Abstract
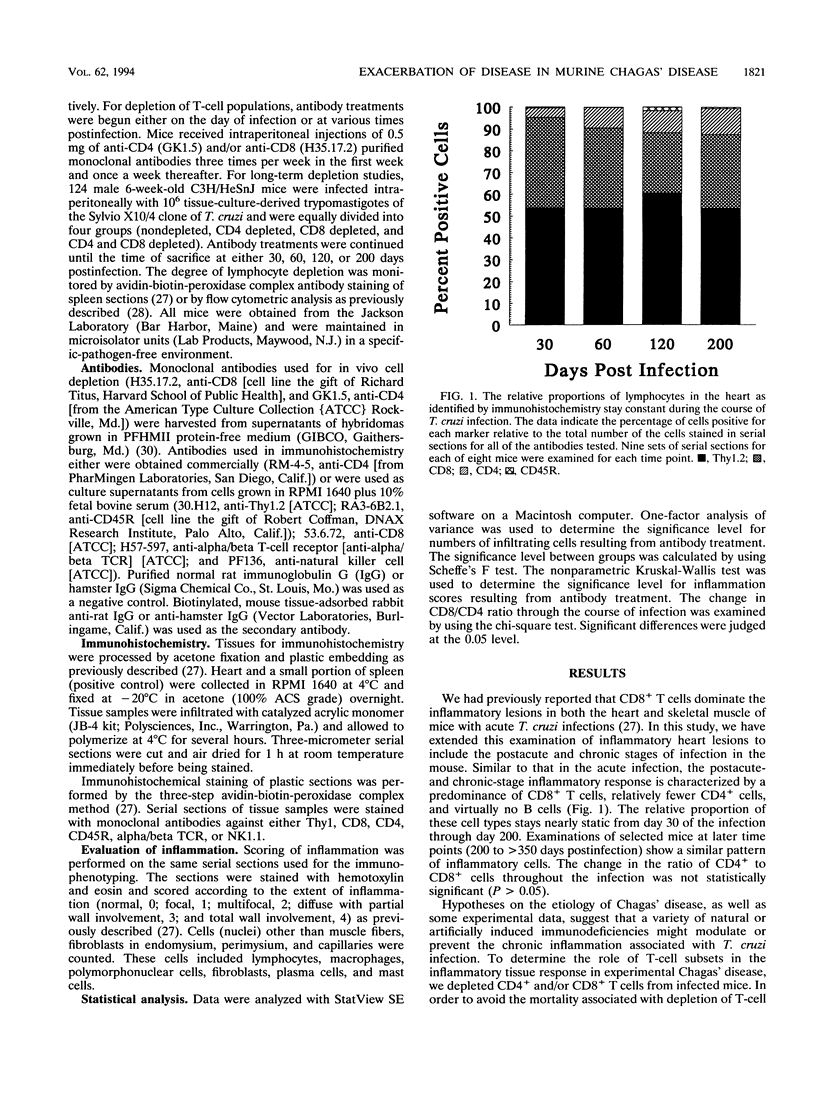
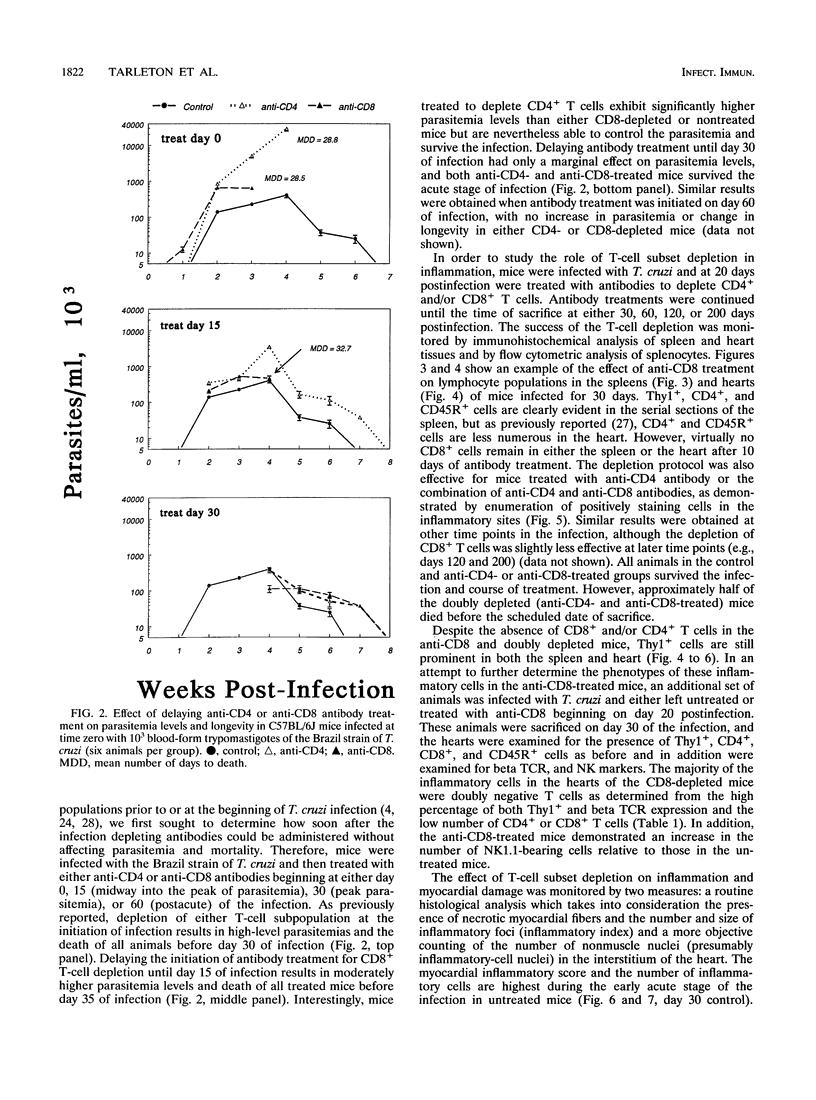
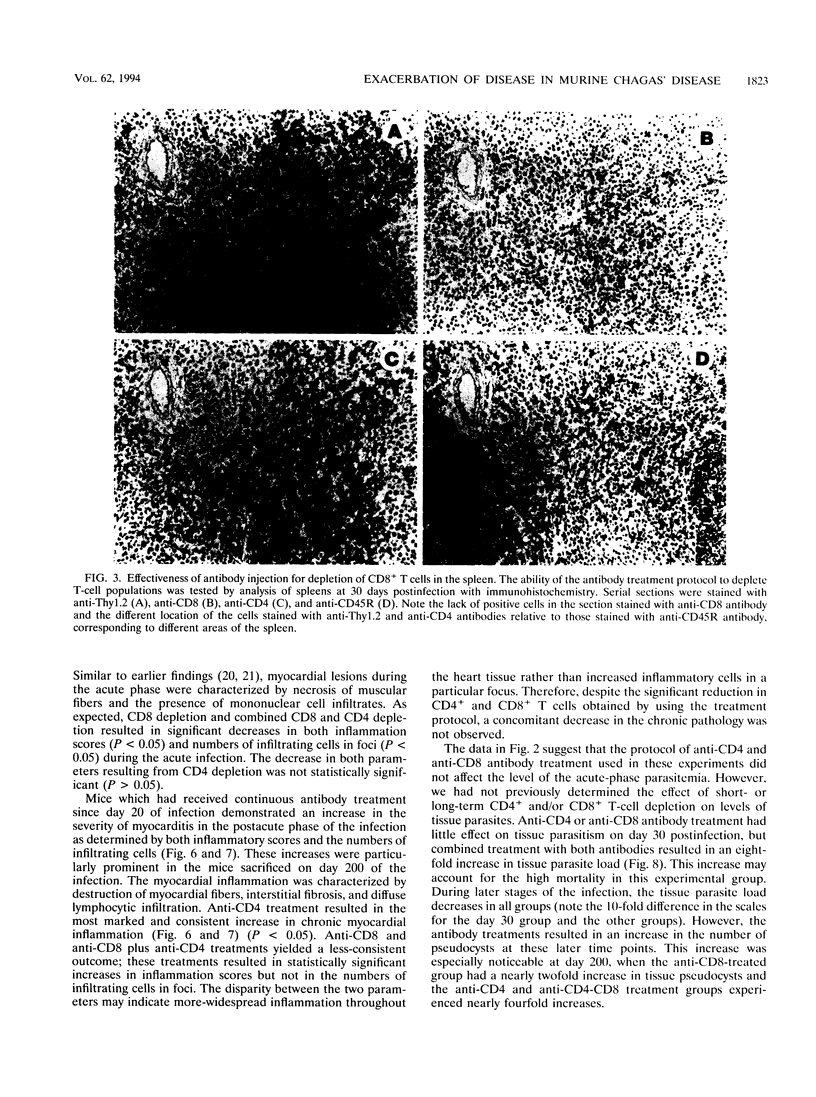
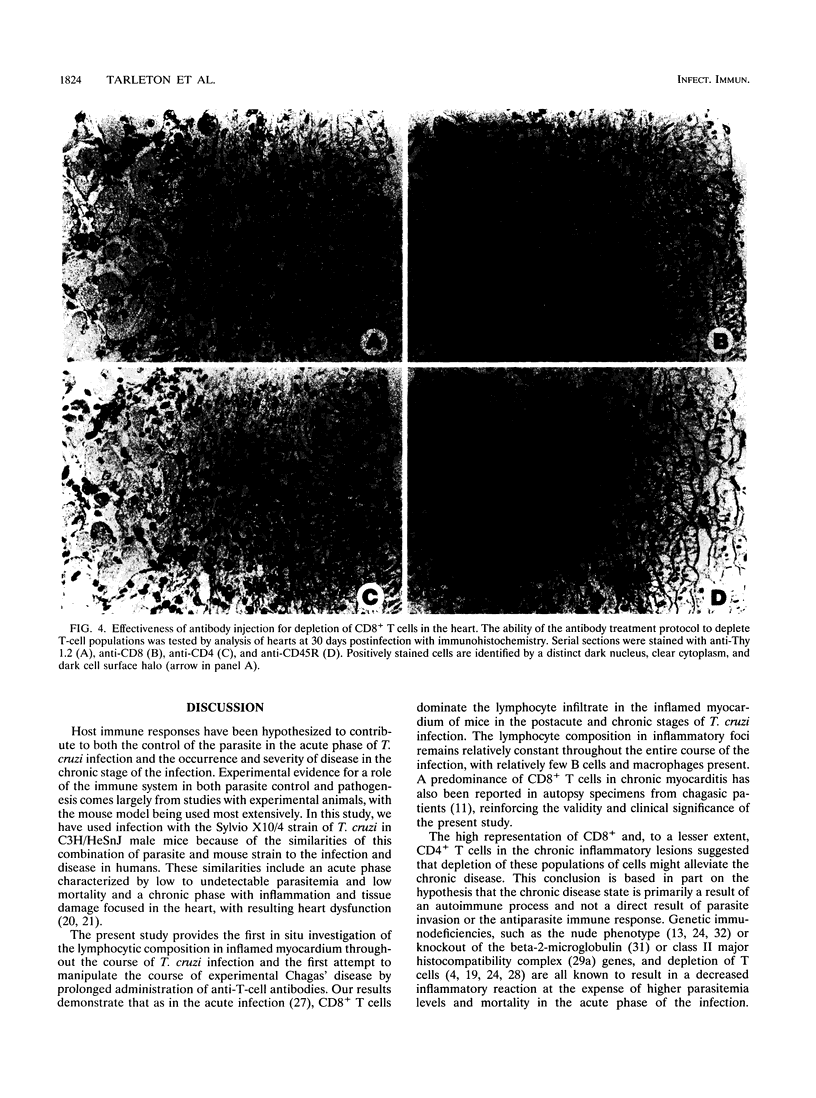
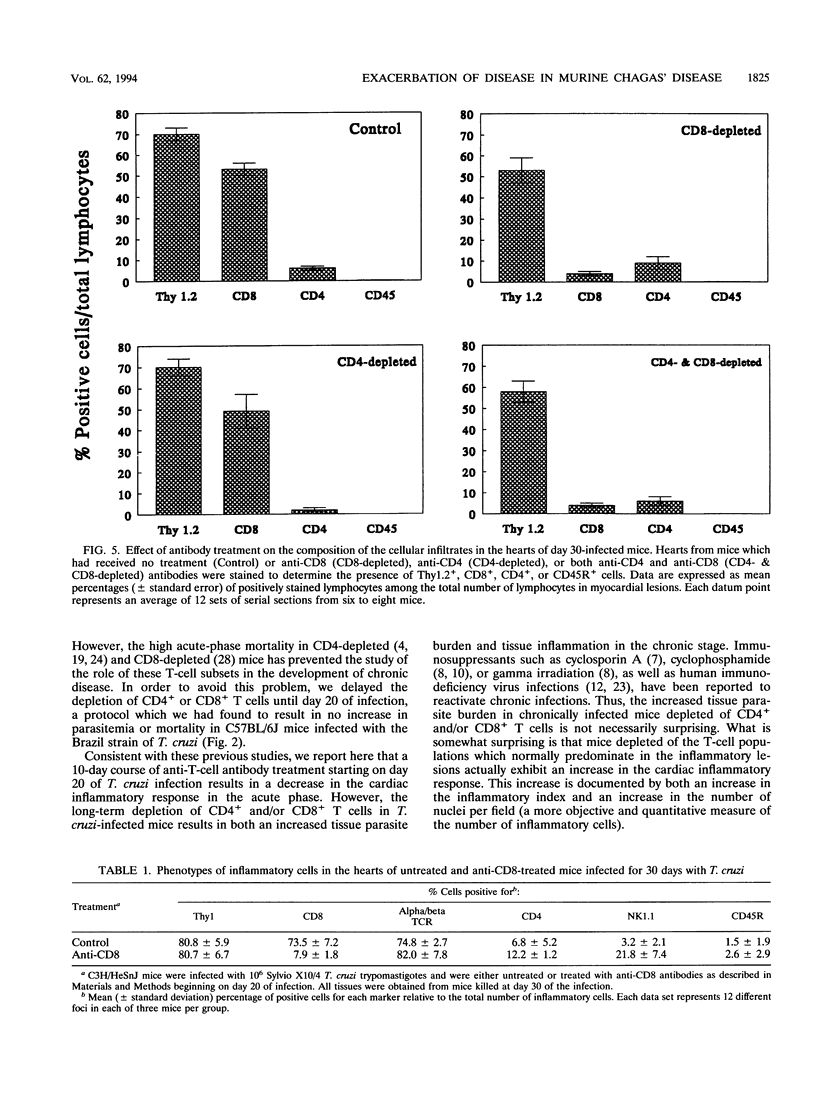
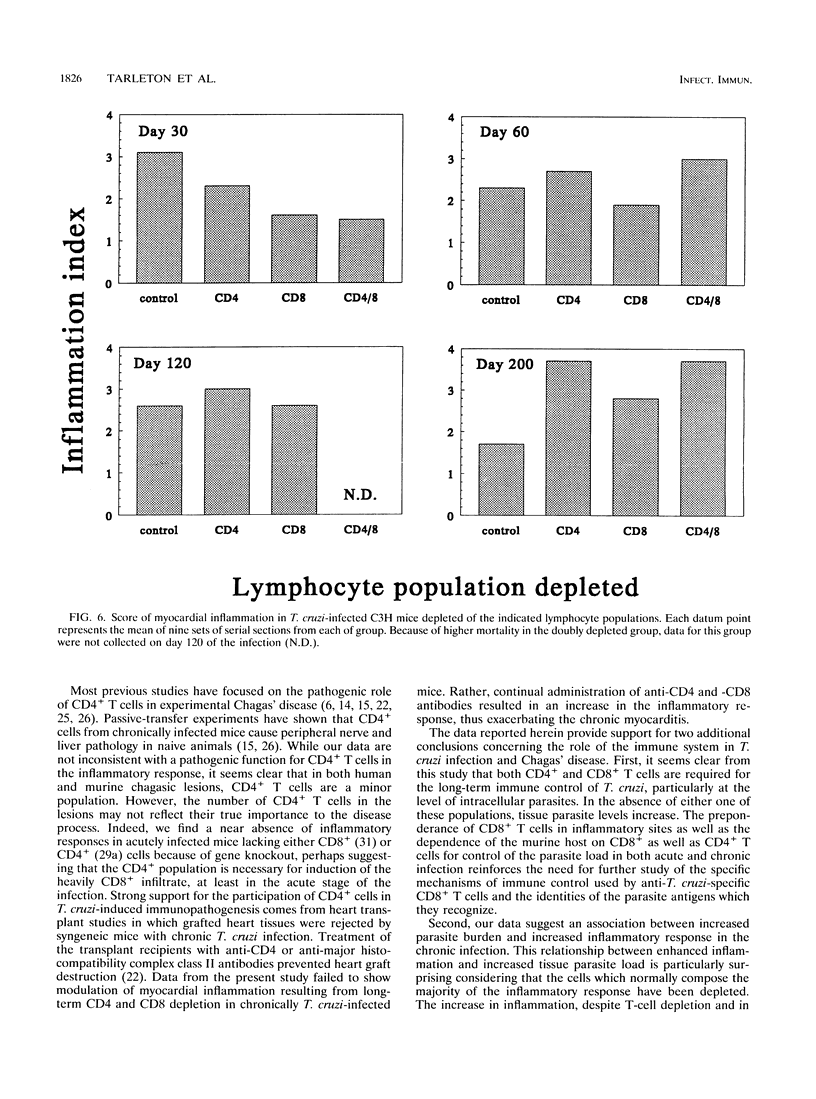
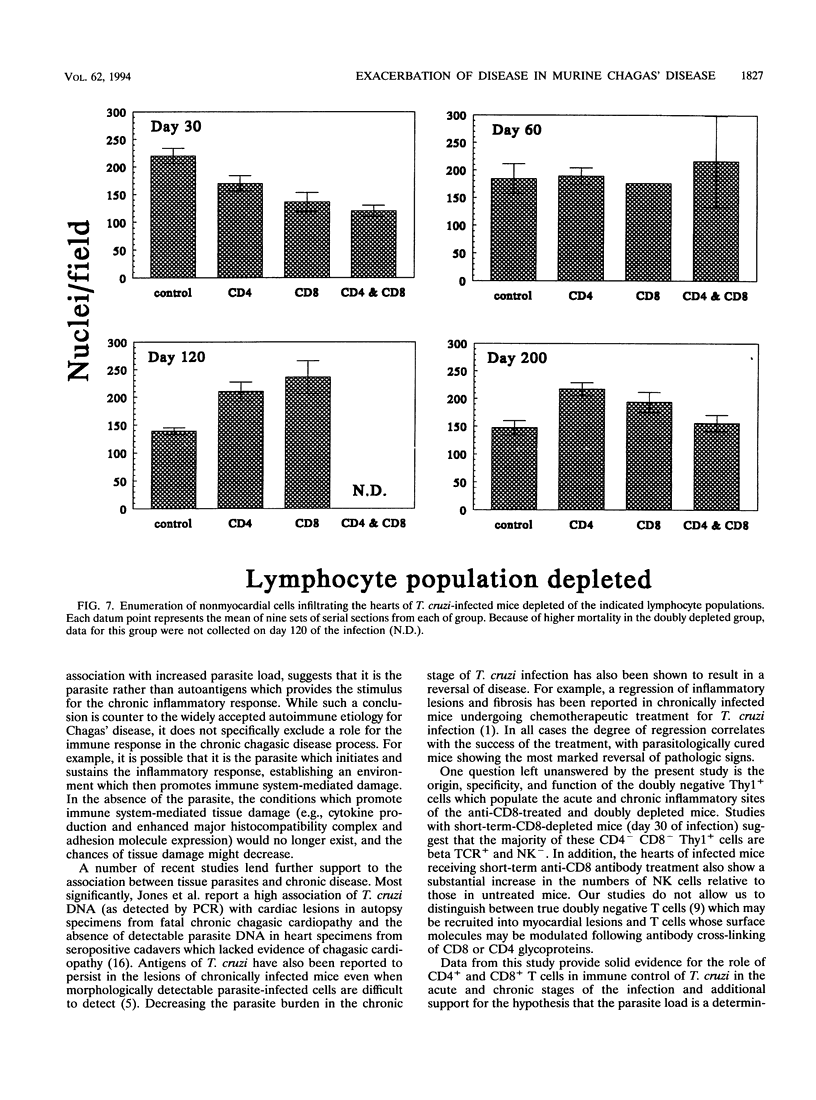
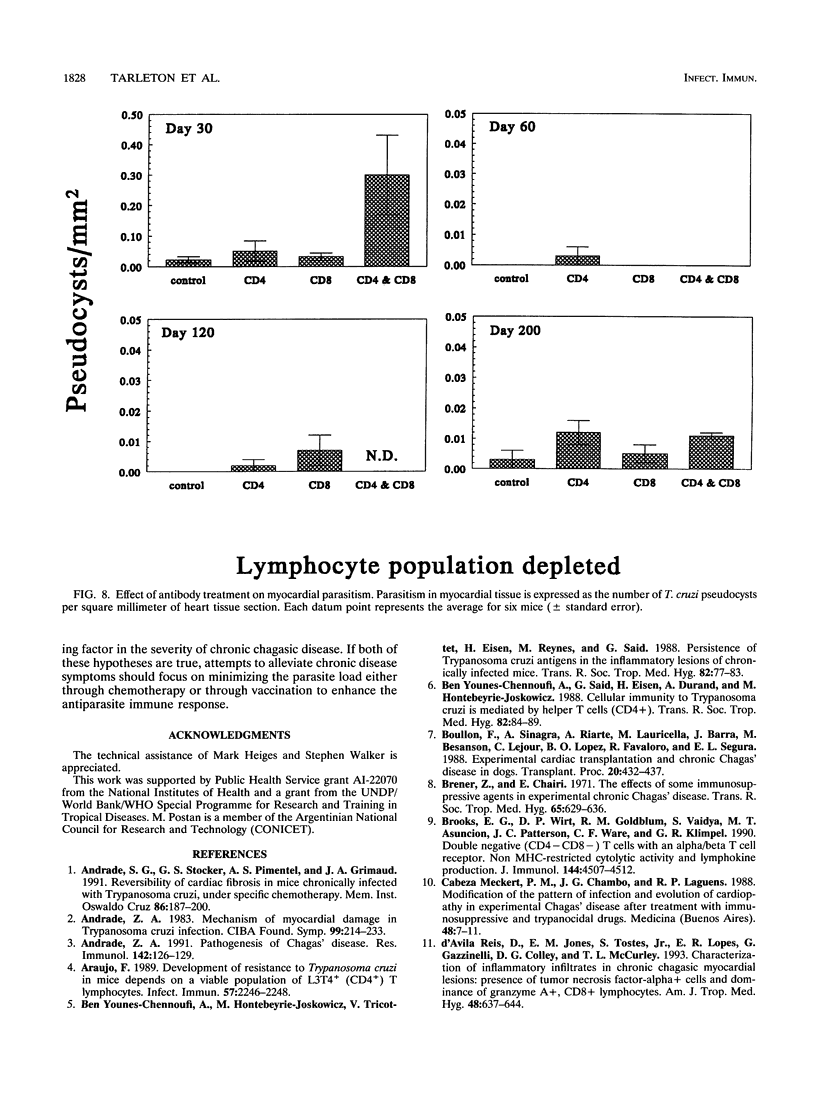
The contribution of T-cell subpopulations to immunopathology in murine Trypanosoma cruzi infection was studied by using in situ localization of lymphocytes and in vivo depletion of T-lymphocyte populations. CD8+ T cells were the major lymphocyte population in the inflamed hearts of C3H/HeSnJ mice infected with the Sylvio X10/4 clone of T. cruzi at all time points of the acute and chronic phases of the infection examined. Depletion of CD8+ and/or CD4+ T cells beginning on day 20 of the infection resulted in a moderate decrease in the inflammation and an increase in parasite burden in the hearts of mice at day 30 of infection. Longer-term depletion, beginning at day 20 and extending as long as 200 days of infection, resulted in an increased inflammatory response in the heart. A large proportion of the inflammatory cells in the hearts of anti-CD8- or anti-CD4- and anti-CD8-treated mice were Thy1+ and CD4- CD8-. At 200 days of infection, the increased inflammation was accompanied by an increase in the parasite load in the heart. These results show that T-cell subset depletion does not prevent the inflammatory response associated with acute and chronic T. cruzi infection. The increased parasite load in T-cell-depleted mice also demonstrates the participation of these T-cell subsets in regulation of parasite load throughout the course of the infection. The increased inflammatory response despite T-cell depletion and in association with increased numbers of tissue parasites suggests that intracellular parasites are a driving force behind the inflammatory response in chronic murine T. cruzi infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. G., Stocker-Guerret S., Pimentel A. S., Grimaud J. A. Reversibility of cardiac fibrosis in mice chronically infected with Trypanosoma cruzi, under specific chemotherapy. Mem Inst Oswaldo Cruz. 1991 Apr-Jun;86(2):187–200. doi: 10.1590/s0074-02761991000200008. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A. Pathogenesis of Chagas' disease. Res Immunol. 1991 Feb;142(2):126–129. doi: 10.1016/0923-2494(91)90021-a. [DOI] [PubMed] [Google Scholar]
- Araujo F. G. Development of resistance to Trypanosoma cruzi in mice depends on a viable population of L3T4+ (CD4+) T lymphocytes. Infect Immun. 1989 Jul;57(7):2246–2248. doi: 10.1128/iai.57.7.2246-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Younès-Chennoufi A., Hontebeyrie-Joskowicz M., Tricottet V., Eisen H., Reynes M., Said G. Persistence of Trypanosoma cruzi antigens in the inflammatory lesions of chronically infected mice. Trans R Soc Trop Med Hyg. 1988;82(1):77–83. doi: 10.1016/0035-9203(88)90269-6. [DOI] [PubMed] [Google Scholar]
- Ben Younès-Chennoufi A., Said G., Eisen H., Durand A., Hontebeyrie-Joskowicz M. Cellular immunity to Trypanosoma cruzi is mediated by helper T cells (CD4+). Trans R Soc Trop Med Hyg. 1988;82(1):84–89. doi: 10.1016/0035-9203(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Boullon F., Sinagra A., Riarte A., Lauricella M., Barra J., Besanson M., Lejour C., Lopez Blanco O., Favaloro R., Segura E. L. Experimental cardiac transplantation and chronic Chagas' disease in dogs. Transplant Proc. 1988 Feb;20(1 Suppl 1):432–437. [PubMed] [Google Scholar]
- Brener Z., Chiari E. The effects of some immunosuppressive agents in experimental chronic Chagas's disease. Trans R Soc Trop Med Hyg. 1971;65(5):629–636. doi: 10.1016/0035-9203(71)90047-2. [DOI] [PubMed] [Google Scholar]
- Brooks E. G., Wirt D. P., Goldblum R. M., Vaidya S., Asuncion M. T., Patterson J. C., Ware C. F., Klimpel G. R. Double-negative (CD4- CD8-) T cells with an alpha/beta T cell receptor. Non-MHC-restricted cytolytic activity and lymphokine production. J Immunol. 1990 Jun 15;144(12):4507–4512. [PubMed] [Google Scholar]
- Cabeza Meckert P. M., Chambó J. G., Laguens R. P. Modification of the pattern of infection and evolution of cardiopathy in experimental Chagas' disease after treatment with immunosuppressive and trypanocidal drugs. Medicina (B Aires) 1988;48(1):7–11. [PubMed] [Google Scholar]
- Ferreira M. S., Nishioka S. de A., Rocha A., Silva A. M., Ferreira R. G., Olivier W., Tostes Júnior S. Acute fatal Trypanosoma cruzi meningoencephalitis in a human immunodeficiency virus-positive hemophiliac patient. Am J Trop Med Hyg. 1991 Dec;45(6):723–727. doi: 10.4269/ajtmh.1991.45.723. [DOI] [PubMed] [Google Scholar]
- Gonçalves da Costa S. C., Lagrange P. H., Hurtrel B., Kerr I., Alencar A. Role of T lymphocytes in the resistance and immunopathology of experimental Chagas' disease. I. Histopathological studies. Ann Immunol (Paris) 1984 May-Jun;135C(3):317–332. doi: 10.1016/s0769-2625(84)80962-9. [DOI] [PubMed] [Google Scholar]
- Hontebeyrie-Joskowicz M. Murine Trypanosoma cruzi infection: a role for TH2 cells in the immunopathology of chronic infection. Res Immunol. 1991 Feb;142(2):141–143. doi: 10.1016/0923-2494(91)90025-e. [DOI] [PubMed] [Google Scholar]
- Hontebeyrie-Joskowicz M., Said G., Milon G., Marchal G., Eisen H. L3T4+ T cells able to mediate parasite-specific delayed-type hypersensitivity play a role in the pathology of experimental Chagas' disease. Eur J Immunol. 1987 Jul;17(7):1027–1033. doi: 10.1002/eji.1830170720. [DOI] [PubMed] [Google Scholar]
- Jones E. M., Colley D. G., Tostes S., Lopes E. R., Vnencak-Jones C. L., McCurley T. L. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg. 1993 Mar;48(3):348–357. doi: 10.4269/ajtmh.1993.48.348. [DOI] [PubMed] [Google Scholar]
- Miles M. A. Letter: Cloning Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1974;68(3):256–256. doi: 10.1016/0035-9203(74)90126-6. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Coutinho A., Spinella S., Hontebeyrie-Joskowicz M. Xid immunodeficiency imparts increased parasite clearance and resistance to pathology in experimental Chagas' disease. Int Immunol. 1991 May;3(5):427–433. doi: 10.1093/intimm/3.5.427. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Eisen H., Joskowicz M., Pereira P., Coutinho A. Suppression of polyclonal antibody production in Trypanosoma cruzi-infected mice by treatment with anti-L3T4 antibodies. J Immunol. 1987 Jul 15;139(2):545–550. [PubMed] [Google Scholar]
- Postan M., Bailey J. J., Dvorak J. A., McDaniel J. P., Pottala E. W. Studies of Trypanosoma cruzi clones in inbred mice. III. Histopathological and electrocardiographical responses to chronic infection. Am J Trop Med Hyg. 1987 Nov;37(3):541–549. doi: 10.4269/ajtmh.1987.37.541. [DOI] [PubMed] [Google Scholar]
- Postan M., Dvorak J. A., McDaniel J. P. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN- mice with two clones isolated from a common source. Am J Trop Med Hyg. 1983 May;32(3):497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- Reis D. D., Jones E. M., Tostes S., Jr, Lopes E. R., Gazzinelli G., Colley D. G., McCurley T. L. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993 May;48(5):637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- Rosemberg S., Chaves C. J., Higuchi M. L., Lopes M. B., Castro L. H., Machado L. R. Fatal meningoencephalitis caused by reactivation of Trypanosoma cruzi infection in a patient with AIDS. Neurology. 1992 Mar;42(3 Pt 1):640–642. doi: 10.1212/wnl.42.3.640. [DOI] [PubMed] [Google Scholar]
- Rottenberg M., Cardoni R. L., Andersson R., Segura E. L., Orn A. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand J Immunol. 1988 Nov;28(5):573–582. doi: 10.1111/j.1365-3083.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Russo M., Starobinas N., Minoprio P., Coutinho A., Hontebeyrie-Joskowicz M. Parasitic load increases and myocardial inflammation decreases in Trypanosoma cruzi-infected mice after inactivation of helper T cells. Ann Inst Pasteur Immunol. 1988 May-Jun;139(3):225–236. doi: 10.1016/0769-2625(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Said G., Joskowicz M., Barreira A. A., Eisen H. Neuropathy associated with experimental Chagas' disease. Ann Neurol. 1985 Dec;18(6):676–683. doi: 10.1002/ana.410180609. [DOI] [PubMed] [Google Scholar]
- Sun J., Tarleton R. L. Predominance of CD8+ T lymphocytes in the inflammatory lesions of mice with acute Trypanosoma cruzi infection. Am J Trop Med Hyg. 1993 Feb;48(2):161–169. doi: 10.4269/ajtmh.1993.48.161. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L., Beyer A. M. Medium-scale production and purification of monoclonal antibodies in protein-free medium. Biotechniques. 1991 Nov;11(5):590–593. [PubMed] [Google Scholar]
- Tarleton R. L. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990 Jan 15;144(2):717–724. [PubMed] [Google Scholar]
- Tarleton R. L., Koller B. H., Latour A., Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992 Mar 26;356(6367):338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- Tarleton R. L. The role of T-cell subpopulations in experimental Chagas' disease. Res Immunol. 1991 Feb;142(2):130–133. doi: 10.1016/0923-2494(91)90022-b. [DOI] [PubMed] [Google Scholar]
- Trischmann T., Tanowitz H., Wittner M., Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978 Aug;45(2):160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- dos Santos R. R., Rossi M. A., Laus J. L., Silva J. S., Savino W., Mengel J. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992 Jan 1;175(1):29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]




