Abstract
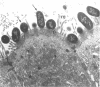
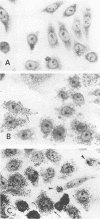
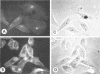
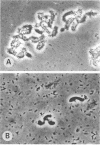
Twelve strains of Escherichia coli previously reported to cause diarrhea in rabbits were examined for properties associated with virulence. Ten strains met the criteria for classification as enteropathogenic E. coli in that they were diarrheagenic strains that evoked attaching-effacing lesions in the small intestine and did not produce detectable enterotoxins or cytotoxins. These bacteria exhibited a variety of patterns when investigated for adherence to HEp-2 epithelial cells. Although several strains displayed localized and/or diffuse adherence to epithelial cells, they did not hybridize with DNA probes that recognize the genes responsible for these phenotypes in diarrheagenic E. coli from humans. The bacteria also varied in their ability to bind to erythrocytes and intestinal brush borders from various animal species. Six strains adhered to rabbit brush borders; two of these also adhered to brush borders from other animals. Two strains that did not adhere to rabbit brush borders adhered to those from guinea pigs or sheep. Only one of the strains investigated carried AF/R1 fimbriae, which are believed to govern the host specificity of this category of diarrheagenic E. coli. This strain was E. coli RDEC-1, which remains the only E. coli strain to date that is known to carry fimbriae of this type. The results indicate that although diarrheagenic E. coli strains from rabbits may have common properties associated with the ability to produce attaching-effacing lesions, they differ from each other and from enteropathogenic E. coli of humans in terms of some of the adhesins that mediate binding to eukaryotic cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry B., Savarino S. J., Vial P., Kaper J. B., Levine M. M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990 Jun;161(6):1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Inman L. R., Blake R. K. Production of diarrhea in the rabbit by a mutant of Escherichia coli (RDEC-1) that does not express adherence (AF/R1) pili. J Infect Dis. 1989 Jul;160(1):136–141. doi: 10.1093/infdis/160.1.136. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Moseley S. L. HeLa cell adherence, actin aggregation, and invasion by nonenteropathogenic Escherichia coli possessing the eae gene. Infect Immun. 1991 Nov;59(11):3924–3929. doi: 10.1128/iai.59.11.3924-3929.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
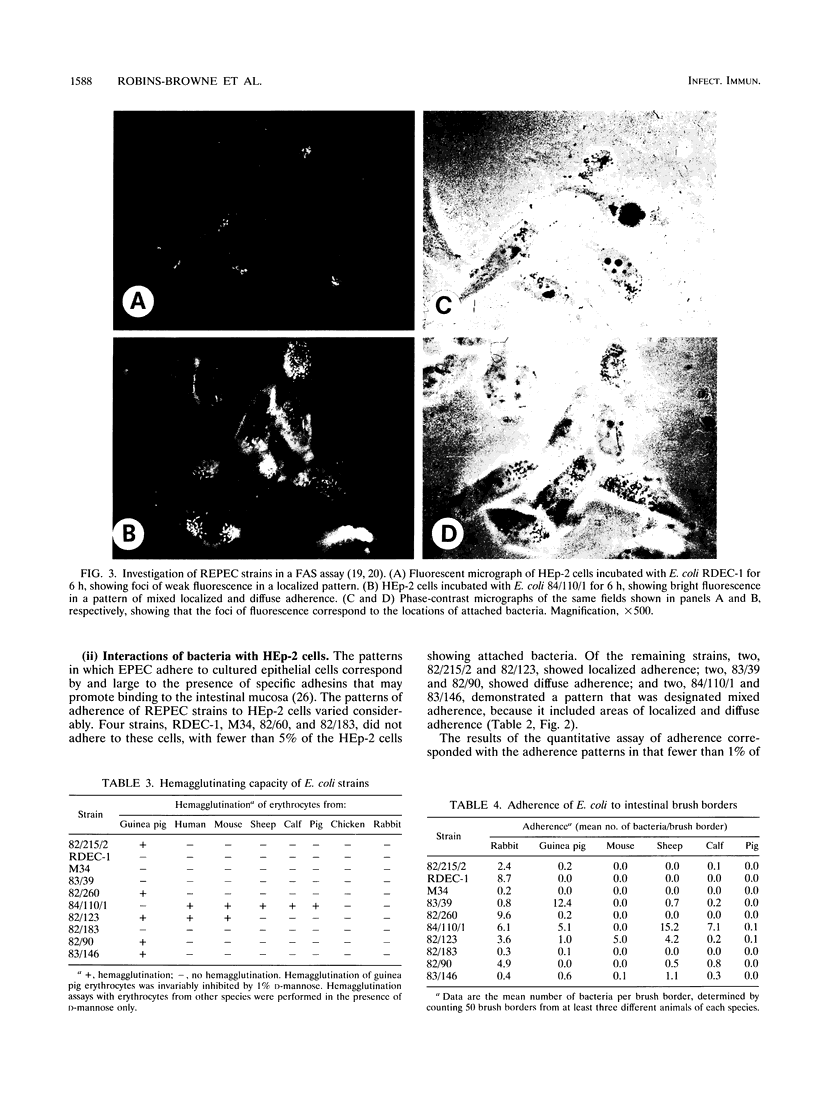
- Cheney C. P., Boedeker E. C., Formal S. B. Quantitation of the adherence of an enteropathogenic Escherichia coli to isolated rabbit intestinal brush borders. Infect Immun. 1979 Nov;26(2):736–743. doi: 10.1128/iai.26.2.736-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C. Rabbit mucosal receptors for an enteropathogenic Escherichia coli strain: appearance of bacterial receptor activity at weaning. Gastroenterology. 1984 Oct;87(4):821–826. [PubMed] [Google Scholar]
- Cheney C. P., Schad P. A., Formal S. B., Boedeker E. C. Species specificity of in vitro Escherichia coli adherence to host intestinal cell membranes and its correlation with in vivo colonization and infectivity. Infect Immun. 1980 Jun;28(3):1019–1027. doi: 10.1128/iai.28.3.1019-1027.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Tacket C. O., James S. P., Losonsky G., Nataro J. P., Wasserman S. S., Kaper J. B., Levine M. M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993 Sep;92(3):1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. N., Saunders J. R., Batt R. M., Embaye H., Getty B., Hart C. A. Attaching effacement of the rabbit enterocyte brush border is encoded on a single 96.5-kilobase-pair plasmid in an enteropathogenic Escherichia coli O111 strain. Infect Immun. 1990 May;58(5):1316–1322. doi: 10.1128/iai.58.5.1316-1322.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. L., Jerse A. E., Kaper J. B., Falkow S. Characterization of interactions of enteropathogenic Escherichia coli O127:H6 with mammalian cells in vitro. J Infect Dis. 1991 Oct;164(4):693–703. doi: 10.1093/infdis/164.4.693. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón J. A., Ho A. S., Schoolnik G. K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991 Nov 1;254(5032):710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Gicquelais K. G., Kaper J. B. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect Immun. 1991 Nov;59(11):3869–3875. doi: 10.1128/iai.59.11.3869-3875.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
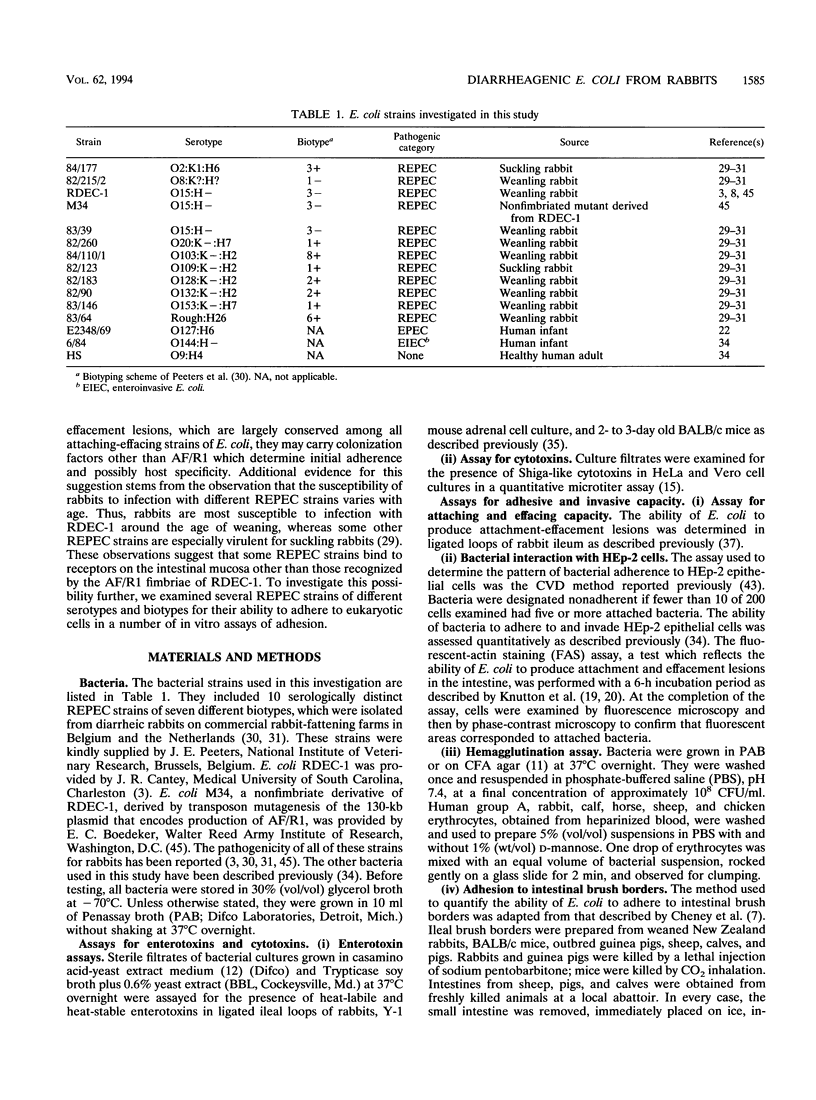
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Phillips A. D., Smith H. R., Gross R. J., Shaw R., Watson P., Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991 Jan;59(1):365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Prado V., Robins-Browne R., Lior H., Kaper J. B., Moseley S. L., Gicquelais K., Nataro J. P., Vial P., Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988 Jul;158(1):224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- Milon A., Esslinger J., Camguilhem R. Adhesion of Escherichia coli strains isolated from diarrheic weaned rabbits to intestinal villi and HeLa cells. Infect Immun. 1990 Aug;58(8):2690–2695. doi: 10.1128/iai.58.8.2690-2695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Newland J. W., Neill R. J. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J Clin Microbiol. 1988 Jul;26(7):1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okerman L. Enteric infections caused by non-enterotoxigenic Escherichia coli in animals: occurrence and pathogenicity mechanisms. A review. Vet Microbiol. 1987 May;14(1):33–46. doi: 10.1016/0378-1135(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Peeters J. E., Charlier G. J., Halen P. H. Pathogenicity of attaching effacing enteropathogenic Escherichia coli isolated from diarrheic suckling and weanling rabbits for newborn rabbits. Infect Immun. 1984 Dec;46(3):690–696. doi: 10.1128/iai.46.3.690-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. E., Geeroms R., Orskov F. Biotype, serotype, and pathogenicity of attaching and effacing enteropathogenic Escherichia coli strains isolated from diarrheic commercial rabbits. Infect Immun. 1988 Jun;56(6):1442–1448. doi: 10.1128/iai.56.6.1442-1448.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. E., Pohl P., Okerman L., Devriese L. A. Pathogenic properties of Escherichia coli strains isolated from diarrheic commercial rabbits. J Clin Microbiol. 1984 Jul;20(1):34–39. doi: 10.1128/jcm.20.1.34-39.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl P. H., Peeters J. E., Jacquemin E. R., Lintermans P. F., Mainil J. G. Identification of eae sequences in enteropathogenic Escherichia coli strains from rabbits. Infect Immun. 1993 May;61(5):2203–2206. doi: 10.1128/iai.61.5.2203-2206.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Bennett-Wood V. Quantitative assessment of the ability of Escherichia coli to invade cultured animal cells. Microb Pathog. 1992 Feb;12(2):159–164. doi: 10.1016/0882-4010(92)90119-9. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Levine M. M., Rowe B., Gabriel E. M. Failure to detect conventional enterotoxins in classical enteropathogenic (serotyped) Escherichia coli strains of proven pathogenicity. Infect Immun. 1982 Nov;38(2):798–801. doi: 10.1128/iai.38.2.798-801.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Miliotis M. D., Cianciosi S., Miller V. L., Falkow S., Morris J. G., Jr Evaluation of DNA colony hybridization and other techniques for detection of virulence in Yersinia species. J Clin Microbiol. 1989 Apr;27(4):644–650. doi: 10.1128/jcm.27.4.644-650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987 Jan-Feb;9(1):28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Yam W. C., O'Gorman L. E., Bettelheim K. A. Examination of archetypal strains of enteropathogenic Escherichia coli for properties associated with bacterial virulence. J Med Microbiol. 1993 Mar;38(3):222–226. doi: 10.1099/00222615-38-3-222. [DOI] [PubMed] [Google Scholar]
- Sohel I., Puente J. L., Murray W. J., Vuopio-Varkila J., Schoolnik G. K. Cloning and characterization of the bundle-forming pilin gene of enteropathogenic Escherichia coli and its distribution in Salmonella serotypes. Mol Microbiol. 1993 Feb;7(4):563–575. doi: 10.1111/j.1365-2958.1993.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Tesh V. L., O'Brien A. D. Adherence and colonization mechanisms of enteropathogenic and enterohemorrhagic Escherichia coli. Microb Pathog. 1992 Apr;12(4):245–254. doi: 10.1016/0882-4010(92)90043-n. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Gibson R., Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989 Apr;57(4):1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Robins-Browne R. M., Gonis G., Hayes J., Withers M., McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985 Jun;26(6):570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial P. A., Mathewson J. J., DuPont H. L., Guers L., Levine M. M. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J Clin Microbiol. 1990 May;28(5):882–885. doi: 10.1128/jcm.28.5.882-885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam T. S., Wolfe M. L., Wachsmuth I. K., Orskov F., Orskov I., Wilson R. A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993 May;61(5):1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
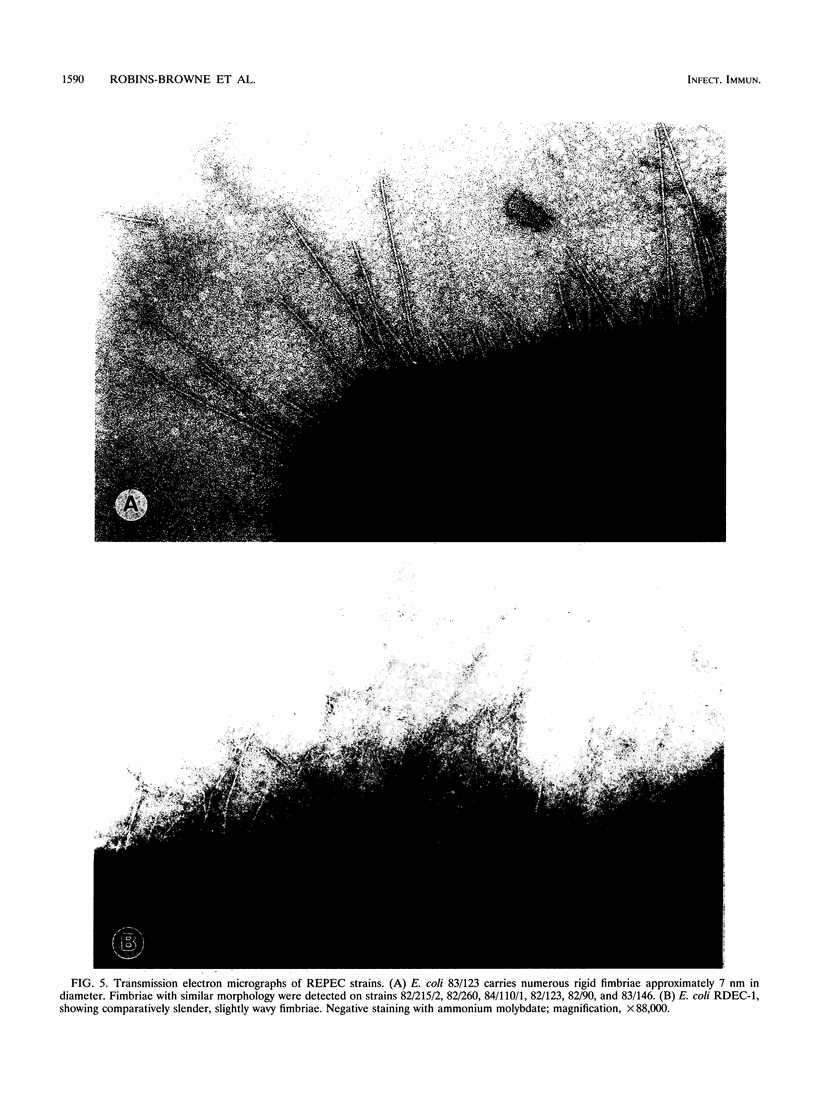
- Wolf M. K., Andrews G. P., Fritz D. L., Sjogren R. W., Jr, Boedeker E. C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988 Aug;56(8):1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Boedeker E. C. Cloning of the genes for AF/R1 pili from rabbit enteroadherent Escherichia coli RDEC-1 and DNA sequence of the major structural subunit. Infect Immun. 1990 Apr;58(4):1124–1128. doi: 10.1128/iai.58.4.1124-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]