Abstract
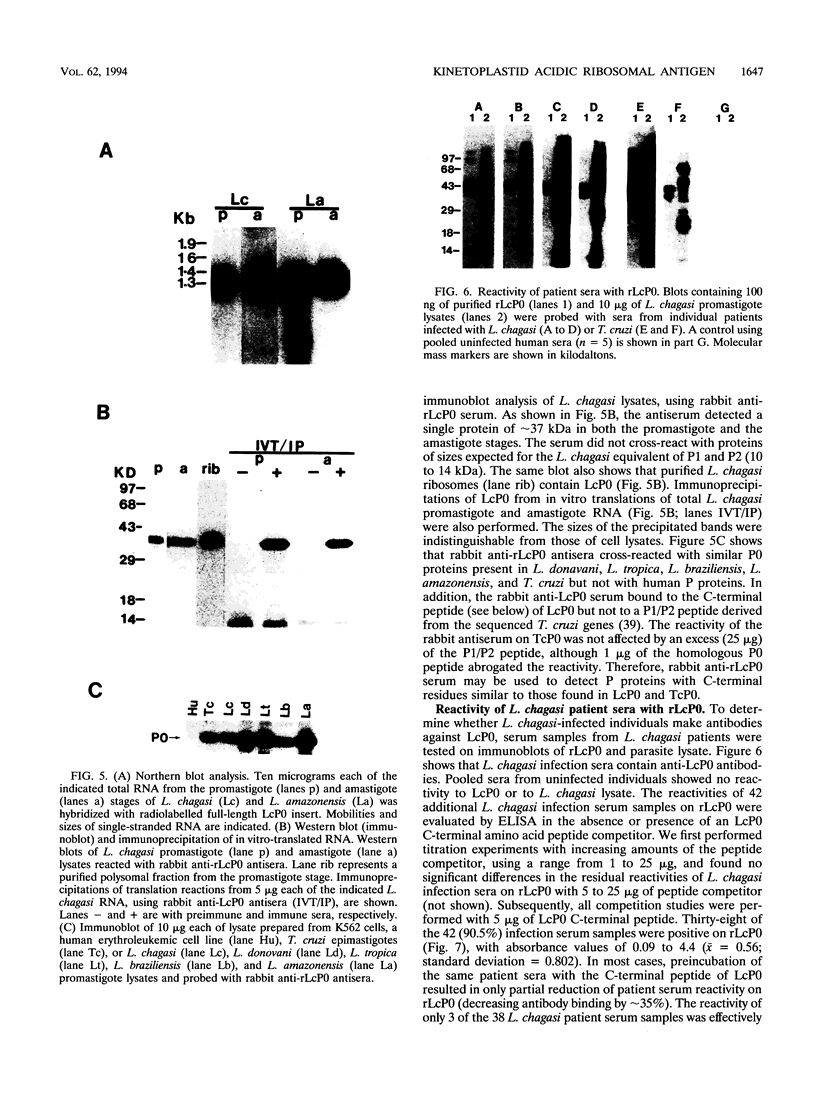
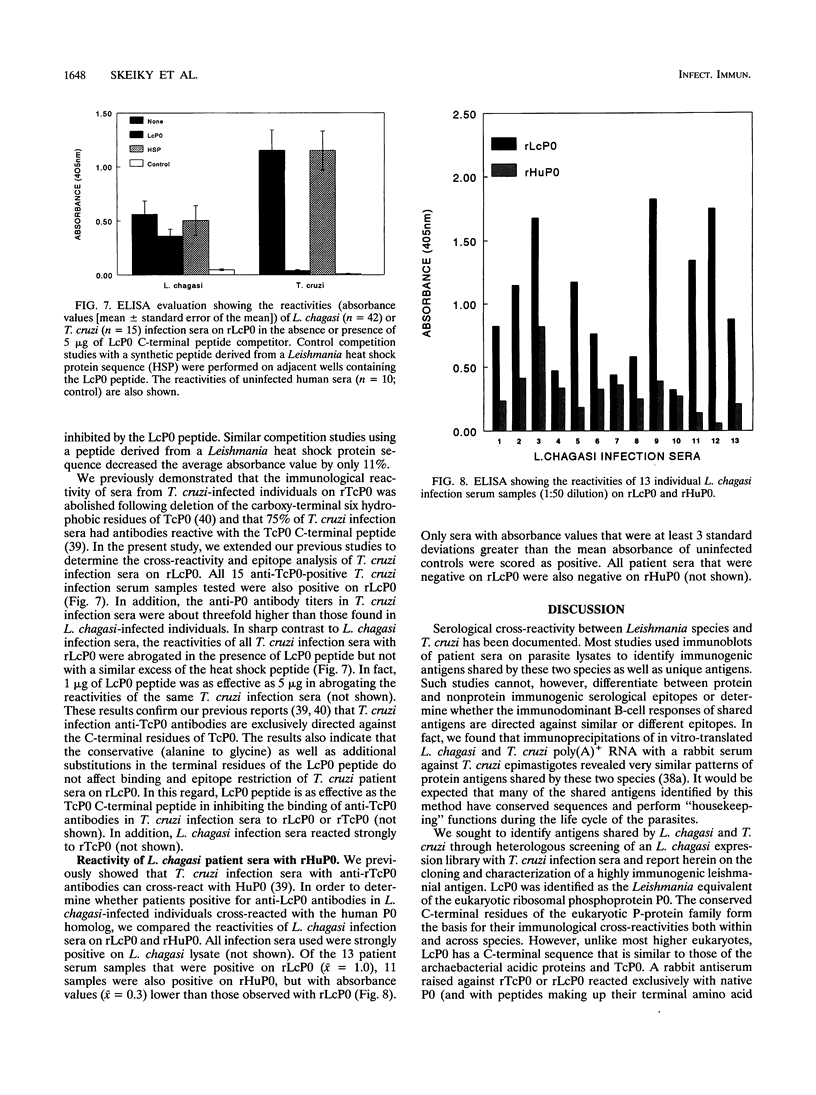
Patients with visceral leishmaniasis produce high levels of immunoglobulin, but the specificities of antibodies produced are not well characterized. In an effort to identify leishmania antigens that are specific to Leishmania species or are cross-reactive with other parasitic protozoa, we have cloned and characterized full-length genomic and cDNA clones encoding a Leishmania chagasi acidic ribosomal antigen, LcP0, recognized during human infections. The protein is homologous to the Trypanosoma cruzi and human ribosomal proteins TcP0 and HuP0, respectively. Unlike most higher eukaryotes, but similar to TcP0, LcP0 has a C-terminal heptapeptide sequence resembling those of the archaebacterial acidic (P-like) proteins. The highly charged C-terminal acidic domain of LcP0 contains a serine residue typically found in most eukaryotes but lacking in all T. cruzi P proteins we have characterized thus far. L. chagasi-infected individuals as well as those with T. cruzi infections have antibodies cross-reactive with recombinant LcP0 and TcP0 as well as HuP0. However, the properties of anti-P0 antibodies in T. cruzi and L. chagasi infection sera are quite different. Through the use of synthetic peptides, we showed that while T. cruzi infection anti-TcP0 antibodies are exclusively directed against the C-terminal domain of TcP0, L. chagasi infection sera contain antibodies reactive with epitopes other than the C-terminal sequence of LcP0. Thus, anti-LcP0 antibodies in L. chagasi infection sera represent the first characterized deviation from the restricted immunodominant C-terminal epitope involved in the generation of anti-P0 antibodies following infection or autoimmune diseases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G. Analysis of Trypanosoma cruzi antigens bound by specific antibodies and by antibodies to related trypanosomatids. Infect Immun. 1986 Jul;53(1):179–185. doi: 10.1128/iai.53.1.179-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaró R., Reed S. G., Barral A., Orge G., Jones T. C. Evaluation of the micro enzyme-linked immunosorbent assay (ELISA) for antibodies in American visceral leishmaniasis: antigen selection for detection of infection-specific responses. Am J Trop Med Hyg. 1986 Jan;35(1):72–78. doi: 10.4269/ajtmh.1986.35.72. [DOI] [PubMed] [Google Scholar]
- Burns J. M., Jr, Scott J. M., Carvalho E. M., Russo D. M., March C. J., Van Ness K. P., Reed S. G. Characterization of a membrane antigen of Leishmania amazonensis that stimulates human immune responses. J Immunol. 1991 Jan 15;146(2):742–748. [PubMed] [Google Scholar]
- Burns J. M., Jr, Shreffler W. G., Benson D. R., Ghalib H. W., Badaro R., Reed S. G. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. M., Jr, Shreffler W. G., Rosman D. E., Sleath P. R., March C. J., Reed S. G. Identification and synthesis of a major conserved antigenic epitope of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1239–1243. doi: 10.1073/pnas.89.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo M. E., Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies. A simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969 Jul;18(4):500–505. doi: 10.4269/ajtmh.1969.18.500. [DOI] [PubMed] [Google Scholar]
- Campos-Neto A., Bunn-Moreno M. M. Polyclonal B cell activation in hamsters infected with parasites of the genus Leishmania. Infect Immun. 1982 Dec;38(3):871–876. doi: 10.1128/iai.38.3.871-876.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Andrews B. S., Martinelli R., Dutra M., Rocha H. Circulating immune complexes and rheumatoid factor in schistosomiasis and visceral leishmaniasis. Am J Trop Med Hyg. 1983 Jan;32(1):61–68. doi: 10.4269/ajtmh.1983.32.61. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller T. M., Samudio M. A., Zoulek G. IgG antibody reactivity with Trypanosoma cruzi and Leishmania antigens in sera of patients with Chagas' disease and leishmaniasis. Am J Trop Med Hyg. 1990 Dec;43(6):650–656. doi: 10.4269/ajtmh.1990.43.650. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Bonfa E., Llovet R., Danho W., Weissbach H., Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Heckman K. J., Lee J. C., Tan E. M. Identification of ribosomal protein autoantigens. J Immunol. 1985 Oct;135(4):2378–2384. [PubMed] [Google Scholar]
- Galvão-Castro B., Sá Ferreira J. A., Marzochi K. F., Marzochi M. C., Coutinho S. G., Lambert P. H. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol. 1984 Apr;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Hasler P., Brot N., Weissbach H., Parnassa A. P., Elkon K. B. Ribosomal proteins P0, P1, and P2 are phosphorylated by casein kinase II at their conserved carboxyl termini. J Biol Chem. 1991 Jul 25;266(21):13815–13820. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hines J. J., Weissbach H., Brot N., Elkon K. Anti-P autoantibody production requires P1/P2 as immunogens but is not driven by exogenous self-antigen in MRL mice. J Immunol. 1991 May 15;146(10):3386–3395. [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacConnell W. P., Kaplan N. O. The activity of the acidic phosphoproteins from the 80 S rat liver ribosome. J Biol Chem. 1982 May 25;257(10):5359–5366. [PubMed] [Google Scholar]
- Marchiori F., Meggio F., Marin O., Borin G., Calderan A., Ruzza P., Pinna L. A. Synthetic peptide substrates for casein kinase 2. Assessment of minimum structural requirements for phosphorylation. Biochim Biophys Acta. 1988 Oct 7;971(3):332–338. doi: 10.1016/0167-4889(88)90149-8. [DOI] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Sather S., Selkirk M., Agabian N. Identification of a small RNA containing the trypanosome spliced leader: a donor of shared 5' sequences of trypanosomatid mRNAs? Cell. 1984 Oct;38(3):721–729. doi: 10.1016/0092-8674(84)90267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K., Nakagawa T., Tsurugi K. The gene and the primary structure of acidic ribosomal protein A0 from yeast Saccharomyces cerevisiae which shows partial homology to bacterial ribosomal protein L10. J Biochem. 1989 Aug;106(2):223–227. doi: 10.1093/oxfordjournals.jbchem.a122836. [DOI] [PubMed] [Google Scholar]
- Nafziger D. A., Recinos R. F., Hunter C. A., Donelson J. E. Patients infected with Leishmania donovani chagasi can have antibodies that recognize heat shock and acidic ribosomal proteins of Trypanosoma cruzi. Mol Biochem Parasitol. 1991 Dec;49(2):325–328. doi: 10.1016/0166-6851(91)90076-i. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Detre J. A., Casnellie J. E., Greengard P. Serum antibodies that distinguish between the phospho- and dephospho-forms of a phosphoprotein. Nature. 1982 Oct 21;299(5885):734–736. doi: 10.1038/299734a0. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Badaro R., Lloyd R. M. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol. 1987 Mar 1;138(5):1596–1601. [PubMed] [Google Scholar]
- Reed S. G., Carvalho E. M., Sherbert C. H., Sampaio D. P., Russo D. M., Bacelar O., Pihl D. L., Scott J. M., Barral A., Grabstein K. H. In vitro responses to Leishmania antigens by lymphocytes from patients with leishmaniasis or Chagas' disease. J Clin Invest. 1990 Mar;85(3):690–696. doi: 10.1172/JCI114493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Shreffler W. G., Burns J. M., Jr, Scott J. M., Orge M. da G., Ghalib H. W., Siddig M., Badaro R. An improved serodiagnostic procedure for visceral leishmaniasis. Am J Trop Med Hyg. 1990 Dec;43(6):632–639. doi: 10.4269/ajtmh.1990.43.632. [DOI] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. Competition between the elongation factors 1 and 2, and phenylalanyl transfer ribonucleic acid for the ribosomal binding sites in a polypeptide-synthesizing system from brain. J Biol Chem. 1973 Apr 25;248(8):2853–2857. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijman A. G., Levitus G., Levin M. J. Characterization of the C-terminal region of a Trypanosoma cruzi 38-kDa ribosomal P0 protein that does not react with lupus anti-P autoantibodies. Immunol Lett. 1992 Jun;33(1):15–20. doi: 10.1016/0165-2478(92)90087-5. [DOI] [PubMed] [Google Scholar]
- Skeiky Y. A., Benson D. R., Guderian J. A., Sleath P. R., Parsons M., Reed S. G. Trypanosoma cruzi acidic ribosomal P protein gene family. Novel P proteins encoding unusual cross-reactive epitopes. J Immunol. 1993 Nov 15;151(10):5504–5515. [PubMed] [Google Scholar]
- Skeiky Y. A., Benson D. R., Parsons M., Elkon K. B., Reed S. G. Cloning and expression of Trypanosoma cruzi ribosomal protein P0 and epitope analysis of anti-P0 autoantibodies in Chagas' disease patients. J Exp Med. 1992 Jul 1;176(1):201–211. doi: 10.1084/jem.176.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M., Requena J. M., Alonso C. Isolation, characterization and analysis of the expression of the Leishmania ribosomal PO protein genes. Mol Biochem Parasitol. 1993 Oct;61(2):265–274. doi: 10.1016/0166-6851(93)90072-6. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Skelly S., Watson T., Elkon K., Weissbach H., Brot N. The inhibition of protein synthesis by IgG containing anti-ribosome P autoantibodies from systemic lupus erythematosus patients. Arch Biochem Biophys. 1988 Nov 15;267(1):398–403. doi: 10.1016/0003-9861(88)90045-8. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F., Reyes R., Conde P., Ballesta J. P. Acidic ribosomal proteins from eukaryotic cells. Effect on ribosomal functions. Eur J Biochem. 1979 Aug 1;98(2):409–416. doi: 10.1111/j.1432-1033.1979.tb13200.x. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Nagakura K., Kaneda Y. Serodiagnosis of Chagas' disease using monoclonal antibody against Trypanosoma cruzi-specific Mr 25,000 antigen. Parasitol Res. 1988;74(5):409–414. doi: 10.1007/BF00535139. [DOI] [PubMed] [Google Scholar]
- Towbin H., Ramjoué H. P., Kuster H., Liverani D., Gordon J. Monoclonal antibodies against eucaryotic ribosomes. Use to characterize a ribosomal protein not previously identified and antigenically related to the acidic phosphoproteins P1/P2. J Biol Chem. 1982 Nov 10;257(21):12709–12715. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugi K., Collatz E., Todokoro K., Ulbrich N., Lightfoot H. N., Wool I. G. Isolation of eukaryotic ribosomal proteins. Purification and characterization of the 60 S ribosomal subunit proteins La, Lb, Lf, P1, P2, L13', L14, L18', L20, and L38. J Biol Chem. 1978 Feb 10;253(3):946–955. [PubMed] [Google Scholar]
- Uchiumi T., Traut R. R., Kominami R. Monoclonal antibodies against acidic phosphoproteins P0, P1, and P2 of eukaryotic ribosomes as functional probes. J Biol Chem. 1990 Jan 5;265(1):89–95. [PubMed] [Google Scholar]
- Uchiumi T., Wahba A. J., Traut R. R. Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by crosslinking. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5580–5584. doi: 10.1073/pnas.84.16.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidales F. J., Robles M. T., Ballesta J. P. Acidic proteins of the large ribosomal subunit in Saccharomyces cerevisiae. Effect of phosphorylation. Biochemistry. 1984 Jan 17;23(2):390–396. doi: 10.1021/bi00297a032. [DOI] [PubMed] [Google Scholar]
- Weintraub J., Gottlieb M., Weinbaum F. I. Leishmania tropica: association of a B-cell mitogen with hypergammaglobulinemia in mice. Exp Parasitol. 1982 Feb;53(1):87–96. doi: 10.1016/0014-4894(82)90095-9. [DOI] [PubMed] [Google Scholar]