Abstract
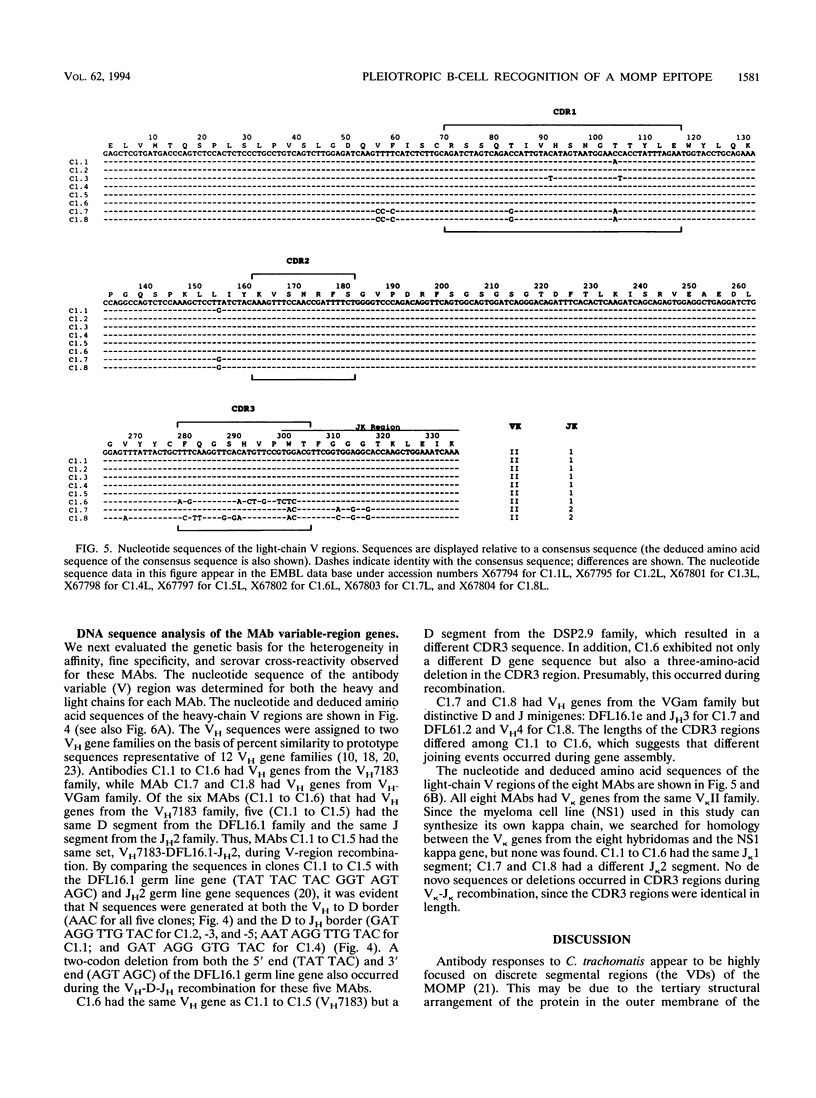
Two BALB/c mice were immunized with serovar C Chlamydia trachomatis elementary bodies, and 63 hybridomas producing monoclonal antibodies to C. trachomatis were recovered. Eight hybridomas which were specific for an identical peptide epitope (AGLQND) in serovar C major outer membrane protein variable domain I were identified. Detailed immunochemical study of the antigen-antibody interaction and genetic characterization of the antibody variable-region gene sequences showed that distinct B-cell clonal lineages were elicited by the epitope sequence. Since each antibody had a distinct pattern of fine specificity for recognition of the epitope and displayed different degrees of cross-reactivity with a related serovar (serovar A), we conclude that B-cell recognition of an immunodominant neutralization epitope can be pleiotropic. Differences in B-cell recognition of a neutralization epitope may delay the emergence by mutation of antigenic-drift variants of the C. trachomatis major outer membrane protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D., Cumano A., Dildrop R., Kocks C., Rajewsky K., Rajewsky N., Roes J., Sablitzky F., Siekevitz M. Timing, genetic requirements and functional consequences of somatic hypermutation during B-cell development. Immunol Rev. 1987 Apr;96:5–22. doi: 10.1111/j.1600-065x.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Plummer F. A., Stephens R. S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect Immun. 1993 Jun;61(6):2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Stephens R. S., Ada G., Caldwell H. D., Su H., Morrison R. P., Van der Pol B., Bavoil P., Bobo L., Everson S. Workshop on in vitro neutralization of Chlamydia trachomatis: summary of proceedings. J Infect Dis. 1993 Aug;168(2):415–420. doi: 10.1093/infdis/168.2.415. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Kofler R. A new murine Ig VH gene family. J Immunol. 1988 Jun 1;140(11):4031–4034. [PubMed] [Google Scholar]
- Rath S., Stanley C. M., Steward M. W. An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J Immunol Methods. 1988 Feb 10;106(2):245–249. doi: 10.1016/0022-1759(88)90204-9. [DOI] [PubMed] [Google Scholar]
- Reininger L., Kaushik A., Izui S., Jaton J. C. A member of a new VH gene family encodes antibromelinized mouse red blood cell autoantibodies. Eur J Immunol. 1988 Oct;18(10):1521–1526. doi: 10.1002/eji.1830181008. [DOI] [PubMed] [Google Scholar]
- Rini J. M., Schulze-Gahmen U., Wilson I. A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992 Feb 21;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Fieser T. M., Lerner R. A., Wilson I. A. Crystal structures of an antibody to a peptide and its complex with peptide antigen at 2.8 A. Science. 1990 May 11;248(4956):712–719. doi: 10.1126/science.2333521. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Caldwell H. D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992 Jan 1;175(1):227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- Zhong G. M., Brunham R. C. Antigenic determinants of the chlamydial major outer membrane protein resolved at a single amino acid level. Infect Immun. 1991 Mar;59(3):1141–1147. doi: 10.1128/iai.59.3.1141-1147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., Reid R. E., Brunham R. C. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990 May;58(5):1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Brunham R. C. Antibody responses to the chlamydial heat shock proteins hsp60 and hsp70 are H-2 linked. Infect Immun. 1992 Aug;60(8):3143–3149. doi: 10.1128/iai.60.8.3143-3149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Toth I., Reid R., Brunham R. C. Immunogenicity evaluation of a lipidic amino acid-based synthetic peptide vaccine for Chlamydia trachomatis. J Immunol. 1993 Oct 1;151(7):3728–3736. [PubMed] [Google Scholar]