Abstract
Rabies is a fatal neurological pathogen that is a persistent problem throughout the developing world where it is spread primarily by domestic dogs. Although the disease has been extensively studied in wildlife populations in Europe and North America, the dynamics of rabies in domestic dog populations has been almost entirely neglected. Here, we demonstrate that rabies epidemics in southern and eastern Africa cycle with a period of 3–6 years and show significant synchrony across the region. The observed period is shorter than predictions based on epidemiological parameters for rabies in domestic dogs. We find evidence that rabies prevention measures, including vaccination, are affected by disease prevalence and show that a simple model with intervention responses can capture observed disease periodicity and host dynamics. We suggest that movement of infectious or latent animals combined with coordinated control responses may be important in coupling populations and generating synchrony at the continental scale. These findings have important implications for rabies prediction and control: large-scale synchrony and the importance of intervention responses suggest that control of canine rabies in Africa will require sustained efforts coordinated across political boundaries.
Keywords: disease dynamics, epidemics, infectious disease, mathematical model, vaccination
Every year at least 55,000 people die from rabies and >7 million are treated for exposure to the virus (1). More than 99% of these deaths occur in the developing world where the disease is a much greater problem, chiefly because rabies is endemic in domestic dog populations (2). Yet examples from around the world have repeatedly shown that canine rabies can be effectively controlled and human deaths can be eliminated through mass vaccination of domestic dogs [see supporting information (SI) Table 1 for a summary of canine rabies control programs]. Despite the availability of safe, effective, and inexpensive tools for the control of rabies in domestic dog populations (3) the disease has been neglected across much of Asia and Africa where it has become an increasing problem (1, 4).
Domestic dogs are the principal reservoir of rabies throughout most of Africa and Asia (5). Although there is evidence that some wild canid populations in Africa can support rabies cycles (6), most outbreaks in wild canids are triggered by epidemics in domestic dogs rather than the converse (7–11), and increasing rabies incidence in Africa and Asia has been largely attributed to population growth of this ubiquitous carnivore (12, 13). Understanding the dynamics of rabies in domestic dogs would therefore aid the design of more effective control measures that could massively reduce human deaths from the disease, of which >95% result from bites by domestic dogs (2).
Rabies is an acute fatal infection. Therefore, intrinsic cycles are expected to occur as disease-induced mortality drives down numbers of susceptibles (14). Epidemic cycles and traveling waves have been well documented for the spread of wildlife rabies in Europe and North America (15–18). In contrast, very little has been published on the spatial and temporal dynamics of rabies in domestic dogs, which we might expect to be different. Surveillance data for rabies in domestic dog populations are scant compared with comprehensive data sets of rabies in wildlife from Europe and North America, and analyses of long-term patterns are consequently limited. Nonetheless, annual data are available from several countries across southern and eastern Africa during the 30-year interval from 1971–2000 (refs. 19–29, www.who.int/rabies/rabnet/en, and SI Text).** Here, we collate these data (Fig. 1) and examine trends in periodicity and synchronicity of rabies in domestic dog populations across the region. We develop a mathematical model that can account for the observed temporal patterns under the influence of reactive control efforts, and we discuss the broader implications for elimination of rabies in domestic dogs.
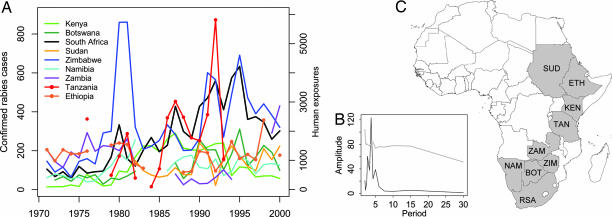
Fig. 1.
Trends in rabies incidence from 1971 to 2000 across southern and eastern Africa. (A) Annual number of laboratory-confirmed rabies cases for each country, apart from Tanzania, where the annual number of people reporting to a hospital for postexposure treatment is shown. Initial inspection suggests 3- to 6-year periodicity and noisy synchrony of rabies epidemics. (B) A periodogram of the combined differenced data from each country with the 97.5th percentile of the spectra of the randomized data shown by a gray solid line illustrates the significant overall 3- to 6-year periodicity. (C) The location of countries included in the analysis. BOT, Botswana; KEN, Kenya; NAM, Namibia; RSA, South Africa; SUD, Sudan; ZIM, Zimbabwe; TAN, Tanzania; ETH, Ethiopia; ZAM, Zambia.
Results
Periodicity.
Inspection of the data and wavelet analyses and periodograms (Fig. 1 and SI Fig. 5) suggest that rabies epidemics cycle with a period of 3–6 years. This finding is consistent with previous observations (11, 21). Using standard spectral analysis techniques, significant periodicity was detected in six of nine countries (P < 0.05 for Botswana, Kenya, Namibia, South Africa, Sudan, and Zimbabwe) and in a combined series based on all nine time series (P = 0.006, one-tailed). These results were robust under a more conservative statistical test using permuted spectra.
Intrinsic epidemic cycles are predicted from “natural enemy” dynamics as a result of feedback between host and pathogen populations (31). However, simple models of rabies parameterized for domestic dog populations generate oscillatory dynamics in disease incidence with periods that are considerably longer than those observed in these data (9, 32) and corresponding large oscillations in dog population densities for which there is no empirical evidence.
The attitudes and actions of dog owners, local communities, and national governments likely play a major role in determining the dynamics of rabies in domestic dog populations and may explain these disparities. Interventions such as dog vaccination and killing of exposed and rabid animals tend to increase with rabies incidence (11, 33). Given the slow time scale of rabies epidemics, reactive vaccination programs and increased killing of exposed and rabid animals have the potential to reduce the final epidemic size. Such responses may reinforce natural cycles of disease incidence and decrease the interepidemic period, because surviving animals continue to breed, replenishing susceptibles more rapidly than in populations depleted by disease-induced mortality. We therefore developed a model to test whether localized, national, and possibly international responses to increasing rabies incidence could act as a strong density-dependent force that may drive cycles over the periods observed.
We found that incorporating vaccination responses into models of rabies in domestic dogs produces epidemic periodicity that is consistent with the frequencies observed (Fig. 2). The delays before implementation of intervention responses lead to reduced oscillations in dog populations to levels that would be barely detectable and greater persistence of oscillations in disease incidence, which otherwise dampen quickly. Although other mechanisms could maintain oscillations in rabies incidence, none of these produce the correspondence between observed dynamics and those predicted from models that include human responses, which suggests that reactive vaccination may play an important role in modulating the dynamics of rabies in domestic dogs.
Fig. 2.
Model output showing the influence of reactive vaccination on the dynamics of rabies and domestic dog populations. Overall population density (black) and densities of susceptible (gray) and infected rabid animals (red) are shown (note the different scales). (A) In the absence of vaccination interventions, the overall population density is indistinguishable from the density of susceptibles, which initially show large damped oscillations during rabies outbreaks. (B) In the presence of reactive vaccination, short sustained oscillations in rabies incidence (on the order of every 4–5 years) are observed and overall dog population sizes remain approximately constant, although detectable cycling of susceptibles occurs. A response ratio (τ) of 70 vaccinations per rabies case detected is modeled with a dynamical delay of 8 months. (C) The effect of the magnitude of the delayed vaccination response to reported levels of rabies incidence on the cycle period (in years) is shown. These results are robust over a reasonable range of uncertainty for parameter estimates (sensitivity analyses are shown in SI Fig. 6).
Available data from Africa are insufficient to test this hypothesis, but data on vaccine use and rabies incidence in Zimbabwe, South Africa, and Kenya lend qualitative support (Fig. 3): vaccine use tends to increase after large outbreaks of rabies and large outbreaks occur after years of very low vaccine coverage. Historical records from domestic dog populations elsewhere in the world are also corroborative. Domestic dog vaccines were first developed in Japan in 1918 and rapidly deployed together with strict licensing laws to bring rabies under control (34). However, in the aftermath of World War II an outbreak occurred that was controlled through reactive measures and increased, sustained vaccination coverage during a second cycle led to its eventual eradication (ref. 35 and Fig. 3). After Japan's initial example, vaccine production was undertaken in many countries around the world. As a result, rabies in domestic dogs was controlled in several regions, including Malaysia, North America, and Europe, and most recently considerable success has been achieved in South America, Mexico, and the Caribbean (36–40). Where measures were insufficient or not sustained, relatively short (≈5 year) cycles are evident (41–43) until the implementation of intensive vaccination programs when cycles disappear (35, 42, 44–47). Conversely, when intensive control programs lapsed, rabies outbreaks rapidly emerged from remnant foci or neighboring endemic regions (refs. 44, 45, and 47–50, Fig. 3, and SI Fig. 7). The few records available from before the vaccination era suggest the occurrence of larger, longer-lived outbreaks (35, 39, 50).
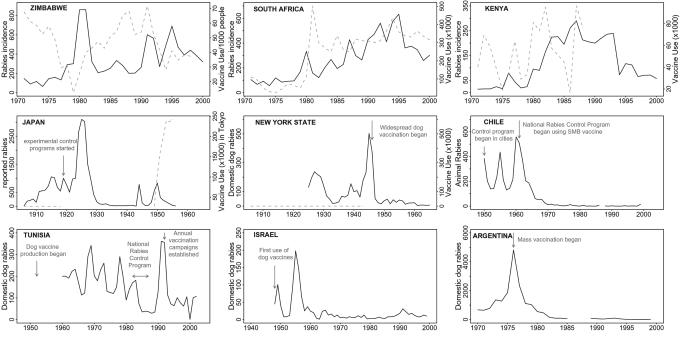
Fig. 3.
Evidence for density-dependent feedbacks between rabies incidence and vaccination responses and the impacts of reactive and proactive vaccination campaigns. Rabies cases (solid black lines) and vaccine use (dotted gray lines) are plotted for Zimbabwe, South Africa, Kenya, Japan, New York, Chile, Tunisia, Israel, and Argentina (from refs. 35, 38, 39, and 42–44). Annotated arrows indicate initiation or changes in vaccination programs. For Zimbabwe, the number of dog vaccinations delivered per 1,000 people is shown, whereas for South Africa, Kenya, and Japan, the total number of vaccines used is plotted. Across Africa, domestic dog populations have been increasing; thus vaccine use would need to increase to maintain the same coverage. The Zimbabwe data therefore provide a better approximation of vaccination coverage, although the human/dog ratio is unlikely to have remained constant over this period. Large rabies outbreaks tend to follow periods with little vaccination use, and vaccine use tends to increase after large outbreaks (rabies incidence predicts 60% of the variation in vaccine use the following year for South Africa, and vaccine use predicts 40% of the variation in incidence the following year for both Zimbabwe and South Africa; P < 0.001 in all cases). Sustained mass vaccination results in declines in domestic dog rabies as shown by the records from Japan, New York, Chile, Israel, and Argentina. There is no evidence of short cycles in Japan, New York, or Argentina before vaccines were deployed. Approximately 5-year cycles occur in Chile, Tunisia, and Israel when vaccination efforts were not uniformly applied at a national level, whereas intensification of mass vaccination controlled dog rabies.
Synchrony.
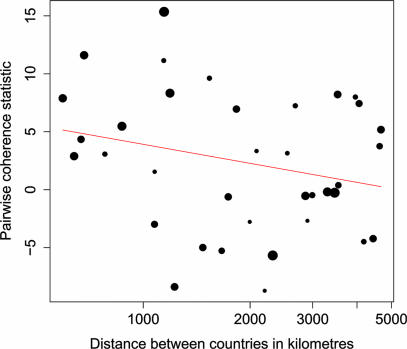
A surprising feature of the data is the degree to which peaks and troughs in cases appear coincident in time. A randomization test of the pairwise correlations in oscillations between all nine time series found significant evidence of phase synchrony (P = 0.008), implying some synchrony between rabies epidemics over extremely large spatial scales. Higher-resolution temporal and spatial data further support these findings; monthly data from distinct regions within Zimbabwe each followed the same oscillations as the nationwide data and were closely synchronized (11); data from Swaziland (51) and Lesotho (52) indicate peaks in rabies cases coincident with those in South Africa, and there is evidence for synchronous rabies outbreaks in northern Tanzania (unpublished data). The degree of phase synchrony tends to decay with distance between countries (Fig. 4), although this relationship is not significant at the 5% level (P = 0.07) when tested with appropriately conservative randomization procedures for nonindependent time series.
Fig. 4.
Relationship between geographic distance and phase synchrony. The pairwise correlation statistic (positive values indicate phase synchrony) is plotted against the log of the pairwise distance between countries. Distance is measured in kilometers. The red line is the weighted regression (using the square root of the number of points in the time series) of the correlation statistic against the log distance between countries (P = 0.072).
The observed synchrony is unlikely to be an artifact of surveillance bias and contagion of reporting. Courtin et al. (21) were unable to find surveillance biases based on levels of (and correlations between) epidemiological surveillance indices in monthly data from the central stock-ranching district of Namibia, a data set that follows the same qualitative patterns as the countrywide data. Furthermore, where data are available, numbers of human rabies deaths and postexposure treatments correlate closely with numbers of confirmed animal cases (33, 53, 55, 56)†† even though medical and veterinary authorities operate independently.
Synchrony of disease cycles usually occurs through external forcing. For example, large-scale synchronous epidemics of measles, cholera, influenza, and meningitis are forced climatically or seasonally by social aggregation (31, 57–59). It is possible that the synchrony observed in rabies outbreaks is driven by a common climate variable, although it is hard to determine the mechanism that would lead to this coupling. The dominant climate driver in east and southern Africa is the sea surface temperature (SST) of the Indian Ocean, which exhibits a strong triannual cycle that modifies the intensity and location of rainfall during the interannual African convergence event (60, 61). We found no relationship between annual averaged Indian Ocean SST and the intensity of rabies outbreaks, suggesting that climate does not play a role in forcing the observed synchronous patterns of rabies outbreaks, although more thorough investigation is required to rule out this possibility.
Dispersal also promotes large-scale population synchrony (62), particularly in oscillating systems (63–65). For example, a recent paper (66) argued that increasing synchrony of syphilis epidemics across American cities was a result of an increasingly interconnected sexual network. In the case of rabies, historical data show that when control measures deteriorate, epidemics rapidly reemerge even in areas that have been rabies-free for long periods, indicating that considerable dispersal must occur from endemic rabies regions (Fig. 4 and SI Table 1). A small proportion of rabid dogs may act as superspreaders (unpublished data), infecting many others or transmitting the disease over large distances. Similarly, transportation of latent, or infectious, dogs, by people as well as infections in wildlife that commute long distances (e.g., hyenas), potentially carry the disease even further. Previous studies have illustrated that even small amounts of relatively local dispersal can generate synchrony in cyclical dynamics over large spatial scales (63) and that the resulting synchrony tends to decline as distance increases and varies through time (64). The pronounced phase synchrony in rabies epidemics for distances up to 1,000 km (Fig. 4) and the lack of relationship across larger distances lends further support to the argument that dispersal could be linking dog rabies epidemics in different countries. Additionally, large-scale control efforts, such as regional or national government vaccination campaigns, could strengthen within-country phase coherence (67), which would increase the rate of disease dispersal across international boundaries during countrywide epidemics. More research is needed to conclusively determine the mechanism synchronizing rabies epidemics, but a combination of dispersal and coordinated responses seems to be the most plausible explanation.
Discussion
Rabies has proved a remarkably valuable system for exploring ecological processes of disease transmission. A vast array of modeling and statistical techniques has been used to investigate dynamics and analyze spatiotemporal trends in rabies surveillance data (14–16, 18). However, these methods have been almost exclusively applied to wildlife rabies in North America and Europe. Although the resolution and length of available time series limit the extent to which the dynamics of rabies in domestic dog populations can be interpreted, our analyses indicate cycles synchronized over very large spatial scales in Africa and should provide impetus for improving surveillance, reporting, and international collaboration. More detailed analysis of higher-resolution data from across scales is necessary to determine the mechanisms that underlie patterns of canine rabies circulation and to develop a more predictive understanding of spatiotemporal dynamics in Africa and elsewhere in the world. This important research goal would promote further pan-African collaboration and provide insight into the longer-term persistence and spatial spread of rabies in Africa.
The finding that rabies dynamics are periodic across much of Africa has important practical implications. Evaluating effectiveness of control efforts is difficult if the disease's natural oscillating tendencies are not considered. We suspect that control efforts generally increase during periods of high rabies incidence and that as incidence declines rabies falls from the agenda. More generally, control efforts for all oscillating pathogens will be prone to such problems to a lesser, or greater, extent, depending on the length of the interepidemic period, the perceived risk, and the capacity of the agencies concerned. The lack of consistent control efforts, together with rapid growth of domestic dog populations, may be responsible for the increase in and general persistence of rabies in Africa, which disconcertingly suggests that the burden of rabies will only worsen unless concerted and prolonged efforts are directed toward its control.
Local vaccination programs in Africa have been effective in lowering rabies incidence in the short term (68), but few have been sustained. The widespread periodicity of rabies epidemics in Africa is consistent with the dynamics of rabies in domestic dog populations in other parts of the world before the effective implementation of sustained control programs. These dynamics highlight the need to maintain vaccination coverage at consistently high levels to prevent epidemics from reoccurring, which typically happens when control programs lapse and would be particularly likely in Africa given the finding of large-scale synchrony. On the other hand, synchronized dynamics also provide optimism for prospects of rabies eradication if control efforts are coordinated across political boundaries. The evidence that proactive, sustained vaccination programs can control domestic dog rabies is overwhelming, and the tremendous recent successes throughout South America, Mexico, and the Caribbean from large-scale domestic dog rabies control programs should provide ample motivation and a practical model for the control of domestic dog rabies in Africa (4, 37, 42, 69, 70).
Methods
Data.
Published data on national annual rabies cases (confirmed wild and domestic animal and human cases from Botswana, Kenya, and Namibia; confirmed canine, bovine, wildlife, and human cases from Zambia; confirmed wild and domestic animal cases from Zimbabwe; and confirmed domestic animal cases from South Africa and Ethiopia), suspect domestic animal cases (from Sudan) and human postexposure treatments (from Tanzania) were collated over the 30-year period 1971–2000 (refs. 19–29, www.who.int/rabies/rabnet/en, and SI Text).** The longest uninterrupted time series was constructed from available data for each country; however, the rabies index used was not consistent between countries (see above). For some countries several data sources were available, and occasionally annual totals from different sources did not exactly match, although the differences were minor. In those instances, we used the figure given in the source for which data were available for the longest interval.
Confirmed rabies cases are underestimates of actual rabies cases, and postexposure treatments are often delivered to victims of bites by healthy animals. Nevertheless, both provide relatively reliable and robust measures of rabies trends over this period. Although data are available for several other countries in the region, data sets were only included if they spanned at least a 10-year continuous period in this interval. For Namibia the time series was constructed by using nationwide data except for the year 1986. Here, data were extrapolated from central Namibia (21) by using the ratio between the nationwide data and the central Namibian data, because an uninterrupted time series was not available for the entire country due to political instability in the north of the country. Where data were available to compare, the central Namibian time series was consistent with nationwide patterns.
The African canid rabies lineage is established throughout the subcontinent (30) and has been identified from several different species, with the vast majority of cases coming from domestic dogs. In parts of southern Africa other variants of rabies have been identified that circulate endemically in specific wildlife hosts to which they appear well adapted (54). We include data from wild and domestic animals in our analysis (except for South Africa and Sudan where only data from domestic animals is presented), and we make no distinctions between rabies variants. However, we expect that because of the widespread abundance of the African canid lineage and the general bias in surveillance toward domestic animals, that most of the cases in our analyses belong to the African canid lineage.
Analysis.
Statistical analysis was done by using R (www.r-project.org). Scripts are available on request. The long-term trends in each time series were removed by taking the residuals from a fitted, locally weighted, polynomial regression (the smoothing parameter included 75% of the observations and assumes Gaussian errors). The detrended data were normalized (mean = 0, variance = 1). The raw and detrended normalized data are presented in Fig. 1 and SI Fig. 5. Although spectral analysis techniques were not ideal for examining periodicity because of the low resolution of the time series, we calculated spectrograms based on first-order differences. The bias in estimated period (66) caused by differencing was minimal. Significance tests were based on comparisons of the spectra and the 97.5th percentile of 1,000 random permutations of the differenced data. Wavelet analyses are also presented, but are limited because of the low resolution and relatively short length of the time series.
To investigate synchrony, pairs of detrended, normalized time series were compared by using the sum of their products through time. Positive products indicate phase synchrony, and negative products indicate phase asynchrony. Simulated pairs were generated with one of the time series shuffled by an offset of between 1 and 30 years for all possible variations, and the sum of their product was calculated. For the country pairs only 30 variations were possible, making significance tests infeasible. An overall statistic, and significance level, was therefore generated from the sum of the pairwise products of all countries included in the analysis. Identical results were obtained by using cross-correlation functions and measures of regionwide synchrony (63). Additionally, the pairwise test statistics were plotted against the log of the distance between countries (from the central point in each country, with distance calculated by using the Haversine formula). To test the a priori hypothesis that correlation between countries decreases with distance we calculated the slope of a weighted regression (by the number of points in each time series) of the pairwise correlation statistic and the log distance between countries. We then randomized the distance between countries and recalculated the slope for 10,000 runs to estimate the probability of generating such a slope by chance.
Where higher-resolution data are available over short intervals, observed oscillations are relatively smooth, providing more support for our coherence and periodicity statistics calculated from low-resolution annual data.
Model.
We extend the classic susceptible-exposed-infectious (SEI) model for rabies (15) to include a vaccinated class (V), with vaccination implemented in response to rabies incidence (Eqs. 1–6). The vaccination rate υ is determined by a response variable (R) that tracks incidence with characteristic time delay η, which represents reporting, logistical, and bureaucratic delays. The vaccination rate is scaled so that each case detected results in the delivery of approximately τ doses at equilibrium (the number is exactly τ in the limit of low equilibrium level of disease). As a consequence of the implementation delay, more vaccinations are delivered in the wake of epidemics than in the early stages.
We parameterized the model by using published estimates of dog demography and rabies transmission (9, 32) and direct estimates from natural infections of rabid dogs in northwest Tanzania (unpublished data). Dog birth (a) and death (b) rates are 0.42 and 0.33 yr−1, respectively, and in the absence of disease the population is assumed to be regulated at some carrying capacity K, which we assume to be 10 dogs per km2. Density-dependent deaths therefore occur at rate γN, where N is the total dog population (S + E + I + V) and γ = a − b/K. We assign the incubation period (1/σ) a length of 25.5 days, the infectious period (1/μ) a length of 5.7 days, and the basic reproductive ratio, R0, a value of 2, giving a transmission rate (β) of 13.2 dogs per km2 per year. The duration of immunity afforded by the vaccine (1/δ) is assumed to be ≈2.5 years, whereupon dogs return to the susceptible class. The long- and medium-term behavior is given by a complex pair of dominant eigenvalues λd = −u ± vi, where u and v are positive real numbers. We calculate the characteristic period and damping time of oscillations as 2π/v and 1/u, respectively, and explore the sensitivity of these predictions to uncertainty in parameter values particularly the effects of variance in rates of vaccine delivery (SI Fig. 6).
Supplementary Material
Acknowledgments
We thank D. Bennett, S. Cleaveland, D. Haydon, P. Hosseini, C. Packer, J. Pulliam, D. L. Smith, and two anonymous reviewers for helpful comments and the Southern and Eastern African Rabies Group. This work was supported by National Institutes of Health/National Science Foundation Ecology of Infectious Diseases Program Grant DEB0225453 (to A.D.), National Science Foundation Grant DEB0513994 (to K.H), Pew Charitable Trusts Award 2000-002558 (to Princeton University), and The Heinz Foundation (K.H).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.L. is a guest editor invited by the Editorial Board.
Seventh Southern and Eastern African Rabies Group/World Health Organization Meeting, May 12–15, 2003, Ezulwini, Swaziland.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609122104/DC1.
Ali, Y. H., Seventh Southern and Eastern African Rabies Group/World Health Organization Meeting, May 12–15, 2003, Ezulwini, Swaziland.
References
- 1.Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin FX. Bull WHO. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World Survey of Rabies for 1998. Geneva: WHO; 1999. [Google Scholar]
- 3.Kayali U, Mindekem R, Hutton G, Ndoutamia AG, Zinsstag J. Trop Med Int Health. 2006;11:1058–1065. doi: 10.1111/j.1365-3156.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 4.Coleman PG, Dye C. Vaccine. 1996;14:185–186. doi: 10.1016/0264-410x(95)00197-9. [DOI] [PubMed] [Google Scholar]
- 5.Cleaveland S, Kaare M, Knobel D, Laurenson MK. Vet Microbiol. 2006;117:43–50. doi: 10.1016/j.vetmic.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Bingham J, Foggin CM, Wandeler AI, Hill FWG. Onderstepoort J Vet Res. 1999a;66:11–23. [PubMed] [Google Scholar]
- 7.Rhodes CJ, Atkinson RPD, Anderson RM, Macdonald DW. Philos Trans R Soc London B. 1998;353:999–1010. doi: 10.1098/rstb.1998.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randall DA, Marino J, Haydon DT, Sillero-Zubiri C, Knobel DL, Tallents LA, Macdonald DW, Laurenson MK. Biol Conserv. 2006;131:151–162. [Google Scholar]
- 9.Cleaveland S, Dye C. Parasitology. 1995;111:S33–S47. doi: 10.1017/s0031182000075806. [DOI] [PubMed] [Google Scholar]
- 10.Brückner GK, Hurter LR, Boshoff JN. J South African Vet Assoc. 1978;49:33–36. [PubMed] [Google Scholar]
- 11.Bingham J, Foggin CM, Wandeler AI, Hill FWG. Onderstepoort J Vet Res. 1999b;66:1–10. [PubMed] [Google Scholar]
- 12.Cleaveland S. Trans R Soc Trop Med Hyg. 1998;92:131–134. doi: 10.1016/s0035-9203(98)90718-0. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Luo M, Zhang S, Fooks AR, Hu R, Tu C. Emerging Infect Dis. 2005;11:1970–1972. doi: 10.3201/eid1112.050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon PJ. Population Dynamics of Rabies in Wildlife. London: Academic; 1985. [Google Scholar]
- 15.Anderson RM, Jackson HC, May RM, Smith AM. Nature. 1981;289:765–771. doi: 10.1038/289765a0. [DOI] [PubMed] [Google Scholar]
- 16.Murray JD, Stanley EA, Brown DL. Proc R Soc London Ser B. 1986;229:111–150. doi: 10.1098/rspb.1986.0078. [DOI] [PubMed] [Google Scholar]
- 17.Russell CA, Smith DL, Waller LA, Childs JE, Real LA. Proc R Soc London Ser B. 2004;271:21–25. doi: 10.1098/rspb.2003.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith DL, Lucey B, Waller LA, Childs JE, Real LA. Proc Natl Acad Sci USA. 2002;99:3668–3672. doi: 10.1073/pnas.042400799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bingham J, Bishop GC, King AA, editors. Proceedings of the Third International Conference of the Southern and Eastern African Rabies Group; Lyon, France: Editions Fondation Marcel Mérieux; 1995. [Google Scholar]
- 20.Bishop GC, editor. Proceedings of the Southern and Eastern African Rabies Group International Symposium; Lyon, France: Editions Fondation Marcel Mérieux; 1993. [Google Scholar]
- 21.Courtin F, Carpenter TE, Paskin RD, Chomel BB. Prev Vet Med. 2000;43:13–28. doi: 10.1016/s0167-5877(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 22.Fekadu M. Am J Epidemiol. 1982;115:266–273. doi: 10.1093/oxfordjournals.aje.a113298. [DOI] [PubMed] [Google Scholar]
- 23.Foggin CM. Rabies and Rabies-Related Viruses in Zimbabwe: Historical, Virological, and Ecological Aspects. Harare, Zimbabwe: University of Zimbabwe; 1988. [Google Scholar]
- 24.Hayles LB, Sawchuk A, Akafekwa GI, Awan MAQ. Bull Anim Health Production Africa. 1977;25:9–16. [Google Scholar]
- 25.King AA, editor. Proceedings of the Southern and Eastern Africa Rabies Group/World Health Organization Meeting; Lyon, France: Editions Fondation Marcel Mérieux; 2001. [Google Scholar]
- 26.King AA, editor. Proceedings of the International Conference on Epidemiology, Control, and Prevention of Rabies in Eastern and Southern Africa; Lyon, France: Editions Fondation Marcel Mérieux; 1992. [Google Scholar]
- 27.Kitala P, Perry B, Barrat J, King AA, editors. Proceedings of the Southern and Eastern African Rabies Group Meeting; Lyon, France: Editions Fondation Marcel Mérieux; 1997. [Google Scholar]
- 28.Kuwert E, Merieux C, Koprowski H, Bogel K. Rabies in the Tropics. Berlin: Springer; 1988. [Google Scholar]
- 29.Rutebarika C, Winyi-Kaboyo R, Barrat J, King AA, editors. Proceedings of the Southern and Eastern African Rabies Group/World Health Organization Meeting; Lyon, France: Editions Fondation Marcel Mérieux; 1999. [Google Scholar]
- 30.Sabeta CT, Bingham J, Nel LH. Virus Res. 2003;91:203–211. doi: 10.1016/s0168-1702(02)00272-1. [DOI] [PubMed] [Google Scholar]
- 31.Grenfell BT, Bjornstad ON, Kappey J. Nature. 2001;414:716–723. doi: 10.1038/414716a. [DOI] [PubMed] [Google Scholar]
- 32.Kitala PM, McDermott JJ, Coleman PG, Dye C. Epidemiol Infect. 2002;129:215–222. doi: 10.1017/s0950268802006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magembe SR. In: Rabies in the Tropics. Kuwert E, Mérieux C, Koprowski H, Bögel K, editors. Berlin: Springer; 1988. pp. 392–398. [Google Scholar]
- 34.Umeno S, Doi Y. Kitasato Arch Exp Med. 1921;4:89–108. [Google Scholar]
- 35.Shimada K. In: Rabies. Nagano Y, Davenport FM, editors. Baltimore: University Park; 1971. pp. 11–28. [Google Scholar]
- 36.Wells CW. Bull WHO. 1957;17:1025–1029. [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. WHO Expert Consultation on Rabies: First Report. Geneva: WHO; 2004. [Google Scholar]
- 38.Larghi OP, Arrosi JC, Nakajata-AJ, Villa-Nova A. In: Developments in Veterinary Virology: Rabies. Campbell JB, Charlton KM, editors. The Netherlands: Kluwer, Dordrecht; 1988. pp. 407–422. [Google Scholar]
- 39.Friend M. NY Fish Game J. 1968;15:71–97. [Google Scholar]
- 40.King AA, Fooks AR, Aubert M, Wandeler AI. Historical Perspective of Rabies in Europe and the Mediterranean Basin. Paris: World Organization for Animal Health; 2004. [Google Scholar]
- 41.Widdowson M-A, Morales GJ, Chaves S, McGrane J. Emerg Infect Dis. 2002;8:458–461. doi: 10.3201/eid0805.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ernst SN, Fabrega GF. Revista Microbiol. 1989;20:121–127. [Google Scholar]
- 43.Chadli A. Arch l'Institut Pasteur Tunis. 1988;65:15–27. [PubMed] [Google Scholar]
- 44.Yakobson B, David D, Aldomy F. In: Historical Perspective of Rabies in Europe and the Mediterranean Basin. King AA, Fooks AR, Aubert M, Wandeler AI, editors. Paris: World Organization for Animal Health; 2004. pp. 171–183. [Google Scholar]
- 45.Osman FB, Haddad N. Rev Infect Dis. 1988;10:S703–S706. doi: 10.1093/clinids/10.supplement_4.s703. [DOI] [PubMed] [Google Scholar]
- 46.Mutinelli F, Stankov S, Hristovski M, Seimenis A, Theoharakou H, Vodopija I. In: Historical Perspectives of Rabies in Europe and the Mediterranean Basin. King AA, Fooks AR, Aubert M, Wandeler AI, editors. Paris: World Organization for Animal Health; 2004. pp. 93–118. [Google Scholar]
- 47.Matter H, Blancou J, Benelmouffok A, Hammami S, Fassi-Fehri N. In: Historical Perspectives of Rabies in Europe and the Mediterranean Basin. King AA, Fooks AR, Aubert M, Wandeler AI, editors. Paris: World Organization for Animal Health; 2004. pp. 185–199. [Google Scholar]
- 48.Yakobson B, Manalo DL, Bader K, Perl S, Haber A, Shahimov B, Shechat N, Orgad U. Isr J Vet Med. 1998;53:114–126. [Google Scholar]
- 49.Kaplan MM, Goor Y, Tierkel ES. Bull WHO. 1954;10:743–752. [PMC free article] [PubMed] [Google Scholar]
- 50.Humphrey GL. In: Sikes K, editor. Proceedings of the National Rabies Symposium; Atlanta, GA: Centers for Disease Control and Prevention; 1966. pp. 65–72. [Google Scholar]
- 51.Dlamini RX. In: King A, editor. Proceedings of the Southern and Eastern Africa Rabies Group/World Health Organization Meeting; Lyon, France: Editions Fondation Marcel Merieux; 2001. pp. 53–54. [Google Scholar]
- 52.Khomari L. In: King A, editor. Proceedings of the International Conference on Epidemiology, Control and Prevention of Rabies in Eastern and Southern Africa; Lyon: Editions Fondation Marcel Mérieux; 1992. pp. 45–46. [Google Scholar]
- 53.Bishop GC. In: Rutebarika C, Winyi-Kaboyo R, Barrat J, King AA, editors. Proceedings of the Southern and Eastern African Rabies Group/World Health Organization Meeting; Lyon, France: Editions Fondation Marcel Mérieux; 1999. pp. 46–50. [Google Scholar]
- 54.Swanepoel R. In: Infectious Diseases of Livestock. Coetzer JAW, Tustin RC, editors. Cape Town, South Africa: Oxford Univ Press; 2004. pp. 1123–1182. [Google Scholar]
- 55.Zyambo GCN, Sinyangwe PG, Bussein NA. In: Rabies in the Tropics. Kuwert E, Mérieux C, Koprowski H, Bögel K, editors. Berlin: Springer; 1988. pp. 415–421. [Google Scholar]
- 56.Mariam SH. In: Rabies in the Tropics. Kuwert EK, Mérieux C, Koprowski H, Bögel K, editors. Berlin: Springer; 1988. pp. 473–480. [Google Scholar]
- 57.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 58.Sultan B, Labadi K, Guegan JF, Janicot S. Plos Med. 2005;2:43–49. doi: 10.1371/journal.pmed.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viboud C, Bjornstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Science. 2006;312:447–451. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 60.Clark CO, Cole JE, Webster PJ. J Climate. 2000;13:2505–2519. [Google Scholar]
- 61.Saji NH, Goswami BN, Vinayachandran PN, Yamagata T. Nature. 1999;401:360–363. doi: 10.1038/43854. [DOI] [PubMed] [Google Scholar]
- 62.Rohani P, Earn DJD, Grenfell BT. Science. 1999;286:968–971. doi: 10.1126/science.286.5441.968. [DOI] [PubMed] [Google Scholar]
- 63.Bjornstad ON, Ims RA, Lambin X. Trends Ecol Evol. 1999;14:427–432. doi: 10.1016/s0169-5347(99)01677-8. [DOI] [PubMed] [Google Scholar]
- 64.Ranta E, Kaitala V, Lundberg P. Oikos. 1998;83:376–382. [Google Scholar]
- 65.Ranta E, Kaitala V, Lindstrom J. Proc R Soc London Ser B. 1999;266:1851–1856. [Google Scholar]
- 66.Grassly NC, Fraser C, Garnett GP. Nature. 2005;433:417–421. doi: 10.1038/nature03072. [DOI] [PubMed] [Google Scholar]
- 67.Earn DJD, Rohani P, Grenfell BT. Proc R Soc London Ser B. 1998;265:7–10. [Google Scholar]
- 68.Cleaveland S, Kaare M, Tiringa P, Mlengeya T, Barrat J. Vaccine. 2003;21:1965–1973. doi: 10.1016/s0264-410x(02)00778-8. [DOI] [PubMed] [Google Scholar]
- 69.Chomel B, Chappuis G, Bullon F, Cardenas E, Debeublain TD, Lombard M, Giambruno E. Rev Infect Dis. 1988;10:S697–S702. doi: 10.1093/clinids/10.supplement_4.s697. [DOI] [PubMed] [Google Scholar]
- 70.Belotto A, Leanes LF, Schneider MC, Tamayo H, Correa E. Virus Res. 2005;111:5–12. doi: 10.1016/j.virusres.2005.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.