, major nuclei subdivision of the amygdala does not occur until around PN7 with major neurogenesis occurring from PN10-20 (Bayer, 1980; Berdel, Morys & Maciejewska, 1997; Mizukawa, Tseng & Otsuka,1989). Maturation of the hippocampus occurs even later, since primary cortical input to the hippocampus from the entorhinal cortex is only beginning to develop during the first postnatal week (Crain, Cotman, Taylor & Lynch, 1973). Indeed, based on behavioral studies, the hippocampus does not appear to be functioning in learning until weaning or later (Rudy & Morledge, 1994; Rudy, Stadler-Morris & Alberts, 1987; Stanton, 2000). Furthermore, the frontal cortex is still undeveloped during this early neonatal period (Vermer, VanVulpen & VanUum, 1996) and there is evidence that the cortex may not be involved in neonatal sensitive period learning (Landers & Sullivan, 1999). The failure of brain areas such as the amygdala, hippocampus and frontal cortex to function during learning may be due to a lack of the functional connectivity not emerging until after the first week and a half of life (Nair & Gonzalez-Lima, 1999). Thus, neural immaturity, along with lack of functional connectivity between brain areas may underlie the infant rat’s reliance on another learning neural circuitry.
Without the adult learning circuitry, the neonate appears to depend upon a unique, simplistic neural circuit for learning. Specifically, based upon the results of a series of experiments from both our lab and others, it appears as though the heightened release of norepinephrine (NE) from the locus coeruleus (LC) into the olfactory bulb underlies the olfactory bulb plasticity responsible for neonatal odor learning.
The importance of Norepinephrine (NE) in neonatal learning
Many neurotransmitters have a role in early olfactory learning in neonatal rats (5-HT— McLean et al.,1993; DA—Weldon et al.,1991; Barr & Wang, 1992; glutamate—Mickley et al., 1998; Lincoln et al., 1988; GABA - Okutani, Yagi & Kaba, 1999; and opiates—Barr & Rossi,1992; Kehoe & Blass, 1988; Roth & Sullivan, in press), although the action of NE appears particularly important in neural plasticity during early development and learning induced-plasticity used in early attachment. We have shown this through manipulating the site of NE action in the olfactory bulb, as well as through the manipulation of the source of NE, the LC.
NE within the olfactory bulb
The notion that NE plasticity underlies the neural and behavioral changes of the rapid, olfactory based learning underlying rat pups’ attachment, has received strong support from a variety of labs. First, work in Michael Leon’s lab using microdialysis showed that olfactory bulb NE increases during infant odor learning but only during the sensitive period (Rangel & Leon, 1995). Second, recordings from olfactory bulb mitral cells during learning indicates that mitral cells maintain their responsiveness to odors in the experimental learning groups, but not control groups (Wilson & Sullivan, 1992). Third, infusion of NE into the olfactory bulb during an odor presentation is sufficient for pups to acquire an odor preference (Langdon, Harley & McLean, 1997; Sullivan, Stackenwalt, Nasr, Lemon & Wilson, 2000b). Fourth, blocking olfactory bulb NE during learning, prevents neonatal odor learning (Sullivan, Zyzak, Skierkowski & Wilson, 1992). Thus, it is the contingent events of increasing olfactory bulb NE and odor stimulation that underlies the neural plasticity responsible for olfactory-based attachment formation.
This olfactory bulb NE is not intrinsic to the olfactory bulb and arrives via LC fibers terminating in the granule cell layer (McLean & Shipley, 1991). The inhibitory interneurons of the granule cell layer modulate the output of the olfactory bulb through the adjacent mitral cells (Lauder & Bloom, 1974; Trombley & Shepherd, 1992; Wilson & Leon, 1988b). Wilson (Wilson & Sullivan, 1992) has shown that activation of the NE input to the infant rat’s olfactory bulb during an odor presentation maintains mitral cell responsiveness to that odor, preventing the habituation these cells normally exhibit to repeated odor presentations. Subsequent work has shown that NE increases mitral cell responses to olfactory nerve input, suggesting that NE functions to increase the signal to noise ratio (Jiang, Griff, Ennis, Zimmer & Shipley, 1996).
Manipulating the source of olfactory bulb NE, the LC
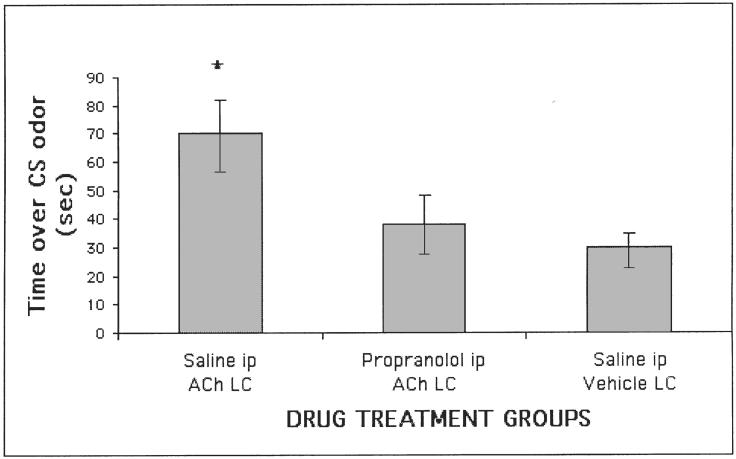
Direct manipulation of the LC is also able to induce or block neonatal odor learning. First, destroying the LC is sufficient to prevent pups from learning (Sullivan, Wilson, Lemon & Gerhardt, 1994; Figure 5). Second, stimulating release of NE by the LC with either idozoxane or ACh (Sullivan, Stackenwalt, Nasr, Lemon & Wilson, 2000b; Figure 4) during an odor presentation is sufficient to induce an odor preference. Thus, NE appears both necessary and sufficient for learning during the neonatal sensitive period.
FIG. 5.
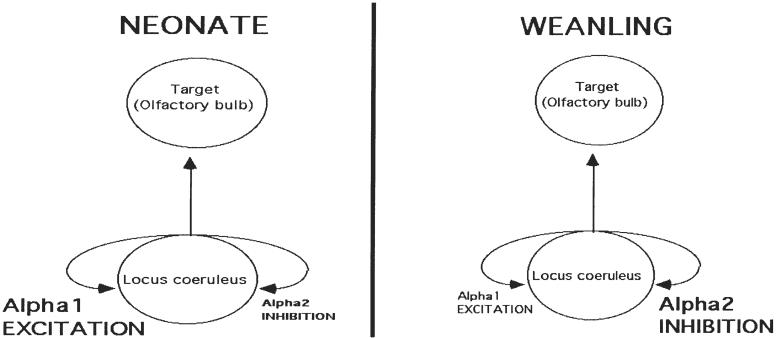
The LC has recurrent collaterals that activate autoreceptors within the LC and modulate its behavior. In the neonate, the LC autoreceptors are primarily excitatory and result in prolonged LC activation (20-30 sec). At around PN10, the LC autoreceptors are functionally primarily inhibitory and result in a very brief excitation (few millisec; Nakamura et al., 1987; Nakamura & Sakaguchi, 1990).
FIG. 4.
Infusing ACh bilaterally into the LC during an odor presentation results in neonatal pups acquiring a subsequent preference for that odor. This results in the LC releasing NE. The effect of the LC infusion can be blocked by injecting pups systemically with propranolol prior to the LC infusion (Sullivan et al., 2000b).
Developmental changes in the LC may account for the end of the sensitive period
Developmental changes within the LC appear to underlie the termination of the infant rat’s unique learning abilities during the first week and a half of postnatal life. In neonatal pups, the LC is not completely mature during the first week of postnatal life, nor is it an immature version of the adult LC. Indeed, it has unique characteristics that result in an enhanced response of neonatal LC neurons to sensory stimulation. The infant LC is more responsive to sensory stimuli than the adult LC. Although the adult LC is activated by sensory stimuli (Aston-Jones, Rajkowski & Cohen, 1999; Aston-Jones, Rajkowski, Kubiak & Alexinsky 1994; Foote, Aston-Jones & Bloom, 1980; Harley & Sara, 1992; Sara, Dyon-Laurent & Herve,1995; Vankov, Herve-Minvielle & Sara, 1995), in comparison to the infant, the adult LC is less likely to respond to non-noxious stimuli (Kimura & Nakamura, 1985; Nakamura, Kimura & Sakaguchi, 1987; Nakamura & Sakaguchi, 1990). Furthermore, the adult LC habituates after repeated presentation of the stimuli (Vankov, Herve-Minvielle & Sara, 1995), whereas the infant LC fails to exhibit habituation (Kimura & Nakamura, 1985; Nakamura & Sakaguchi, 1990). Even more striking is the duration of the response of the neonatal LC as compared to the preweanling/adult LC. Indeed, a 1 sec presentation of tactile stimulation is likely to cause a few ms response in the adult LC, but a 20-30 sec response in the infant LC (Kimura & Nakamura, 1985; Nakamura & Sakaguchi, 1990). Finally, the early infant LC shows far more extensive electrotonic coupling, a process that appears to potentiate the neonatal LC’s response (Christie & Jelinek,1993; Marshall, Christie, Finlayson & Williams, 1991). The infant LC appears to take on adult characteristics at around PN9-10 (Nakamura et al., 1987; Nakamura & Sakaguchi, 1990; Marshall et al., 1991). Together, these results suggest that the infant rat’s olfactory bulb receives a uniquely large input of NE from the LC during the first 9 days of life. When the LC matures, it brings to a close the early sensitive period for the imprinting like learning underlying attachment in this species.
As illustrated in Figure 5, the change in LC functioning around PN10 appears to be based on the functional development of the α2 inhibitory autoreceptors on LC dendrites and cell bodies. Specifically, the LC contains recurrent collaterals which feedback onto the LC, potentiating the neonatal response, but shutting down the older LC response to stimulation via the α2 inhibitory autoreceptors (Kimura & Nakamura, 1985; Nakamura et al., 1987; Nakamura & Sakaguchi, 1990). Reduction in LC electrotonic coupling (Marshall, Christi, Finlayson & Williams, 1991), as well as changes in tyrosine hydroxylase levels (Bezin, Marcel, Rousset, Pujolm & Weissmann, 1994a; 1994b) at PN10 may also contribute to LC maturational changes underlying the massive reduction in LC NE release after the sensitive period (Rangel & Leon, 1995).
After the sensitive period, NE is no longer necessary for learning but remains sufficient
This effect of LC/NE in the neonate is in sharp contrast to the modulatory or attentional role in learning of LC/NE function in older pups and adults (Harris & Fitzgerald, 1991; Ferry, Roozendaal & McGaugh, 1999; Liang,1998; McGaugh, Cahill & Roozendall, 1996; Moffat, Suh & Fleming, 1993; Sara et al., 1995). Additionally, electrophysiological studies on the adult LC during learning clearly support the notion that NE is involved in adult learning but appears to modulate behavior and attention (Sara et al., 1995).
However, direct infusions of NE into the site of plasticity within the brain still remains sufficient for learning. Recent work in our lab has shown that the infusion of NE directly into the olfactory bulb, even after the sensitive period has ended, still results in an odor preference (Sullivan, Stackenwalt, Nasr, Lemon & Wilson, 2000b; Moriceau & Sullivan, 2000). This is consistent with work from a number of other laboratories where direct infusion of NE into the brain was able to support learning in a variety of sensory systems (Dahl & Li, 1994; Harley, 1998; Lacaille & Harley, 1985).
IV. The failure of the amygdala to participate in odor-shock conditioning may underlie pups’ difficulties in learning odor aversions
The amygdala is a brain area strongly implicated in adult and preweanling rat conditioned fear (i.e. odor-shock conditioning; Cahill, Weinberger, Roozendaal & McGaugh, 1999; Fanselow & LeDoux, 1999; Fanselow & Rudy, 1998; Hunt & Campbell, 1999; Fendt & Fanselow, 1999; Sananes & Campbell, 1989; Schoenbaum, Chiba & Gallagher, 1999). Evidence suggests that the lack of a functional amygdala during the neonatal period may underlie pups’ difficulty in learning and expressing fear. First, behaviors associated with amygdala function emerge around PN10 (inhibitory conditioning and passive avoidance: Blozovski & Cudennec, 1980; Blozovski & Hennocq, 1982; Collier, Mast & Meyer, 1979; review Myslivecek, 1997). Second, amygdala lesions during the neonatal sensitive period (PN1-9) do not prevent the acquisition of an odor preference, although it can slightly retard odor preference conditioning with no effect on specific conditioned responses (Sullivan & Wilson, 1993). Third, the amygdala does not appear to participate in the odor-shock induced odor preference during the sensitive period (Figure 6; Sullivan et al., 2000a). However, following the termination of the sensitive period, when odor-shock conditioning produces an odor aversion, the amygdala is involved in learning. Our data indicates that during the neonatal rat’s sensitive period, when aversions are difficult to learn, the amygdala may not be active.
FIG. 6.
The amygdala does not appear to participate in the odor preference induced by odor-shock conditioning during the sensitive period. However, following the termination of the sensitive period, when pups easily learn an odor aversion, the amygdala is activated during odor-shock training (Sullivan et al,2000a)
Other unlearned behaviors associated with the amygdala, such as immobility to a novel male odor (males eat pups) also emerges at PN10 (Takahashi, 1994a; Wiedenmayer & Barr, 1998). However, certain amygdala related olfactory conditioned behaviors emerge later in development, such as heart rate changes (PN15, Sananes, Gaddy & Campbell, 1988). Moreover, amygdala related behaviors in other sensory systems emerge even later in development, with immobility to auditory and visual stimuli emerging at PN16 and 18 respectively (Hunt & Campbell, 1999; Hunt, Hess & Campbell, 1997). Thus, microcircuitry within the amygdala or connections within each sensory specific system exhibits different developmental progression making global statements concerning amygdala function difficult.
IV. Summary and Conclusion
Based on the content of this review, the immature brain is designed to maximize attachment to the mother. Specifically, during the sensitive period, ontogenetically unique characteristics of the neonatal LC appear to underlie the neonates propensity for odor preference learning. On the other hand, the failure of the amygdala to participate in odor-shock conditioning may underlie pups’ difficulties in learning odor aversions, and underlie the odor preference expressed by older pups.
Human infant attachment is also characterized by the infant seeking proximity to the caregiver and maintaining contact with the caregiver regardless of the quality of the care they receive (Bowlby, 1965). As has been reviewed above, this is true for a wide range of species including humans and rats. Based on our work on rats, the neural basis of attachment may be due to reduced amygdala function and heightened LC function. Although human attachment may not use the same neural circuitry as the rat, or use the circuitry in a different manner, these studies provide us with a new conceptual framework in which to explore human attachments.
Acknowledgements
Support for this work was provided by NICHO and NSF-IBN.
References
- Ader R, Peck JH. Early learning and retention of a conditioned taste aversion. Developmental Psychobiology. 1977;10:213–218. doi: 10.1002/dev.420100305. [DOI] [PubMed] [Google Scholar]
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Developmental Psychobiology. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky R. Locus coeruleus neurons in the monkey are selectively activated by attended stimuli in a vigilance task. Journal of Neuroscience. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Wang S. Peripheral and central administration of cocaine produces conditioned odor preferences in the infant rats. Brain Research. 1992;599:181–185. doi: 10.1016/0006-8993(92)90389-q. [DOI] [PubMed] [Google Scholar]
- Barr GA, Rossi G. Conditioned place preference from ventral tegmental injections of morphine in neonatal rats. Developmental Brain Research. 1992;66:133–136. doi: 10.1016/0165-3806(92)90149-q. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Quantitivative 3H-thimidine radiographic analysis of neurogenesis in the rat amygdala. The Journal of Comparative Neurology. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. International Journal of Developmental Neuroscience. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Bezin L, Marcel D, Rousset C, Pujol JF, Weissmann D. Quantitative study of tyrosine hydroxylase protein levels within the somatic area of the rat locus coeruleus during postnatal development. Journal of Neuroscience. 1994b;14:7502–7510. doi: 10.1523/JNEUROSCI.14-12-07502.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezin L, Marcel D, Rousset C, Pujol JF, Weissmann D. Ontogeny of tyrosine hydroxylase levels in the neuropil close to locus coeruleus. Neuroreport. 1994a;5:1809–12. doi: 10.1097/00001756-199409080-00031. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Developmental Psychobiology. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Hennocq N. Effects of antimuscarinic cholinergic drugs injected systemically or into the hippocampal-entorhinal area upon passive avoidance learning in young rats. Psychopharmacology. 1982;76:351–358. doi: 10.1007/BF00449124. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Animal Behavior. 1965;12:427–441. [Google Scholar]
- Bowlby J. Attachment. Basic Books; New York: 1965. [Google Scholar]
- Brake S. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science. 1981;211:506–508. doi: 10.1126/science.7192882. [DOI] [PubMed] [Google Scholar]
- Brennen PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Progress in Neurobiology. 1997;51:451–457. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Alberts JR. Olfactory stimulation induces filial preferences for huddling in rat pups. Journal of Comparative and Physiological Psychology. 1979;93:548–555. doi: 10.1037/h0077571. [DOI] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendall B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Campbell BA. Reflections on the ontogeny of learning and memory. In: Kail R, Spear NE, editors. Comparative perspectives on the development of memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1984. pp. pp23–35. [Google Scholar]
- Christie MJ, Jelinek HF. Dye-coupling among neurons of the rat locus coeruleus during postnatal development. Neuroscience. 1993;56:127–129. doi: 10.1016/0306-4522(93)90568-z. [DOI] [PubMed] [Google Scholar]
- Collier AC, Mast J, Meyer DR, Jacobs CE. Approach-avoidance conflict in preweanling rats: a developmental study. Animal Learning & Behavior. 1979;7:514–520. [Google Scholar]
- Cornwell-Jones C, Sobrian SK. Development of odor-guided behavior in Wistar & Sprague-Dawley rat pups. Physiology and Behavior. 1977;19:685–688. doi: 10.1016/0031-9384(77)90044-0. [DOI] [PubMed] [Google Scholar]
- Crain B, Cotman C, Taylor D, Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Research. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Dahl D, Li J. Induction of long-lasting potentiation by sequenced applications of isoproterenol. Neuroreport. 1994;31:657–660. doi: 10.1097/00001756-199401000-00032. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Interactions between perinatal and neonatal associative learning defined by contiguous olfactory and tactile stimulation. Neurobiology of Learning and Memory. 1999;71:272–288. doi: 10.1006/nlme.1998.3882. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear N, Spear L. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Developmental Psychobiology. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Rudy JW. Convergence of experimental & developmental approaches to animal learning and memory processes. In: Carew TJ, Menzel R, Shatz CJ, editors. Mechanistic relationships between development and learning. J. Wiley & Sons; New York: 1998. pp. 15–28. [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience and Biobehavioral Review. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biological Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Fillion TJ, Blass EM. Infantile experience with suckling odors determined adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkey is a function of sensory stimulation and arousal. Proceedings of the National Academy of Science. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef GG, Kaner HC. Establishment & maintenance of preference for natural & artificial olfactory stimuli in juvenile rats. Journal of Comparative & Physiological Psychology. 1980;94:588–595. doi: 10.1037/h0077693. [DOI] [PubMed] [Google Scholar]
- Gemberling GA, Domjan M. Selective associations in one-day-old rats: taste-toxicosis and texture-shock aversion learning. Journal of Comparative Physiological Psychology. 1982;96:105–13. doi: 10.1037/h0077855. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Tobach E. Behavior modification in infant rats. Animal Behavior. 1967;15:559–562. doi: 10.1016/0003-3472(67)90058-9. [DOI] [PubMed] [Google Scholar]
- Harley CW. Noradrenergic long-term potentiation in the dentate gyrus. Advances in Pharmacology. 1998;42:952–956. doi: 10.1016/s1054-3589(08)60905-9. [DOI] [PubMed] [Google Scholar]
- Harley CW, Sara SJ. Locus coeruleus burst induced by glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Experimental Brain Research. 1992;89:581–587. doi: 10.1007/BF00229883. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schrier A, Harlow HF, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 2. Academic Press; New York: 1965. [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Harris GC, Fitzgerald RD. Locus coeruleus involvement in the learning of classically conditioned bradycardia. Journal of Neuroscience. 1991;11:2314–2320. doi: 10.1523/JNEUROSCI.11-08-02314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer ME, Kempe RS, Krugman RD. The battered child. 1997. University of Chicago Press. [Google Scholar]
- Hess EH. Ethology: An approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess EH, Mendler G, Holt, editors. New directions in psychology. Rinehart & Winston; New York: 1962. [Google Scholar]
- Hofer MA. The roots of human behavior. W.H. Freeman & Co; New York: 1981. [Google Scholar]
- Hoffman H, Spear NE. Facilitation and impairment of conditioning in the preweanling rat after prior exposure to the conditioned stimulus. Animal Learning and Behavior. 1989:1763–1769. [Google Scholar]
- Hudson R. Olfactory imprinting. Current Opinions in Neurobiology. 1993:3548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- Hunt P, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, Motivation, and Cognition: The functional behaviorism of Robert C Bolles. Washington, DC: 1999. [Google Scholar]
- Hunt PS, Hess MF, Campbell BA. Behaivoral Neuroscience. 1997;111:1257–64. [Google Scholar]
- Jiang MR, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. Journal of Neuroscience. 1996;16:6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;2054:19–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher M. Classical conditioning of an odor preference in 3-day-old rats. Behavioral and Neural Biology. 1980;29:132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Research. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass E. Central nervous system mediation of positive and negative reinforcement in neonatal albino rats. Developmental Brain Research. 1986;27:69–75. doi: 10.1016/0165-3806(86)90233-6. [DOI] [PubMed] [Google Scholar]
- Kimura F, Nakamura S. Locus coeruleus neurons in the neonatal rat: Electrical activity and responses to sensory stimulation. Developmental Brain Research. 1985;23:301–305. doi: 10.1016/0165-3806(85)90055-0. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Harley CW. The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Research. 1985;358:210–220. doi: 10.1016/0006-8993(85)90965-5. [DOI] [PubMed] [Google Scholar]
- Landers M, Sullivan RM. Vibrissae evoked behavior and conditioning before functional ontogeny of the somatosensory vibrissae cortex. Journal of Neuroscience. 1999;19:5131–5137. doi: 10.1523/JNEUROSCI.19-12-05131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon PE, Harley CW, McLean JH. Increased ß adrenoceptor activation overcomes conditioned olfactory learning induced by serotonin depletion. Developmental Brain Research. 1997;102:291–293. doi: 10.1016/s0165-3806(97)00090-4. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. Journal of Comparative Neurology. 1974;155:469–482. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Leon M. Dietary control of maternal pheromone in the lactating rat. Physiol Behav. 1975;14:311–319. doi: 10.1016/0031-9384(75)90039-6. [DOI] [PubMed] [Google Scholar]
- Liang KC. Pretraining infusion of DSP-4 into the amygdala impaired retention in the inhibitory avoidance task: Involvement of norepinephrine but not serotonin in memory facilitation. Chinese Journal of Physiology. 1998;41:223–33. [PubMed] [Google Scholar]
- Lincoln J, Coopersmith R, Harris EW, Cotman CW, Leon M. NMDA receptor activation and early olfactory learning. Brain Research. 1988;467:309–312. doi: 10.1016/0165-3806(88)90036-3. [DOI] [PubMed] [Google Scholar]
- Marshall KC, Christi MM, Finlayson PG, Williams JT. Developmental aspects of the locus coeruleus-noradrenaline system. Progress in Brain Research. 1991;88:173–185. doi: 10.1016/s0079-6123(08)63807-8. [DOI] [PubMed] [Google Scholar]
- Martin LT, Alberts JR. Taste aversion to mother’s milk: the age-related role of nursing in acquisition and expression of a learned association. Journal of Comparative and Physiological Psychology. 1979;93:430–445. doi: 10.1037/h0077568. [DOI] [PubMed] [Google Scholar]
- Martin LT, Alberts JR. Associative learning in neonatal rats revealed by cardiac response patterns. Journal of Comparative and physiological psychology. 1982:668–75. doi: 10.1037/h0077901. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Rooszendall B. Involvement of the amygdala in memory storage Interaction with other brain systems. Proceeding of the National Academy of Science. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postnatal development of the noradrenergic projection from the locus coeruleus to the olfactory bulb in the rat. Journal of Comparative Neurology. 1991;304:469–477. doi: 10.1002/cne.903040310. [DOI] [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Sullivan RM, King SR. Serotonergic influences on olfactory learning in the neonatal rat. Behavioral and Neural Biology. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW, Darby-King A, Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learning & Memory. 1999;6:608–618. doi: 10.1101/lm.6.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Schaldach MA, Snyder KJ, Balagh SA, Len T, Neimanis K, Gaulis P, Hug J, Sauchak K, Remmers-Roeber DR, Walker C, Yamamoto BK. Ketamine blocks a conditioned taste aversion in neonatal rats. Physiology and Behavior. 1998;64:381–390. doi: 10.1016/s0031-9384(98)00097-3. [DOI] [PubMed] [Google Scholar]
- Miller JS, Jagielo JA, Spear NE. Age-related differences in short-term retention of separable elements of an odor aversion. Journal of Experimental Psychology. 1989;15:194–201. [PubMed] [Google Scholar]
- Mizukawa K, Tseng I-Ming, Otsuka N. Quantitative electron microscopic analysis of postnatal development of zinc-positive nerve endings in the rat amygdala using Timm’s sulphide silver technique. Developmental Brain Research. 1989;50:197–203. doi: 10.1016/0165-3806(89)90195-8. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Suh EJ, Fleming A. Noradrenergic involvement in the consolidation of maternal experience in postpartum rats. Physiology and Behavior. 1993;53:805–811. doi: 10.1016/0031-9384(93)90192-i. [DOI] [PubMed] [Google Scholar]
- Moore CL, Jordan L, Wong L. Early olfactory experience, novelty and choice of sexual partner by male rats. Physiology and Behavior. 1996;60:1361–1367. doi: 10.1016/s0031-9384(96)00249-1. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Sullivan RM. Reinstating the neonatal sensitive period for olfactory learning. International Society for Developmental Psychobiology; New Orleans: 2000. [Google Scholar]
- Myslivecek J. Inhibitory learning and memory in newborn rats. Progress in Neurobiology. 1997;53:399–430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: Development of functional coupling between septal, hippocampal, and ventral tegmental regions. Journal of Neuroscience. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus coeruleus. Journal of Neurophysiology. 1987;58:510–24. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Nakamura ST, Sakaguchi T. Development and plasticity of the locus coeruleus. A review of recent physiological and pharmacological experimentation. Progress in Neurobiology. 1990;34:505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- Okutani F, Yagi F, Kaba H. Gabaergic control of olfactory learning in young rats. Neuroscience. 1999;93:1297–300. doi: 10.1016/s0306-4522(99)00224-9. [DOI] [PubMed] [Google Scholar]
- Pager J. A selective modulation of olfactory bulb electrical activity in relation to the learning of palatability in hungry and satiated rats. Physiology and Behavior. 1974;12:189–195. doi: 10.1016/0031-9384(74)90172-3. [DOI] [PubMed] [Google Scholar]
- Pedersen P, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. Journal of Experimental Psychology: Animal Behavior Process. 1982;8:329–341. [PubMed] [Google Scholar]
- Rajecki DW, Lamb ME, Obmascher P. The Behavioral and Brain Sciences. 1978;3:417–464. [Google Scholar]
- Rangel S, Leon M. Early odor preference training increases olfactory bulb norepinephrine. Developmental Brain Research. 1995;85:187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- Roth T, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Developmental Psychobiology. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Cheatle MD. Odor aversion learning in neonatal rats. Science. 1977;198:845–846. doi: 10.1126/science.918668. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Cheatle MD. A role for conditioned stimulus duration in toxiphobia conditioning. Journal of Experimental Psychology: Animal Behavior Process. 1978;4:399–411. doi: 10.1037//0097-7403.4.4.399. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. The ontogeny of contextual fear conditioning: Implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Stadler-Morris S, Alberts PA. Ontogeny of spatial navigation behaviors in the rat: Dissociation of “proximal-” and “distal-cue” based behaviors. Behavioral Neuroscience. 1987;101:62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Salzen EA, Aronson LR. Imprinting and environmental learning. In: Tobach E, Lehrman DS, Rosensblatt J, editors. Development and Evolution of Behavior. W.H. Feeman; San Francisco: 1970. [Google Scholar]
- Sananes CB, Campbell BA. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behavioral Neuroscience. 1989;103:519–525. [PubMed] [Google Scholar]
- Sananes CB, Gaddy JR, Campbell BA. Ontogeny of conditioned heart rate to an olfactory stimulus. Developmental Psychobiology. 1988;21:117–33. doi: 10.1002/dev.420210202. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Dyon-Laurent D, Herve A. Novelty seeking behavior in the rat is dependent upon the integrity of the of the noradrenergic system. Cognitive Brain Research. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear NE, Kucharski D, Miller JS. The CS—effect in simple conditioning and stimulus selection during development. Animal Learning and Behavior. 1989;17:70–82. [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behavioral Brain Research. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Campbell BA. Habituation of the forelimb-withdrawal response in neonatal rats. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Developmental Psychobiology. 1986a;19:625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcement in infancy: Classical conditioning using tactile stroking or intra-oral milk infusions as UCS. Developmental Psychobiology. 1988;20:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986b;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: Ontogeny of conditioned fear and the amygdala. Nature. 2000a;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Developmental Brain Research. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Role of the amygdala complex in early olfactory associative learning. Behavioral Neuroscience. 1993;107:254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Dissociation of behavioral and neural correlates of early associative learning. Developmental Psychobiology. 1995;28:213–219. doi: 10.1002/dev.420280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic ß-receptors or locus coeruleus stimulation is sufficient to produce learned approach response to that odor in neonatal rats. Behavioral Neuroscience. 2000b;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Lemon C, Gerhardt GA. Bilateral 6-OHDA lesions of the locus coeruleus impair associative olfactory learning in newborn rats. Brain Research. 1994;643:306–309. doi: 10.1016/0006-8993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral olfactory bulb responses to maternal odors in preweanling rats. Developmental Brain Research. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak D, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Developmental Brain Research. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Stimulus control of behavioral inhibition in the preweanling rat. Physiology and Behavior. 1994;55:717–21. doi: 10.1016/0031-9384(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Terry LM, Johanson IB. Effects of altered olfactory experiences on the development of infant rats’ responses to odors. Developmental Psychobiology. 1996;29:353–377. doi: 10.1002/(SICI)1098-2302(199605)29:4<353::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. Journal of Neuroscience. 1992;12:3985–3991. doi: 10.1523/JNEUROSCI.12-10-03985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. European Journal of Neuroscience. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Van Vulpen EH, Van Uum JF. Prefrontal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. Journal of Comparative Neurology. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Weldon DA, Travis ML, Kennedy DA. Postraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behavioral Neuroscience. 1991;105:450–458. doi: 10.1037//0735-7044.105.3.450. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Ontogeny of defensive behavior and analgesia in rat pups exposed to an adult male rat. Physiology and Behavior. 1998;63:261–9. doi: 10.1016/s0031-9384(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. Journal of Neurophysiology. 1988a;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Developmental Brain Research. 1988b;42:69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward II: Norepinephrine mediates a specific component of the bulb response to reward. Behavioral Neuroscience. 1991;105:843–849. doi: 10.1037//0735-7044.105.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Blockade of mitral/tufted cell habituation to odors by association with reward: A preliminary note. Brain Research. 1992;594:143–145. doi: 10.1016/0006-8993(92)91039-h. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. Journal of Neuroscience. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward. I. Neurobehavioral consequences. Developmental Brain Research. 1990;53:215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Sensitive period for neural and behavioral responses to learned odors. Developmental Brain Research. 1988;36:309–313. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. Journal of Comparative Neurology. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]