Abstract
Chronic Granulomatous Disease (CGD) is a rare disorder caused by mutations in the NADPH oxidase. The CGD phenotype includes granuloma formation and susceptibility to infection with microorganisms including Aspergillus. The immune adjuvant interferon-γ and the antifungal agent itraconazole have reduced the incidence of infections in CGD. Studies using CGD phagocytes have shown that reactive oxygen species (ROS), products of the NAPDH oxidase, are critical for killing Aspergillus hyphae. But despite lack of ROS production, CGD patients generally only get infected with Aspergillus after heavy exposure. To study why CGD patients are not infected with Aspergillus more frequently we studied host defense against this ubiquitous mold further. We found that neutrophil lactoferrin is fungistatic for Aspergillus fumigatus spores by chelation of iron, an essential growth factor. Thus, the neutrophil employs both nonoxidative (lactoferrin) and oxidative (hydrogen peroxide) defense mechanisms against A. fumigatus spores and hyphae, respectively.
Introduction
Aspergillus was first described by Pietro Antonio Micheli, an Italian botanist who lived from 1679–1737. The organism was named because of the morphological similarity of its conidiophore, the specialized spore-producing hyphae, to the Aspergillum, a device used in the Catholic Church to disperse holy water. Aspergillus species are ubiquitous and it is estimated that the average person is exposed to 200–2000 Aspergillus spores (conidia) per day (1). There are 185 species of Aspergillus and about 20 of these are opportunistic pathogens in humans. Risk factors for susceptibility to Aspergillus include neutropenia (2), organ transplantation (3), chemotherapy for cancer (4), and conditions of iron overload as in liver transplantation (5) stem cell transplantation (6) and in certain hematologic malignancies (7). In addition, there is markedly increased susceptibility to aspergillosis in the phagocytic defect, chronic granulomatous disease of childhood (CGD) (8). Despite significant advances in antifungal therapy, and prophylaxis in particular, the overall mortality from invasive Aspergillus infection in all patients remains high and was reported recently to be 18% in immunocompromised children (9). The purpose of this paper is to review host defenses against Aspergillus as learned from studies of patients with CGD and to review therapeutic strategies to treat this infection and these patients.
Chronic Granulomatous Disease
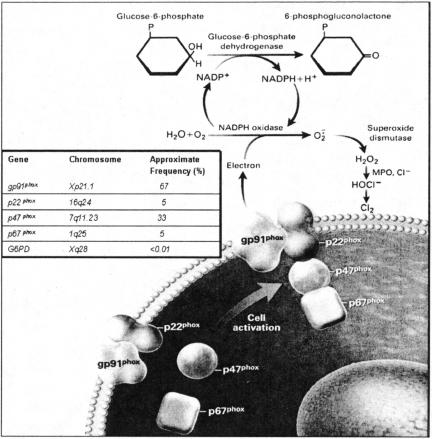
Chronic Granulomatous Disease is a rare disorder of phagocytic cells that occurs in about 1:200,000 live births (10). It has a mortality of about 2% per year and about ⅓ of the deaths are caused by Aspergillus infection. The defect in CGD results from mutation of one of four essential genes of the complex enzyme, NADPH oxidase, termed the phagocyte oxidase (Fig. 1). The protein components of the phagocyte oxidase are named for their molecular mass; gp91phox, p22phox, p47phox and p67phox. Due to the indiscriminate toxicity of reactive oxygen species (ROS), the assembly and activation of the NADPH oxidase complex is under tight regulatory control. Two proteins, gp91phox and p22phox, exist as a noncovalent complex stored in the membranes of PMN secondary granules and are translocated to the plasma membrane or phagocytic membrane upon cellular activation. NADPH oxidase activation, the details of which are beyond the scope of this review, occurs when two cytosolic components, p67phox and p47phox, translocate to this membrane-bound complex to form a b-cytochrome that enables the passage of electrons through the plasma membrane to cause the univalent reduction of oxygen to superoxide anion. Superoxide is converted by superoxide dismutase to hydrogen peroxide. In the presence of myeloperoxidase, which is a neutrophil primary granule protein, hydrogen peroxide is converted to hypochlorous acid (bleach) which then gets converted to chlorine. The intact NADPH oxidase, therefore, stands upstream of a pathway leading to the production of several potent antimicrobial compounds used in innate host defenses. Mutations in any of these components, as occurs in CGD, thereby remove a major component of immunity resulting in increased susceptibility to infection.
Fig. 1.
The membrane-bound phagocyte oxidase components, the 91-kd glycoprotein (gp91phox) and the 22-kd protein (p22phox), interact with the cytoplasmic components, the 47-kd protein (p47phox) and the 67-kd protein (p67phox). Glucose-6-phosphate dehydrogenase (G6PD) converts glucose-6-phosphate to 6-phosphogluconolactone, generating NADPH and a hydrogen ion from NADP+. The genes for the components of NADPH oxidase, their chromosomal locations, and the frequency of mutations as a cause of chronic granulomatous disease are indicated in the box. (Reproduced from reference (10), with permission).
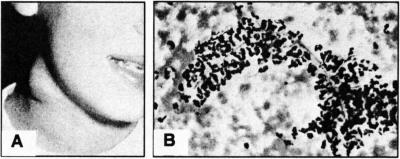
CGD has two major manifestations, a propensity to granuloma formation and susceptibility to infection. The granulomas can involve lymph nodes of the neck (e.g., Fig. 2A), which can suppurate, or granulomas can involve organs such as the liver and lungs and other vital structures, such as the gastrointestinal tract or genitourinary tract, all of which can lead to life threatening situations. Infections are most commonly due to bacteria such as Staphylococcus aureus, Burkholderia species, Serratia marcescens, Nocardia species, and fungi such as Aspergillus (10). Often these present clinically as indolent infections, e.g., we have observed Aspergillus presenting as small cutaneous lesions that were asymptomatic or lesions within the lung with minimal symptoms. Often in CGD patients with Aspergillus infection, the fungal hyphae can be seen enshrouded with neutrophils without degradation of the organism (Fig. 2B).
Fig. 2.
A) Large granuloma in the neck of a CGD patient (reproduced from reference (10) with permission). B) Morphology of aspirate from lung of CGD patient showing branched hyphae enshrouded with neutrophils (reproduced from reference (11), with permission.
The indolent presentation of Aspergillus is often followed by severe, life threatening infection, which underscores both the need for better therapeutic options and a better understanding of the process of fungal pathogenesis.
Host Defenses Against Aspergillus
Aspergillus conidia exist in high numbers everywhere in the home and because of their physical properties, remain airborne for long periods of time. Humans are constantly exposed and have developed a variety of overlapping and normally redundant host defenses against this mold. As mentioned in the previous section, cutaneous infections by Aspergillus can occur, however, for most CGD patients and immunosuppressed individuals, Aspergillus infection occurs through inhalation of spores. Respiratory mucosae and the cilia lining the respiratory tree are therefore the most important primary host defenses against infection. Normal neutrophils and monocytes inhibit Aspergillus growth, and this inhibition is abnormal in neutrophils and monocytes from patients with CGD (10–12). Reconstitution of CGD neutrophils defective in killing Aspergillus can be attained by mixing as few as one normal neutrophil with 15 CGD neutrophils (13). It is now accepted that the correction of CGD neutrophils by normal cells is by virtue of hydrogen peroxide and related species that diffuse into the CGD cell (14). This also suggests that ROS-dependent mechanisms act in synergy with non-oxidative mechanisms in cells to effect immunity in the whole cell.
CGD patients have a 25–40% lifetime probability of Aspergillus infection (15). Given that Aspergillus occurs ubiquitously in the human environment, we wondered why 60–75% of CGD patients do not develop Aspergillus infections. We reported recently that whereas CGD neutrophils fail to inhibit A. fumigatus hyphae normally, they are able to inhibit the growth of A. fumigatus conidia as well as normal neutrophils. This suggests that host defenses against Aspergillus involve both oxidative mechanisms (hydrogen peroxide suppression of hyphae) and nonoxidative mechanisms (16).
In addition to oxidative killing by the NADPH oxidase, neutrophils have abundant nonoxidative antimicrobial agents packaged in their primary (azurophilic) and secondary (specific granules) (17 and summarized in Table 1).
TABLE 1.
Antimicrobial Factors in Neutrophil Granules
| Location | Protein | Mechanism of Action |
|---|---|---|
| Primary (Azurophil) granules | ||
| BPI1 | membrane active, neutralizes LPS2 | |
| Lysozyme | muramidase, neutralizes LPS | |
| Defensins | pore forming, neutralizes LPS | |
| Serprocidins | protease | |
| Secondary (Specific) granules | ||
| Lysozyme | muramidase, neutralizes LPS | |
| Cathelicidins | membrane active, neutralizes LPS | |
| Transcobalamin | sequesters Vitamin B12 | |
| Lactoferrin | iron depletion |
1BPI-bactericidal/permeability-increasing protein
2LPS-endotoxin lipopolysaccharide
We have determined that the secondary granule protein lactoferrin is capable of inhibiting A. fumigatus conidial growth by over 50% at concentrations around 10 μg/ml (16). We found that lactoferrin works by chelation of iron, an essential growth factor for A. fumigatus (16).
As shown in Table 2, lactoferrin is not just a neutrophil protein, but is also abundant in certain body fluids, at concentrations much greater than are required for in vitro activity against A. fumigatus conidia (18–23).
TABLE 2.
Human Lactoferrin Levels In Vivo
We speculate that 60%–70% of CGD patients do not get aspergillosis because lactoferrin and possibly other, as yet unidentified, nonoxidative killing mechanisms inhibit infection in CGD patients most of the time. CGD patients who do get infected usually report a history of working with mulch piles or horse manure, two particularly rich sources of A. fumigatus conidia. This suggests that non-oxidative mechanisms are able to control all but the largest inocula. Mucus clearance likely plays an essential role in removing inhaled conidia as has been suggested for bacteria (24). Resident alveolar macrophages likely play an important role in preventing invasion, however, it has been shown recently in mouse studies (25) that neutrophil recruitment is essential in surviving Aspergillus infection. Neutropenia would remove both the potential for neutrophil immigration to the lung and also, possibly, the appearance of lactoferrin in the airway fluid. Since iron-saturated lactoferrin is less potent in inhibiting Aspergillus (and other microbes), conditions with high plasma concentrations of iron (5–7) may also decrease the efficacy of host defenses.
Prevention and Treatment of Aspergillus Infection in CGD
The first recommendation for managing patients with CGD is to minimize exposure to environmental hazards rich in Aspergillus. Therefore, we recommend CGD patients avoid mulch, horse manure, rotting plants or smoking marijuana, all of which have been associated with increased susceptibility to Aspergillus infection. Likewise, HEPA filtration in hospital transplantation wards has been adopted to help decrease the incidence of aspergillosis in these immunosuppressed populations (26).
The prophylactic use of the antibiotic, bactrim (trimethoprim plus sulfamethoxazole), has reduced significantly bacterial infections in CGD without increasing susceptibility to fungal infections (27). Interferon-γ treatment has been associated with partial correction of the defect in hydrogen peroxide production in some, but not all, CGD patients (28,29). In a double blind study, interferon-γ was shown to decrease the incidence of serious life-threatening infections in CGD by 70% (30), and neutrophils from patients receiving interferon-γ produced significantly more damage to A. fumigatus hyphae than those from the placebo group (31). Based on the successful clinical trial, interferon-γ was licensed in the United States for use in CGD patients. Importantly, a post-marketing study failed to reveal serious adverse consequences of long term use of interferon-γ in CGD patients (32). As a result, interferon-γ prophylaxis is recommended for CGD patients.
Despite interferon-γ prophylaxis in CGD patients, life threatening Aspergillus infections remain a problem and represent the most common cause of mortality from infection in CGD patients (15). Therefore, we performed a study assessing the efficacy of the antifungal agent itraconazole in preventing fungal infections in CGD patients. The data from this ten year study indicated that itraconazole significantly reduced fungal infections in CGD patients without serious adverse consequences (33). As a result, we offer itraconazole prophylaxis to many of our CGD patients.
Infection with Aspergillus in CGD patients requires aggressive treatment. For isolated lesions, surgical debridement may be helpful, but prolonged treatment with the best available antifungal agents is usually required. In the past, we routinely have used high doses of amphotericin B with mixed success. Prolonged courses of high dose amphotericin B are toxic, and renal complications can occur, often requiring renal transplantation. The availability of new antifungal agents has improved treatment success and decreased complications of treatment. However, the mortality per case of Aspergillus remains unacceptably high (34,35). The possible use of lactoferrin or other iron chelators to either prevent or treat active aspergillosis in CGD or other clinical conditions is intriguing and a subject of ongoing investigation in our laboratory.
Several therapeutic approaches to correcting the underlying defects in CGD have been attempted and perhaps promise a more normal life to CGD patients. Bone marrow transplants have shown some remarkable success but are accompanied by their own significant risks (36). Gene therapy, where a normal copy of the defective gene is introduced to correct the mutation, has been fraught with challenges but remains the focus of significant effort (37). While these efforts to correct the underlying cause of CGD potentially represent the best curative approach, we are left in the mean time with the struggle to better understand how the human immune system fights microbial infections in these patients. Recent sequencing of the A. fumigatus genome (38) has allowed construction of DNA microarrays that can be used to probe how the fungus recognizes and responds to attack by the human immune system. Given the difficulties in developing antibiotics against eukaryotic pathogens such as Aspergillus, this new spectrum of information may reveal novel targets to exploit in combating this prevalent infectious agent.
Conclusions
Chronic granulomatous disease is a rare defect of the NADPH oxidase that results in impaired generation of hydrogen peroxide and related oxygen species that are critical in host defense against certain infections. As a consequence these patients have increased susceptibility to several microbes, especially Aspergillus which represents the most common cause of death from infection in CGD patients. Through studies of phagocytes from CGD patients it is well established that host defense against Aspergillus requires oxygen dependent pathways involving superoxide derivatives. We have recently discovered that nonoxidative antimicrobial suppression of A. fumigatus by lactoferrin sequestration of iron is also important, depriving the organism of an essential growth factor. Management of CGD patients requires minimizing exposure to Aspergillus by avoiding environmental risk factors such as mulch, rotting plants and animal manure. Prophylaxis with interferon-γ and itraconazole can reduce the incidence of fungal infections in CGD. Once infection occurs, aggressive treatment with surgical debridement and antifungal agents can be curative in some but not all patients. Although aspergillosis remains a major cause of morbidity and mortality in CGD patients, it is, numerically speaking, a much more common problem in iatrogenically immunosuppressed patients (e.g., transplant and cancer). Novel antifungal therapeutic approaches are desperately needed, and the possibility of prevention or treatment of aspergillosis by use of iron chelators is under study.
ACKNOWLEDGMENTS
The authors thank Harry L. Malech, M.D. and Steven M. Holland, M.D. for making available clinical materials from their patients who participated in our clinical protocols. We also thank Janyce Sugui, Ph.D. and June Kwon-Chung, Ph.D. who partnered with us on the lactoferrin studies.
DISCUSSION
Mackowiak: Baltimore: Fascinating, Dr. Gallin. Thank you. Presumably lactoferrin works by binding iron in such a way that it is unavailable for metabolism by the organism. Help me understand why since there is no free iron in the body, lactoferrin in white cells might suppress Aspergillus the way it does? Does it bind iron stronger? Does it take iron from the organism?
Gallin: Bethesda: The siderophores which the organism has are relatively weak chelators of iron. So lactoferrin could work by removing iron from the pathogen. However, in highly pathogenic organisms, we now know that siderophores may be more prevalent than in non-pathogenic organisms, and, presumably, that is how the organism subverts host defense.
Boxer: Ann Arbor: Very nice talk, John. What are the risks of Aspergillosis in the X-link versus the autosomal recessive forms as CGD, and have you had a chance to compare killing in the two murine models, the one that you have and the other from Indianapolis?
Gallin: Let me answer the first question. Of the four types of CGD, the X-linked form is the most common and generally has the most severe phenotype. In our hands, there is heterogeneity to the genotype of the X-linked group of patients; about two-thirds of the patients have a nonsense mutation and a third have a missense mutation. Those who have a missense mutation do better clinically and respond better to gamma interferon. In response to your second question, we have not looked at the murine models in this system.
Nathan: Boston: Well, needless to say, I enjoyed that, John. The question I have is about the iron. We do see an interesting thing in thalassemia. There is a high incidence of serious pulmonary infections, some of it aspergillosis, in very iron-overloaded patients. Interestingly, it is also seen in chelated patients, which causes confusion in the field. My question is; what about the use of chelators, at least in vitro? What do you see in your system of killing if you go in with a chelator, like Desferal, which can permeate cells or diferiprone that really permeates cells and chelates as well.
Gallin: We have used desferroxamine in vitro. That's the only one that we have looked at and it interferes with lactoferrin.
Nathan: But will it chelate iron from the organism?
Gallin: We haven't looked at that.
Nathan: It would be interesting to know because this L1 is very highly cell permeable and it might be a prophylactic. The newer ones are very effective as chelators but they don't get into cells well.
Cohen: Chapel Hill: Thanks for your talk, John. I know that you and Larry Boxer have a small group of patients with lactoferrin deficiency also called neutrophil-specific granule deficiency. Do those patients get Aspergillus infections?
Gallin: Interestingly, patients with neutrophil-specific granule deficiency, who do not have neutrophil lactoferrin, do not have increased susceptibility to Aspergillus infections. Those patients have normal lactoferrin in their tears and in their salivary fluids and the mutation is not due to a deficiency in the ability to synthesize lactoferrin. The mutation in a gene called C/EBP epsilon results in an inability of neutrophils to properly package the specific granule components. Presumably, the reason patients with neutrophil-specific granule deficiency don't get fungal infections is that the nasal and upper airway lactoferrin levels are completely normal.
Boyer: New Haven: Do you know whether neutrophils from patients with iron overload syndromes, such as hemochromatosis, have impaired bacterial clearance? Certainly those patients have increased serious infections.
Gallin: We've not looked at that. That's a good question.
REFERENCES
- 1.Millner PD, Olenchock SA, Epstein E, et al. Bioaerosols associated with composting facilities. Compost Science & Utilization. 1994;2:6–57. [Google Scholar]
- 2.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infec Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 3.Montoya JG, Chaparro SV, Celis D, et al. Invasive aspergillosis in the setting of cardiac transplantation. Clin Infec Dis. 2003;37(Suppl 3):S282–292. doi: 10.1086/376527. [DOI] [PubMed] [Google Scholar]
- 4.Latge JP. Aspergillus fumagatus and aspergillosis. Clin Microbio Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander J, Limaye AP, Ko CW, et al. Association of hepatic iron overload with invasive fungal infection in liver transplant recipients. Liver Transpl. 2006;12:1799–1804. doi: 10.1002/lt.20827. [DOI] [PubMed] [Google Scholar]
- 6.Altes A, Remach AF, Sarda P, et al. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant. 2004;34:505–509. doi: 10.1038/sj.bmt.1704628. [DOI] [PubMed] [Google Scholar]
- 7.Iglesias-Osma C, Gonzalez-Villaron L, San Miguel JF, et al. Iron metabolism and fungal infections in patients with haematological malignancies. J Clin Pathol. 1995;48:223–225. doi: 10.1136/jcp.48.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Isturiz RE, Malech HL, et al. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981;71:59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- 9.Zaoutis TE, Heydon K, Chu J, et al. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. 2006;117:e711–e716. doi: 10.1542/peds.2005-1161. [DOI] [PubMed] [Google Scholar]
- 10.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. New Eng J Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 11.Segal BH, Leto TL, Gallin JI, et al. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine. 2000;79(3):170–2000. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Levitz SM, Diamond RD. Mechanisms of resistance of Aspergillus fumigatus Conidia to killing by neutrophils in vitro. J Infec Dis. 1985;152:33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Rex JH, Bennett JE, Gallin JI, et al. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infec Dis. 1990;162:523–528. doi: 10.1093/infdis/162.2.523. [DOI] [PubMed] [Google Scholar]
- 14.Ohno Y, Gallin JI. Diffusion of extracellular hydrogen peroxide into intracellular compartments of human neutrophils: Studies utilizing the inactivation of myeloperoxidase by hydrogen peroxide and azide. J Biol Chem. 1985;260:8438–8446. [PubMed] [Google Scholar]
- 15.Winkelstein JA, Marion MC, Johnston RB, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Zarember KA, Sugui J, Zerfas P, et al. Chronic granulomatous disease (CGD) and normal human neutrophils arrest the growth of Aspergillus fumigatus conida: a new fungistatic role for lactoferrin. J Immunol. 2006;176(supplement):S70. [Google Scholar]
- 17.Bainton DF. Developmental biology of neutrophils and eosinophils. Inflammation: Basic Principles and Clinical Correlates. In: Gallin JI, Snyderman, editors. 3rd Ed. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 18.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 19.Brown RD, Yuen E, Rickard PC, et al. Plasma lactoferrin in patients with neutropenia. Blut. 1986;52:289–295. doi: 10.1007/BF00320792. [DOI] [PubMed] [Google Scholar]
- 20.Kijlstra A, Jeruissen SH, Koning KM. Lactoferrin levels in normal human tears. Br J Ophthalmol. 1983;67:199–202. doi: 10.1136/bjo.67.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirai Y, Kawakata N, Satoh K, et al. Concentrations of lactoferrin and iron in human milk at different stages of lactation. J Nutr Sci Vitaminol (Tokyo) 1990;36:531–544. doi: 10.3177/jnsv.36.531. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AB, Bohling T, Payvandi F, et al. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990;115:148–158. [PubMed] [Google Scholar]
- 23.Jacquot J, Tournier JM, Carmona TG, et al. Proteines des secretions bronchiques dans la mucoviscidose. Role de l'infection. Bull europ Physiopath Resp. 1983;19:453–458. [PubMed] [Google Scholar]
- 24.Knowles MR, Boucher R. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnett CR, Cornish EJ, Harmsen AJ, et al. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect Immun. 2006;74:6528–6539. doi: 10.1128/IAI.00909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn T, Cummings KM, Michalek AM, et al. Efficacy of HEPA filters in Preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Congrol Hosp Epidemiol. 2002;23:525–531. doi: 10.1086/502101. [DOI] [PubMed] [Google Scholar]
- 27.Margolis DH, Melnick DA, Alling DW, et al. Trimethoprim-sulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infec Dis. 1990;162:723–726. doi: 10.1093/infdis/162.3.723. [DOI] [PubMed] [Google Scholar]
- 28.Ezekowitz RAB, Orkin SH, Newburger PE. Recombinant interferon gamma augments phagocyte superoxide production and X-linked variant chronic granulomatous disease. J Clin Invest. 1987;80:1009–1016. doi: 10.1172/JCI113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sechler JMG, Malech HL, White CJ, et al. Recombinant human interferon-γ reconstitutes defective phagocyte function in patients with chronic granulomatous disease of childhood. Proceed Natl Acad Sci, USA. 1988;85:4874–4878. doi: 10.1073/pnas.85.13.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. New Eng J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 31.Rex JH, Bennett JE, Gallin JI, et al. In vivo Interferon-γ therapy augments the in vitro ability of chronic granulomatous disease neutrophils to damage Aspergillus hyphae. J Infec Dis. 1991;163:849–852. doi: 10.1093/infdis/163.4.849. [DOI] [PubMed] [Google Scholar]
- 32.Marciano BE, Wesley R, DeCarlo ES, et al. Long-term interferon-γ therapy for patients with chronic granulomatous disease. Clin Infec Dis. 2004;39:692–699. doi: 10.1086/422993. [DOI] [PubMed] [Google Scholar]
- 33.Gallin JI, Alling DW, Malech HL, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. New Eng J Med. 2003;348:2416–2422. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 34.Fourneret-Vivier A, Lebeau B, Mallaret MR. Hospital-wide prospective mandatory surveillance of invasive aspergillosis in a French teaching hospital (2000–2002) J Hosp Infec. 2006;62:22–28. doi: 10.1016/j.jhin.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Mantakakis E, Samonis G. Novel preventative strategies against invasive aspergillosis. Med Mycol. 2006;44:S327–S332. doi: 10.1080/13693780600849113. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz ME, Barrett J, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoetic allograft. New Eng J Med. 2001;344:881–888. doi: 10.1056/NEJM200103223441203. [DOI] [PubMed] [Google Scholar]
- 37.Malech HL. Progress in gene therapy for chronic granulomatous disease. J Infec Dis. 1999;179:S318–325. doi: 10.1086/513852. [DOI] [PubMed] [Google Scholar]
- 38.Nierman WC, Pain A, Anderson MJ, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]